Abstract
Profound thrombocytopenia occurs in humans with sepsis and in mice administered lipopolysaccharide (LPS). Growing evidence indicates that platelets may contribute to these abnormalities, but whether that is a direct result of LPS activation of platelets or an indirect result of other inflammatory mechanisms remains unclear. Here we demonstrate that although platelets do not increase P-selectin expression in response to LPS, platelets bind more avidly to fibrinogen under flow conditions in a Toll-like receptor-4 (TLR4)-dependent manner. In addition, we find that CD41+ megakaryocytes grown from fetal livers and adult circulating platelets express significant amounts of TLR4. LPS induced thrombocytopenia in wild-type mice but not in TLR4-deficient (TLR4def) mice. Wild-type platelets accumulated in the lungs of wild-type mice in response to LPS; TLR4def platelets did not. However, wild-type platelets did not accumulate in the lungs of LPS-treated TLR4def mice. Neutrophils also accumulated in the lungs, and this preceded platelet accumulation. Neutrophil depletion completely abolished LPS-induced platelet sequestration into the lungs, but platelet depletion did not affect neutrophil accumulation. Thus, our data show for the first time that platelets do express functional levels of TLR4, which contribute to thrombocytopenia through neutrophil-dependent pulmonary sequestration in response to LPS. (Blood. 2005;106:2417-2423)
Introduction
Lipopolysaccharide (LPS) or endotoxin is the main structural component of Gram-negative bacteria and an important player in the ability of host cell detection of these foreign pathogens. LPS may also play a fundamental role in sepsis. LPS recognition by mammalian cells occurs through a multiprotein interaction. First, a plasma protein, LPS-binding protein (LBP), binds LPS and transfers LPS monomers to CD14.1 CD14 is a high-affinity receptor for LPS present both as a soluble form in blood or as a glycophosphoinositol (GPI)-anchored protein on the surfaces of myeloid lineage cells. Indeed, CD14-/- mice are at least 100 times more resistant to LPS-induced death.2 However, LPS signaling in cells can only occur if the transmembrane molecule TLR4 is activated. TLR4 belongs to the family of Toll-like receptors that are type 1 transmembrane proteins characterized by an extracellular domain containing multiple leucine-rich repeats, a single transmembrane domain, and an intracellular Toll/interleukin-1 (IL-1) receptor (TIR) domain. Stimulation of TLR4 by LPS activates a signaling cascade that is characterized by the production of proinflammatory cytokines and subsequent immune response. The importance of TLR4 in LPS-induced signaling is emphasized by the fact that TLR4def mice (C57BL/10ScCr), TLR4 knockout mice, and mice with a single point mutation in the TLR4 gene (C3H/HeJ) are resistant to the immunostimulatory and pathophysiologic effects of LPS.3-5
TLR4 is present in many different cell types, including dendritic cells, neutrophils, macrophages, epithelial cells, keratinocytes, and endothelial cells.6-12 Interestingly, dendritic cells express undetectable levels of TLR4 receptor yet they respond avidly to LPS.6 In fact, as few as 100 surface molecules per dendritic cell are sufficient to induce a biologic response. In addition, there are cells that express detectable levels of TLR4 (eg, cardiac myocytes) and yet respond weakly or not at all to LPS.12 Clearly, understanding which cells express TLR4 and, more important, which cells respond to LPS will provide insight into the complex pathology in endotoxemia and perhaps sepsis.
Platelets are among the most understudied cells in sepsis, yet it has been well-described for more than 30 years that patients with sepsis often have low platelet counts and that intravenous injection of LPS into mice induces rapid thrombocytopenia.13 Under these conditions, platelets localize to lung and liver microvasculature.14 Although the mechanisms of activation and recruitment remain unclear, they are thought to be related to the cascade of endogenous mediators released rather than to a direct response to LPS. In fact, the one study that has examined LPS, TLR4, and platelets could detect no surface TLR4 expression.15 A number of other studies, however, have reported some subtle responses of platelets to certain types of LPS and more avid responses to fragments of Gram-negative bacteria.16
Clearly, a reassessment of the mechanism of platelet activation in endotoxemia is warranted in light of recent reports that platelets are potentially important in different inflammatory scenarios, including sepsis.13,17,18 Indeed, 2 studies have shown beneficial effects of GPIIb/IIIa receptor inhibition in patients in endotoxemic shock.19,20 The beneficial effects of GPIIb/IIIa inhibition could be related to reduced recruitment of activated platelets into lungs and liver, where they could cause tissue damage. Indeed, platelets have the capacity to release many different inflammatory mediators to affect local tissue and other inflammatory cells. In addition, platelets can adhere to neutrophils or monocytes to induce transcellular biosynthesis between the 2 cell types to produce mediators that each cell is unable to synthesize alone.21 These mediators could cause ample tissue dysfunction. Finally, immobilized platelets on various substrata can express P-selectin, which can recruit neutrophils.22 Presumably the opposite is also true: adherent neutrophils could also recruit activated platelets either through P-selectin or through other adhesive interactions, including Mac-1/fibrinogen/GPIIb/IIIa and Mac-1/GPIbα.
Therefore, it is important to know the mechanism by which platelets are activated in sepsis. We used a simple model of endotoxemia and the availability of TLR4def mice to elucidate whether platelets could respond to LPS in vivo, whether this occurs through a direct mechanism of platelet TLR4 or is an indirect result of mediators released by other inflammatory cells. We also examined whether wild-type and TLR4def platelets homed to lungs and liver and whether this required activation of the target organs (ie, did the lungs and liver have to be TLR4+?). Finally, we examined a possible role for neutrophils in platelet recruitment into lungs. Our data suggest a very selective activation of platelet TLR4, leading to a P-selectin-independent, neutrophil-dependent recruitment of platelets into lungs.
Materials and methods
Mice
C57Bl/6 mice were purchased from Charles River Laboratories (Montreal, Quebec, Canada). TLR4def mice (C57BL/10ScCr) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice (weight range, 20-35 g; age range, 4-6 weeks) were maintained in a pathogen-free environment. The mice had access to food and water ad libitum. All procedures were performed in accordance with the University of Calgary Animal Care Committee and the Canadian Guidelines for Animal Research.
In vitro experiments
Platelet isolation from mouse blood. Platelets were obtained by a method previously described by Frenette et al.23 Blood from anesthetized mice was obtained by cardiac puncture and collected in citric acid-citrate-dextrose (ACD; 1:9 blood vol/vol). The blood was centrifuged at room temperature for 8 minutes at 228g. Plasma was removed and subsequently centrifuged at 228g for 3 minutes to yield platelet-rich plasma, which was filtered through a Sepharose 2B column (Sigma-Aldrich, Saint Louis, MO) equilibrated with piperazine diethanesulfonic acid (PIPES) buffer (pH 7.0). The activation state of the isolated platelets was checked by examining surface P-selectin expression using FACScan analysis. Only nonactivated gel-filtered platelet preparations were used for the experiments. Platelets were incubated with LPS (5 μg/mL) in the presence of 10% autologous serum for 30 minutes. This dose was chosen to treat the platelets with approximately the same concentration of LPS per milliliter of blood that was used in the in vivo experiments. Thrombin-stimulated platelets were used as positive controls.
Expression of TLR4, P-selectin, and CD41 by flow cytometry. Expression of different molecules on platelets and megakaryocytes was determined in a BD FACScan flow cytometer (BD Biosciences, Mountain View, CA) using CellQuest Pro software (Becton Dickinson Immunocytometry Systems, San Jose, CA). Briefly, 1 × 107 platelets/mL were untreated or were treated with LPS (5 μg/mL) or thrombin (0.2 U/mL) for 30 minutes at room temperature. To determine TLR4 expression, platelets from wild-type and TLR4def mice were stained with phycoerythrin (PE) anti-mouse TLR4/MD2 monoclonal antibody (mAb) (MTS510; eBioscience, San Diego, CA) or an immunoglobulin (Ig) isotype control (PE rat IgG2a; eBioscience). To determine TLR4 expression in human platelets, platelets were stained with PE anti-human TLR4 (HTA125; eBioscience) or an immunoglobulin isotype control (PE mouse IgG2a isotype control; eBioscience). In the TLR4 expression experiments, platelets were first fixed with 1% formalin for 1 hour before labeling. To determine P-selectin expression, platelets were stained with FITC-conjugated rat anti-mouse P-selectin mAb (RB40.34; PharMingen BD Biosciences, San Diego, CA). Fluorescein isothiocyanate (FITC)-conjugated rat IgG1 was used as a monoclonal immunoglobulin isotype control (PharMingen BD Biosciences). To determine TLR4 and CD41 expression on cultured megakaryocytes from fetal mouse liver, megakaryocytes were double stained with PE anti-mouse TLR4/MD2 mAb and FITC rat anti-mouse CD41 (Integrin αIIb) (MWReg30; PharMingen BD Biosciences) or immunoglobulin isotype controls (PharMingen BD Biosciences). After incubation, the platelets and the megakaryocytes were washed in phosphate-buffered saline (PBS) and read on the flow cytometer.
Expression of TLR4. TLR4 expression on the platelet surface was also quantified by using a radiolabeled antibody technique. The mAbs anti-TLR4/MD2 (MTS510; eBioscience) and purified rat IgG2a isotype control (eBioscience) were labeled with sodium iodide I 125 (125I) using the iodogen method, as previously described.24,25 Briefly, 1 × 107 platelets from wild-type and TLR4def mice were labeled with 10 μg/mL 125I-anti-TLR4 or 125I-rat IgG2a isotype control for 30 minutes at room temperature. Then platelets were washed twice with 1 mL PBS underlay with 100 μL heat-inactivated fetal bovine serum (FBS), and the 125I activity was counted. Specific binding was expressed as 125I-anti-TLR4 cpm minus 125I-rat IgG2a isotype control cpm, as previously described.26 In these experiments, platelets from TLR4def mice were used as control.
Determination of platelet adhesion. Platelet interaction with fibrinogen was observed by using a parallel-plate flow chamber (GlycoTech, Rockville, MD) mounted on the stage of an Axiovert 25 (Zeiss, Jena, Germany) microscope with a × 10 eyepiece and a × 40 objective lens for the platelet observation, as previously described.27 Plates were coated with monolayers of fibrinogen (25 μg/mL) at room temperature for 20 minutes just before the experiments. Gel-filtered platelets were fluorescently labeled with calcein am (25 μg/mL; Molecular Probes, Eugene, OR). A fluorescence camera (Pieper GmbH, Berlin, Germany) was used to project the images onto a monitor, and the images were recorded for playback analysis using a videocassette recorder. Untreated, LPS-treated (5 μg/mL for 30 minutes), or thrombin-treated (0.2 U/mL for 30 minutes) platelets diluted in Hanks balanced salt solution (HBSS) (1 × 106/mL) were used in these experiments. Perfusion of platelets onto fibrinogen-coated plates was performed at relative shear force of 0.5 dyne/cm2 for 5 minutes. This shear force was chosen because we observed an optimal platelet interaction with immobilized fibrinogen with the thrombin-treated platelets. The chamber was once again perfused with HBSS to clear nonattached platelets. All images were recorded for playback analysis using a videocassette recorder. The number of firmly adherent platelets was assessed by counting the cells that did not move or detach from the surface for at least 20 seconds.
Megakaryocyte culture. Fetal liver cells were dissociated in PBS from 13.5-day-old embryos. Cells were washed and resuspended in Dulbecco modified Eagle medium (Gibco BRL, Bethesda, MD) with 10% horse serum, 50 ng/mL mouse thrombopoietin (TPO), 20 ng/mL stem cell factor (SCF), 2 mM glutamine, and antibiotic (50 U/mL penicillin and 50 μg/mL streptomycin). These culture conditions lead to primarily CD41+, acetylcholinesterase-positive megakaryocytes, as previously described.28 By 6 days in culture, large megakaryocytic-like cells could be seen on morphologic examination. These large cells proved to be CD41+ on FACScan analysis (see “Results”).
In vivo experiments
Experimental protocol. In the in vivo experiments, each mouse received 0.5 mg/kg or approximately 12 μg LPS intraperitoneally. This dose was chosen from our previous dose-response work. It averts any deaths during the study period, even after anesthesia. LPS from Escherichia coli 0111:B4 (Calbiochem, La Jolla, CA), dissolved in nonpyrogenic water, was added to 0.2 mL sterile saline. This purified LPS elicited identical responses to highly purified LPS (less than 0.0008% contaminating bacterial proteins), as previously reported.29 Moreover, this LPS had no detectable effects in the TLR4def mice. One group of mice received 50 μL rabbit anti-mouse thrombocyte serum per mouse (Accurate Chemical and Scientific Corp, Westbury, NY) intraperitoneally 2 hours before LPS treatment. The anti-mouse thrombocyte serum depleted circulating platelets by 96%. Another group of mice each received 150 μg antineutrophil mAb (RB6-8C5; PharMingen BD Biosciences) 24 hours before LPS treatment. Mice were anesthetized by intraperitoneal injection of a mixture of xylazine hydrochloride (10 mg/kg; MTC Pharmaceuticals, Cambridge, ON, Canada) and ketamine hydrochloride (200 mg/kg; Rogar/STB, London, ON, Canada).
Determination of lung myeloperoxidase activity. Lung samples were weighed, frozen on dry ice, and processed for determination of myeloperoxidase (MPO) activity. MPO is an enzyme found in cells of myeloid origin, and it has been used extensively as a biochemical marker of granulocyte (mainly neutrophil) infiltration into the lung.30,31 Samples were stored at -20°C for no more than 1 week before the MPO assay was performed. MPO activity was determined using an assay described previously, with the volumes of each reagent modified for use in 96-well microtiter plates.32 Change in absorbance at 450 nm over a 90-second period was determined using a kinetic microplate reader (Molecular Devices, Sunnyvale, CA).
51Cr labeling of isolated platelets. Chromium Cr 51 (51Cr) labeling of platelets was performed by a method previously described.33 Isolated platelets suspended in citric ACD were incubated with 51Cr (0.1 mCi [3.7 MBq]) for 1 hour at room temperature. Platelets were then washed in PBS and counted. 51Cr-labeled platelets were diluted with PBS to inject 4 to 5 × 108 platelets per mouse in 0.2 mL. To determine the localization of platelets in untreated and LPS-treated mice (for 4 hours), 51Cr-labeled platelets were injected intravenously and allowed to circulate for 1 hour. A blood sample was obtained from the carotid artery catheter, and then the mice were exsanguinated and the tissues were perfused with PBS. Lungs, heart, liver, pancreas, spleen, mesentery, stomach, small bowel, large bowel, muscle, and skin were harvested and weighed. Platelet sequestration in the different organs was evaluated by analyzing 51Cr activity. 51Cr was measured in plasma and tissue samples. 51Cr activity was calculated per gram of tissue and per milliliter of blood.
Lung electron microscopy. Untreated mice or mice treated with LPS for 4 hours were killed, and lungs were fixed with 2.5% glutaraldehyde by tracheal injection for 1 hour, harvested, resuspended in 2.5% glutaraldehyde, and processed for electron microscopy as previously described.34 Briefly, samples were postfixed for 2 hours at 4°C with 1% osmium tetroxide and subsequently dehydrated in a graded series of acetone solutions. Tissues were then embedded in Epon 812, and ultrathin sections were obtained using an ultramicrotome equipped with a diamond knife (Ultracut E; Reichert-Jung, Vienna, Austria). Sections were stained with uranyl acetate and lead citrate and then were viewed through a transmission electron microscope (TEM H-7000; Hitachi, Tokyo, Japan). Posterior image processing was done with Adobe Photoshop 7 (Adobe Systems, San Jose, CA).
Quantitation of endothelial P-selectin. Expression of the adhesion molecule P-selectin was quantified using a modified dual-radiolabeled antibody technique, as we previously described.5 To study P-selectin, anesthetized animals were injected intravenously with a mixture of 10 μg 125I-anti-labeled P-selectin (RB40.34), and a variable dose of 131I-labeled nonbinding antibody (A110-1). Antibodies were allowed to circulate for 5 minutes, and then a blood sample was obtained from a carotid artery catheter. Mice were exsanguinated by blood withdrawal through the carotid artery catheter and simultaneous intravenous infusion with bicarbonate-buffered saline. Lungs were harvested and weighed. Activities of 131I and 125I were measured in plasma and tissue samples.
P-selectin expression was calculated per gram of tissue by subtracting the accumulated activity of the nonbinding antibody (131I-labeled antibody) from the accumulated activity of the binding antibody (125I-labeled antibody). Data for P-selectin were represented as a percentage of injected dose of antibody per gram of tissue (%ID/g). It has been previously demonstrated that this approach provides reliable quantitative values of adhesion molecule expression.25 The technique is sufficiently sensitive that very small basal levels of P-selectin can be detected in wild-type mice relative to P-selectin-deficient mice, in which values are zero.25 Moreover, adding excess cold antibody has previously been shown to displace all radiolabeled antibody.35
Circulating leukocyte and platelet counts. Whole blood from anesthetized mice was drawn by cardiac puncture. Total leukocyte and platelet counts were performed using a bright-line hemacytometer (Hausser Scientific, Horsham, PA). The number of platelets was counted using an automatic blood cell machine (STK-S; Coulter, Seattle, WA).
Statistical analysis. All data are displayed as mean plus or minus SEM. All data were analyzed using the Student t test with Bonferroni correction for multiple comparisons. A P value below .05 was considered statistically significant.
TLR4 expression on platelets by flow cytometry. Platelets from wild-type (WT) and TLR4def (TLR4def) mice were isolated and stained for TLR4 expression (see “Materials and methods”). (A) Representative histogram obtained by flow cytometry for TLR4 stain. In each histogram, the percentage of positive cells for TLR4 is shown. (B) Mean fluorescence intensity obtained by flow cytometry. (C) TLR4 expression observed by using 125I-anti-TLR4 mAb. Data are expressed as the arithmetic mean ± SEM from 3 to 6 separate donors of platelets in each group. *P < .01 relative to wild-type mice.
TLR4 expression on platelets by flow cytometry. Platelets from wild-type (WT) and TLR4def (TLR4def) mice were isolated and stained for TLR4 expression (see “Materials and methods”). (A) Representative histogram obtained by flow cytometry for TLR4 stain. In each histogram, the percentage of positive cells for TLR4 is shown. (B) Mean fluorescence intensity obtained by flow cytometry. (C) TLR4 expression observed by using 125I-anti-TLR4 mAb. Data are expressed as the arithmetic mean ± SEM from 3 to 6 separate donors of platelets in each group. *P < .01 relative to wild-type mice.
Results
Murine platelets express TLR4 receptor
Expression of TLR4 on resting murine platelets was demonstrated by flow cytometry. Figure 1A shows representative histograms of flow cytometry for TLR4 stain on platelets from wild-type and TLR4def mice. On average, 45.7% ± 2.6% TLR4+ platelets were seen in wild-type mice. This was accompanied by a significant increase in the mean fluorescence intensity in wild-type mice (Figure 1B). In TLR4def mice, TLR4 was not detected on platelets (Figure 1A-B). Specific binding of TLR4 was also confirmed by using 125I-anti-TLR4 mAb. We observed that platelets from wild-type mice expressed TLR4 whereas platelets from TLR4def mice did not (Figure 1C). It is noteworthy that platelets had to be fixed before they were stained with TLR4 antibody. Adding the TLR4 antibody before fixing dramatically increased the variability of TLR4 expression, generally resulting in less TLR4 expression. It is conceivable that some TLR4 internalization or cleavage of the receptor occurred with the addition of antibody, as previously described for GPVI.36 We also found significant expression of TLR4 on human platelets (37% ± 5%, SEM; n = 4), suggesting the finding is not restricted to rodents. The expression of TLR4 was also demonstrated in megakaryocytes (Figure 2), in which we found that the percentage of positive cells for TLR4 expression increased as the megakaryocytes matured (increased CD41 expression).
TLR4 expression on developing megakaryocytes by flow cytometry. Cultured and harvested megakaryocytes from fetal liver were double stained for CD41 and TLR4 expression. (A) TLR4 expression for isotype control and the different levels (low, medium, high) of CD41 expression. (B) Mean fluorescence intensity obtained for the low, medium, and high levels of CD41 expression.
TLR4 expression on developing megakaryocytes by flow cytometry. Cultured and harvested megakaryocytes from fetal liver were double stained for CD41 and TLR4 expression. (A) TLR4 expression for isotype control and the different levels (low, medium, high) of CD41 expression. (B) Mean fluorescence intensity obtained for the low, medium, and high levels of CD41 expression.
LPS increases platelet adhesion to fibrinogen but not P-selectin expression
The effect of LPS on platelet adhesion to fibrinogen at low shear rates was studied. Under basal conditions, few platelets adhered to fibrinogen. However, treatment of the platelets with LPS significantly increased platelet adhesion (Figure 3). LPS was approximately 50% as effective as thrombin in inducing adhesion. LPS-induced platelet adhesion was dependent on TLR4 because platelets from TLR4def mice treated with LPS did not exhibit increased adhesion (Figure 3). However, TLR4def platelets could bind fibrinogen because platelets from TLR4def mice exhibited increased adhesion in response to thrombin (data not shown).
Interestingly, LPS did not induce platelet P-selectin expression (mean fluorescent intensity, 10.1 ± 2.7 vs 11.0 ± 3.2; LPS-treated platelets vs untreated platelets; n = 5). However, thrombin-stimulated platelets increased surface P-selectin expression significantly (mean fluorescent intensity, 153.3 ± 33.4 vs 11.0 ± 3.2; thrombin-treated platelets vs untreated platelets; P < .01, n = 5).
P-selectin expression was also determined in vivo using the dual-radiolabeled antibody technique. Consistent with previous data, LPS induced a significant increase in lung P-selectin expression (I.D./g tissue, 0.11% ± 0.06% vs 4.38% ± 0.92%, untreated vs LPS treated; n = 5; P < .01). Interestingly, when mice were rendered thrombocytopenic before LPS treatment, the lungs showed the same levels of P-selectin expression (I.D./g, 4.25% ± 0.39%; n = 4) observed in platelet-sufficient LPS-treated mice. Clearly, in vivo platelets did not contribute to P-selectin despite their accumulation in lung (see the next section).
Systemic LPS induces thrombocytopenia and platelet sequestration into the lungs through a TLR4-dependent mechanism in vivo
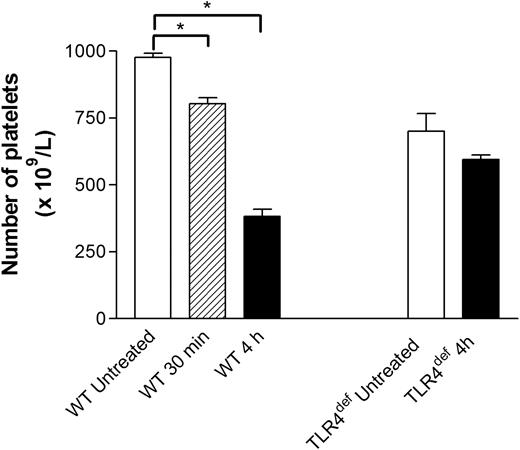
The number of circulating platelets was analyzed in untreated and LPS-treated wild-type mice. Intraperitoneal injection of LPS induced mild (15%) thrombocytopenia within 30 minutes (Figure 4). However, by 4-hour treatment, a 60% decrease in the number of circulating platelets was observed. Although there were consistently approximately 20% fewer platelets in TLR4def mice than in wild-type mice, no decrease was noted with LPS at 30 minutes (data not shown) or at 4 hours (Figure 4).
Effects of LPS on platelet adhesion to fibrinogen-coated plates. Isolated platelets (1 × 106/mL) from wild-type mice or TLR4def mice were untreated or treated with LPS (5 μg/mL) or thrombin (0.2 U/mL). Adhesion of platelets to fibrinogen-coated plates was analyzed by using a parallel-plate flow chamber (see “Materials and methods”). Data are expressed as the arithmetic mean ± SEM from 4 separate donors of platelets in each group, in which 4 to 6 fields were analyzed. *P < .05 versus untreated platelets from wild-type mice. #P < .01 versus thrombin-treated platelets from wild-type mice.
Effects of LPS on platelet adhesion to fibrinogen-coated plates. Isolated platelets (1 × 106/mL) from wild-type mice or TLR4def mice were untreated or treated with LPS (5 μg/mL) or thrombin (0.2 U/mL). Adhesion of platelets to fibrinogen-coated plates was analyzed by using a parallel-plate flow chamber (see “Materials and methods”). Data are expressed as the arithmetic mean ± SEM from 4 separate donors of platelets in each group, in which 4 to 6 fields were analyzed. *P < .05 versus untreated platelets from wild-type mice. #P < .01 versus thrombin-treated platelets from wild-type mice.
Effect of systemic LPS on circulating platelet counts in wild-type and TLR4def mice. Wild-type and TLR4def mice were untreated or treated with LPS for 30 minutes or for 4 hours. At these times, samples of blood were drawn by cardiac puncture to assess the number of circulating platelets (see “Materials and methods”). Data are expressed as the arithmetic mean ± SEM from 5 to 8 mice in each group. *P < .01 versus untreated wild-type mice.
Effect of systemic LPS on circulating platelet counts in wild-type and TLR4def mice. Wild-type and TLR4def mice were untreated or treated with LPS for 30 minutes or for 4 hours. At these times, samples of blood were drawn by cardiac puncture to assess the number of circulating platelets (see “Materials and methods”). Data are expressed as the arithmetic mean ± SEM from 5 to 8 mice in each group. *P < .01 versus untreated wild-type mice.
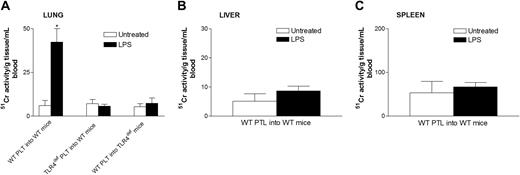
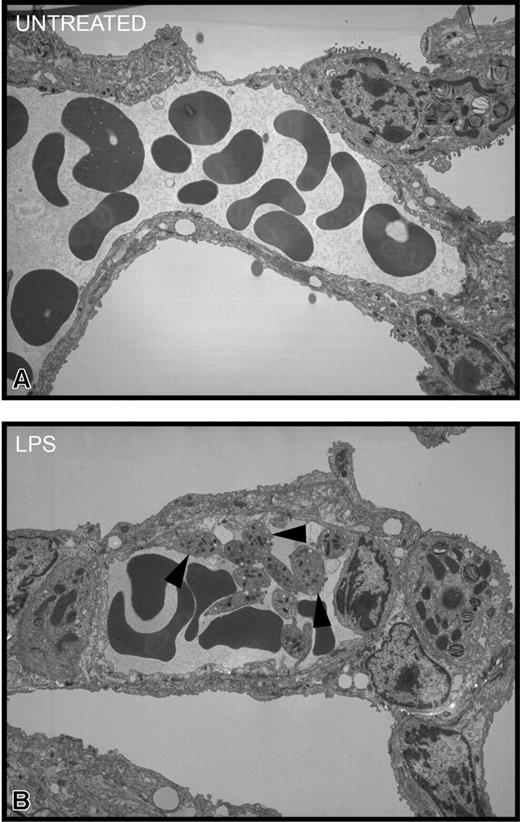
To determine where the platelets localized during endotoxemia, we injected 51Cr-labeled platelets into mice and waited 1 hour before assessing the accumulation of radioactive platelets in tissues. In untreated mice, there was no selective accumulation of platelets into any tissues (Figure 5). However, a significant accumulation of wild-type platelets was noted in lungs of LPS-treated wild-type mice (Figure 5A). By contrast, there was no significant platelet accumulation into the liver (Figure 5B), spleen (Figure 5C), or any other organ (not shown) in LPS-treated wild-type mice. Clearly, unlike previous studies using high quantities of LPS,37 the 12 μg LPS per mouse used in our study did not induce recruitment into liver. Most important, platelets from TLR4def mice did not accumulate into the lungs of LPS-treated wild-type mice, suggesting a potential role for TLR4 on platelets in the accumulation of these cells in the pulmonary microvasculature. In spite of the fact that the wild-type mice had been activated for 4 hours with LPS, allowing ample time for the release of various mediators from macrophages and other cells, the TLR4def platelets did not adhere in lungs. Clearly, even at 4 hours of endotoxemia, LPS was the predominant stimulus for platelet accumulation in lung. To determine whether platelets activated by LPS in vivo predominantly homed to lungs (regardless of stimulus status of the lungs), TLR4def mice received LPS,29 and, not surprisingly, wild-type platelets did not accumulate into the lungs of TLR4def mice (Figure 5). These data show that the presence of TLR4 on cells in the lungs is also critical for platelet accumulation into the lungs. To confirm these 51Cr studies, we performed electron microscopy and observed platelet accumulation in the pulmonary microvasculature of LPS-treated, but not untreated, wild-type mice (Figure 6).
Effect of systemic LPS on platelet sequestration in lung, liver, and spleen in wild-type and TLR4defmice. Wild-type and TLR4def mice were untreated or treated with LPS for 3 hours. At this time, the mice received 4 to 8 × 10851Cr-labeled platelets in 0.2 mL per mouse that were allowed to circulate for 1 hour. Untreated and LPS treated wild-type mice received platelets (PLT) from wild-type mice (WT PLT into WT mice) or from TLR4def mice (TLR4def PLT into WT mice). Untreated and LPS-treated TLR4def mice received platelets from wild-type mice (WT mice into TLR4def mice). Lung (A), liver (B), spleen (C), and other organs were then harvested and radioactivity was analyzed, as explained in “Materials and methods.” Platelet sequestration in the different organs was evaluated by analyzing 51Cr activity (see “Materials and methods”). Data are expressed as the arithmetic mean ± SEM from 4 mice in each group. *P < .005 versus untreated wild-type mice.
Effect of systemic LPS on platelet sequestration in lung, liver, and spleen in wild-type and TLR4defmice. Wild-type and TLR4def mice were untreated or treated with LPS for 3 hours. At this time, the mice received 4 to 8 × 10851Cr-labeled platelets in 0.2 mL per mouse that were allowed to circulate for 1 hour. Untreated and LPS treated wild-type mice received platelets (PLT) from wild-type mice (WT PLT into WT mice) or from TLR4def mice (TLR4def PLT into WT mice). Untreated and LPS-treated TLR4def mice received platelets from wild-type mice (WT mice into TLR4def mice). Lung (A), liver (B), spleen (C), and other organs were then harvested and radioactivity was analyzed, as explained in “Materials and methods.” Platelet sequestration in the different organs was evaluated by analyzing 51Cr activity (see “Materials and methods”). Data are expressed as the arithmetic mean ± SEM from 4 mice in each group. *P < .005 versus untreated wild-type mice.
Transmission electron micrographs of pulmonary interstitium taken at a magnification of × 3500. (A) Representative electron micrograph of untreated mice. This section shows a small arteriole/venule in the interstitium containing numerous red blood cells and plasma. Neither platelets nor neutrophils were apparent. Occasionally, solitary platelets were seen in other sections. (B) Representative electron micrograph of LPS-treated wild-type mice for 4 hours. This section shows, in addition to red blood cells and plasma, numerous platelets (arrowheads), many of which are adherent to the endothelial surface and to each other. There is no evidence of fibrin thrombus formation, and the platelets are not degranulating.
Transmission electron micrographs of pulmonary interstitium taken at a magnification of × 3500. (A) Representative electron micrograph of untreated mice. This section shows a small arteriole/venule in the interstitium containing numerous red blood cells and plasma. Neither platelets nor neutrophils were apparent. Occasionally, solitary platelets were seen in other sections. (B) Representative electron micrograph of LPS-treated wild-type mice for 4 hours. This section shows, in addition to red blood cells and plasma, numerous platelets (arrowheads), many of which are adherent to the endothelial surface and to each other. There is no evidence of fibrin thrombus formation, and the platelets are not degranulating.
Neutrophils are essential for platelet sequestration into the lungs, but platelets are not essential for neutrophil recruitment
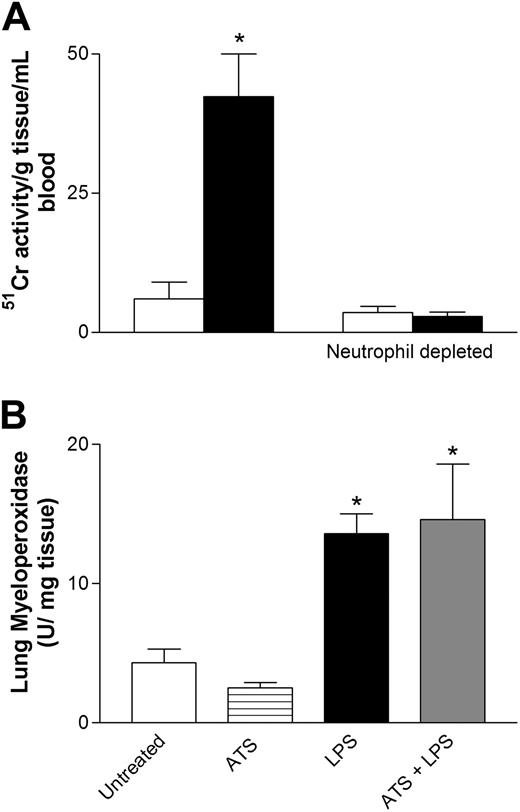
As can be seen by these results, we showed that the presence of TLR4 on other cell types is critical for platelet accumulation into the lungs. In addition, we had previously demonstrated that LPS induced rapid neutropenia accompanied by neutrophil accumulation into the lungs of wild-type mice.5 It has also been reported that platelet-to-neutrophil adhesion is enhanced in patients with septicemia.38 In this study neutrophils infiltrated the lung within 30 minutes and were retained for at least 4 hours (data not shown). Thus, to test whether neutrophils are the critical cell type in LPS-induced thrombocytopenia and platelet sequestration into the lungs, mice were rendered neutropenic before LPS treatment. Anti-neutrophil antibody pretreatment of mice was found to deplete neutrophils by 90%. Interestingly, when these mice were rendered neutropenic before LPS treatment, 51Cr-labeled platelets from wild-type mice did not accumulate into the lungs of LPS-treated wild-type mice (Figure 7A). The results of these experiments implicate neutrophils as critical contributors in LPS-induced platelet sequestration into the lungs. Conversely, when mice were depleted of platelets, the accumulation of neutrophils in response to LPS was not reduced. Figure 7B illustrates that MPO as an index of neutrophil presence is increased significantly at 4 hours of LPS administration within the lung, regardless of platelet depletion.
Platelet recruitment into endotoxemic lungs with or without neutrophils. (A) Amount of wild-type platelet recruitment into the lung in untreated and LPS-treated mice. In a second series of experiments, mice received anti-neutrophil antibody and then received LPS and wild-type 51Cr-labeled platelets. □ indicates untreated; ▪, LPS. (B) Summary of the amount of neutrophil influx (myeloperoxidase) into lungs in response to LPS in mice that did or did not receive antithrombocyte serum (ATS). Data are expressed as the arithmetic mean ± SEM from 4 mice in each group. *P < .05 versus groups not receiving LPS.
Platelet recruitment into endotoxemic lungs with or without neutrophils. (A) Amount of wild-type platelet recruitment into the lung in untreated and LPS-treated mice. In a second series of experiments, mice received anti-neutrophil antibody and then received LPS and wild-type 51Cr-labeled platelets. □ indicates untreated; ▪, LPS. (B) Summary of the amount of neutrophil influx (myeloperoxidase) into lungs in response to LPS in mice that did or did not receive antithrombocyte serum (ATS). Data are expressed as the arithmetic mean ± SEM from 4 mice in each group. *P < .05 versus groups not receiving LPS.
Discussion
It has been known for more than 30 years that patients with sepsis have a high incidence of thrombocytopenia. Moreover, thrombocytopenia is closely associated with an increased mortality rate.39,40 Although thrombocytopenia has also been reported in animals given LPS, the mechanisms responsible for its development are not fully understood. In this study we showed that intraperitoneal injection of LPS at 4 hours induced a 60% decrease in the number of circulating platelets in wild-type mice but not in TLR4def mice, suggesting that the thrombocytopenia was TLR4 dependent. The platelets appeared to accumulate primarily in the pulmonary microvasculature. One likely possibility was that the activation of macrophages and other immune cells caused increased cytokine production that indirectly activated the platelets to accumulate in lungs. Our data, however, eliminate this possibility inasmuch as platelets from TLR4def mice did not accumulate into the lungs of LPS-treated wild-type mice, whereas wild-type platelets did adhere in endotoxemic lungs. In fact, we were able to detect TLR4 on platelets and megakaryocytes and could induce selective adhesion (not general activation) of platelets directly by LPS. These data for the first time raise the possibility that platelet TLR4 is an essential molecule for LPS-induced platelet accumulation into the lungs.
There is some discrepancy regarding whether platelets express TLR4. Although Montrucchio et al15 report no TLR4 on platelets, Cognasse et al41 report that human platelets do express a number of TLRs, including TLR4. When we tried to measure TLR4 using standard flow cytometry techniques, the results were variable and of much lower expression than if we first fixed the platelets. Fixing the platelets revealed consistent, significant TLR4 levels on wild-type but not TLR4def platelets. Because megakaryocytes, obtained through in vitro culture, expressed TLR4 as they became CD41high (a marker of platelets), the data suggest that platelets acquire expression of this receptor during their genesis as protoplatelets from megakaryocyte membranes. Our data in this study also further extend the work to suggest that the TLR4 on platelets had clear functional significance.
Montrucchio et al15 have reported negative results for TLR4 on platelets and no P-selectin expression in response to LPS. Nevertheless, other laboratories have observed increased platelet aggregation but not P-selectin expression in response to LPS, whereas fragments of Gram-negative bacteria induced both.16 Although P-selectin is often considered a good marker of platelet activation, clearly our study and others have observed platelet adhesion independent of P-selectin in response to LPS. Our own results support the lack of P-selectin expression in response to LPS, but our results also suggest that LPS can activate the platelet sufficiently to adhere to fibrinogen in vitro. Indeed, the adhesion of platelets to fibrinogen involves GPIIb/IIIa (CD41/CD61) and not P-selectin interactions.42 In addition, Carvalho-Tavares et al43 reported that platelet adhesion to endothelium helped to tether neutrophils to inflamed brain microvessels and that P-selectin-deficient platelets retain their ability to induce this recruitment. Similarly, adhesion to endothelium involves P-selectin-independent binding of platelets to fibrinogen through GPIIb/IIIa and to von Willebrand factor through GP1bα.44 Consistent with these observations, we also did not discern a difference in P-selectin expression in vivo, when mice had normal platelet counts or were rendered thrombocytopenic and then given LPS. These data as a whole suggest that P-selectin expression levels may not always necessarily be a good marker of activated platelets in vivo, particularly during endotoxemia-associated conditions.
It is interesting that platelets would “home” to lungs during systemic LPS administration, particularly given that all the vascular beds are activated and would therefore be predicted to support platelet recruitment. This was particularly intriguing in light of previous observations that neutrophils also primarily accumulate in the pulmonary microvasculature but not in other tissues.29 Previously, platelet-neutrophil aggregates have been described in endotoxemic and septic blood, which would likely have been “filtered” or trapped within the pulmonary microvasculature.38 Indeed, removing neutrophils prevented platelet accumulation in lungs, strongly implicating the neutrophil as a key player in platelet accumulation. However, our own data suggest that neutrophils already adherent in the lung are responsible for the subsequent recruitment of platelets. First, kinetic work in this and a previous study29 demonstrated that most neutrophils were recruited into lung as early as 30 minutes after LPS administration. By contrast, circulating platelet counts did not decrease to the same extent until significantly later time points, suggesting platelet accumulation after neutrophil accumulation. Second, the depletion of platelets from the circulation (to prevent accumulation in the lung) did not inhibit neutrophil accumulation into endotoxemic lungs, whereas removal of neutrophils from the circulation (so they did not accumulate in lungs) prevented subsequent platelet accumulation into endotoxemic lungs. Overall these data are consistent with the hypothesis that accumulating neutrophils facilitate the binding of platelets within the lung. Whether the platelets bind directly to neutrophils or whether the neutrophils activate the endothelium on adherence to induce platelet binding to endothelium remains unknown. Our electron micrograph data suggest that platelets bind to endothelium and to each other.
In summary, we have shown for the first time that platelets express functional levels of TLR4. The presence of TLR4 on platelets is essential for the LPS-induced thrombocytopenia and platelet accumulation in the lungs.
Prepublished online as Blood First Edition Paper, June 16, 2005; DOI 10.1182/blood-2005-03-0916.
Supported by a grant from Canadian Institutes of Health Research (CIHR).
G.A. is a Canadian Association of Gastroenterology (CAG)-CIHR-AstraZeneca Inc fellow. K.M. is a Michael Smith Foundation for Health Research Scientist. K.D.P. is an Alberta Heritage Foundation for Medical Research Senior Scholar and a Canada Research Chair recipient. P.K. is an Alberta Heritage Foundation for Medical Research Scientist and a Canada Research Chair recipient.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Krista McRae and Lori Zbytnuik for technical assistance and Dr Francis Green, a pathologist, for assessment of electron micrographs.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal