Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a hematologic disorder characterized by clonal expansion of red blood cells (RBCs) lacking the ability to inhibit complement-mediated hemolysis. Eculizumab, a humanized monoclonal antibody that binds the C5 complement protein, blocks serum hemolytic activity. This study evaluated the long-term safety and efficacy of eculizumab in 11 patients with PNH during an open-label extension trial. After completion of an initial 12-week study, all patients chose to participate in the 52-week extension study. Eculizumab, administered at 900 mg every 12 to 14 days, was sufficient to completely and consistently block complement activity in all patients. A dramatic reduction in hemolysis was maintained throughout the study, with a decrease in lactate dehydrogenase (LDH) levels from 3110.7 IU/L before treatment to 622.4 IU/L (P = .002). The proportion of PNH type III RBCs increased from 36.7% at baseline to 58.4% (P = .005). The paroxysm rate of days with gross evidence of hemoglobinuria per patient each month decreased from 3.0 during screening to 0.2 (P < .001) during treatment. The median transfusion rate decreased from 1.8 U per patient each month before eculizumab treatment to 0.3 U per patient each month (P = .001) during treatment. Statistically significant improvements in quality-of-life measures were also maintained during the extension study. Eculizumab continued to be safe and well tolerated, and all patients completed the study. The close relationship between sustained terminal complement inhibition, hemolysis, and symptoms was demonstrated. (Blood. 2005; 106:2559-2565)
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a hematopoietic stem-cell disorder characterized by red blood cell (RBC) destruction, anemia, hemoglobinuria, and thrombosis. The intravascular hemolysis in PNH is continuous, with episodes of dark urine, or paroxysms, occurring at times of particularly brisk hemolysis. Ongoing hemolysis and/or insufficient hematopoiesis often result in transfusion dependence. Hemolysis in patients with PNH can be monitored by levels of the enzyme lactate dehydrogenase (LDH), which is typically elevated and can exceed 20 times the upper limit of normal during severe paroxysms.1-3 There is no effective treatment for the ongoing hemolysis in PNH.
PNH results from the clonal expansion of somatically mutated hematopoietic stem cells. The predominant mutation results in a functional deficiency in phosphatidylinositol glycan class A (PIG-A), a protein that is critical for the biosynthesis of the glycosylphosphatidylinositol (GPI) anchor, a mechanism by which various proteins are attached to the cell membrane.4,5 Consequently, there is a partial (type II) or complete (type III) deficiency of GPI-anchored proteins on the surfaces of PNH hematopoietic stem cells and their progeny. Two such proteins are the complement inhibitors CD55 and CD59. CD55 inhibits complement at the level of C3, whereas CD59 prevents terminal complement components from forming the hemolytic membrane pore (C5b-9).6-8 Deficiency of these complement inhibitors renders PNH RBCs sensitive to complement-mediated lysis.7
Eculizumab is a humanized monoclonal antibody that specifically targets the complement protein C5 and prevents its cleavage.9 C5 is the point at which the pathways of complement activation converge, and it is the first protein of terminal complement assembly. Complement inhibition at this stage blocks the generation of C5a and the formation of C5b-9 while it preserves early complement components that are critical for the clearance of microorganisms and immune complexes.10
We previously reported the outcome of an open-label study of eculizumab in patients with PNH.2 Results of this 12-week study demonstrated a dramatic reduction in hemolysis and a concomitant increase in the proportion of PNH type III RBCs. In addition, this initial study showed a marked decrease in the rates of paroxysms and blood transfusions and an improvement in quality of life. Here we report the results of a 1-year follow-up study designed to assess the long-term efficacy and safety of eculizumab in patients with PNH.
Patients, materials, and methods
Trial design
The acute-phase study was an initial 12-week, open-label trial of eculizumab in 11 patients with PNH and has been described previously in detail.2 The current study was an open-label extension of that acute-phase study. All 11 patients from the acute-phase study enrolled in the extension study. Patients were allowed concomitant therapy—with the exception of whole blood, which contains C5—at the discretion of their treating physicians. Two of 11 patients had a history of thrombosis before eculizumab treatment, and 6 of 11 patients were on warfarin therapy before and during the trial.
The trial was approved by the Leeds (West) Research Ethics Committee, United Kingdom, and was performed according to the International Conference on Harmonisation and Good Clinical Practice Standards. All patients gave written informed consent and were treated with eculizumab.
Eculizumab administration
All patients entered the extension study on a maintenance dose of eculizumab (at the conclusion of the acute-phase study). This maintenance dose of 900 mg intravenously every 14 days was continued throughout the extension study period. Two patients, however, required that the dosing interval be shortened to every 12 days so that consistent and complete complement inhibition could be maintained.
Clinical investigations
As in the acute-phase study, data were obtained in the open-label extension study on the pharmacokinetics (PK), pharmacodynamics (PD), and immunogenicity of eculizumab, indicators of hemolysis, PNH clone size, paroxysm and transfusion rates, and quality-of-life measurements. The trigger for transfusion before and during the study remained unchanged for each patient and was based on a combination of hemoglobin levels and the occurrence of symptoms resulting from anemia, hemolysis, or both. This information has been described in detail elsewhere.2
Statistical analysis
Biochemical values were compared using the paired Student t test, change of transfusion and paroxysm rates were analyzed using the Wilcoxon signed rank test, quality-of-life measurements were analyzed using mixed-effect analysis of variance, and comparison of the number of days with paroxysms was analyzed using the Fisher exact test.
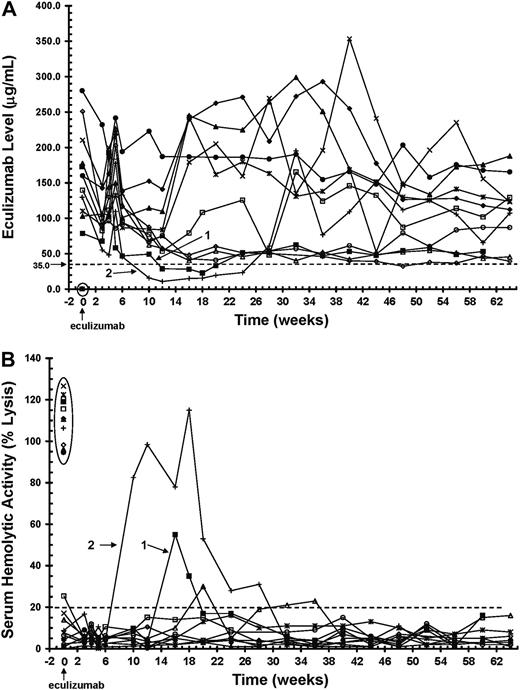
PK and PD analyses of eculizumab in patients with PNH. Initiation of eculizumab dosing is indicated at time 0 on the x axis. (A) Serum levels of eculizumab in 11 patients with PNH during the 64 weeks of treatment. The dashed line indicates the level of eculizumab required to completely block complement activity (≥ 35 μg/mL). Time 0 shows levels of eculizumab before (encircled) and 1 hour after dosing, whereas all other time points represent trough values. Two patients with trough levels of eculizumab below 35 μg/mL during the maintenance dosing are identified (patients 1 and 2). (B) Serum hemolytic activity (PD) during the 64-week treatment period, as determined by the ability of serum to lyse antibody-presensitized chicken erythrocytes. The dashed line indicates the percentage of hemolytic activity at which complement is considered effectively inhibited (≤ 20%). Time 0 shows serum hemolytic activity before (encircled) and 1 hour after dosing, whereas all other time points represent trough values. Two patients with trough serum hemolytic activity values above 20% are identified (patients 1 and 2).
PK and PD analyses of eculizumab in patients with PNH. Initiation of eculizumab dosing is indicated at time 0 on the x axis. (A) Serum levels of eculizumab in 11 patients with PNH during the 64 weeks of treatment. The dashed line indicates the level of eculizumab required to completely block complement activity (≥ 35 μg/mL). Time 0 shows levels of eculizumab before (encircled) and 1 hour after dosing, whereas all other time points represent trough values. Two patients with trough levels of eculizumab below 35 μg/mL during the maintenance dosing are identified (patients 1 and 2). (B) Serum hemolytic activity (PD) during the 64-week treatment period, as determined by the ability of serum to lyse antibody-presensitized chicken erythrocytes. The dashed line indicates the percentage of hemolytic activity at which complement is considered effectively inhibited (≤ 20%). Time 0 shows serum hemolytic activity before (encircled) and 1 hour after dosing, whereas all other time points represent trough values. Two patients with trough serum hemolytic activity values above 20% are identified (patients 1 and 2).
Results
Pharmacokinetics and pharmacodynamics
In the acute-phase study, 10 of 11 patients maintained sufficient levels of eculizumab (≥ 35 μg/mL) for terminal complement to be sufficiently inhibited (≤ 20% serum hemolytic activity) for the duration of the 12-week treatment period (Figure 1, weeks 0-12). During the extension study, 9 of 11 patients continued to show complete complement blockade throughout the 52-week treatment period (Figure 1, weeks 12-64). The 2 patients whose serum eculizumab levels decreased below 35 μg/mL (Figure 1A, lines 1-2) showed a return of serum hemolytic activity (Figure 1B, lines 1-2). However, a reduction in the dosing interval from 900 mg every 14 days to 900 mg every 12 days (initiated between weeks 18 and 24) in the 2 breakthrough patients was adequate to keep trough levels of eculizumab higher than 35 μg/mL (Figure 1A), thereby completely blocking serum complement activity for the remainder of the extension study (Figure 1B).
Measures of hemolysis
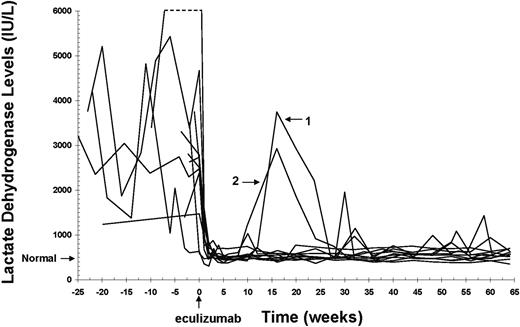
Lactate dehydrogenase (LDH) is a standard biochemical measure of intravascular hemolysis, and levels are frequently elevated in patients with PNH.1-3 The immediate reduction in LDH levels observed in all patients during the acute-phase study was maintained during the extension study (Figure 2). Two patients demonstrated transient increases in LDH levels (Figure 2, lines 1-2) that correlated temporally with the breakthroughs in serum complement activity shown in Figure 1. As complement blockade was restored, LDH levels returned to near-normal values.
Levels of various markers of hemolysis and platelet counts during eculizumab treatment are shown in Table 1. LDH levels decreased from a mean of 3110.7 ± 598.4 IU/L during the 52 weeks before treatment to 594.0 ± 31.7 IU/L and 622.4 ± 41.1 IU/L (normal range at Leeds Teaching Hospitals, 150-480 IU/L) during the 12 and 64 weeks of treatment, respectively (P = .002 for 64-week comparison in all patients). Similarly, aspartate aminotransferase (AST) levels, another marker of hemolysis, decreased from a mean baseline value of 76.2 ± 16.0 IU/L to 26.2 ± 2.3 IU/L and 30.1 ± 3.2 IU/L (normal range at Leeds Teaching Hospitals, 10-40 IU/L) during the 12 and 64 weeks of treatment, respectively (P = .02 for 64-week comparison in all patients). The dramatic reduction in hemolysis during eculizumab treatment was demonstrated in both noncytopenic (platelet count ≥ 150 × 109/L) and cytopenic (platelet count < 150 × 109/L) patient populations. Levels of haptoglobin, hemoglobin, and bilirubin and numbers of reticulocytes and platelets did not change significantly in the comparison of prestudy values and 64-week treatment values.
Changes in levels of various markers of hemolysis and platelet counts during eculizumab therapy
. | Normal range . | Time of analysis∥ . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Biochemical marker . | . | Before study† . | 12 wk . | 64 wk . | P* . | ||
| LDH, IU/L | |||||||
| All patients | 150-480 | 3110.7 ± 598.4 | 594.0 ± 31.7 | 622.4 ± 41.1 | .002 | ||
| Patients without cytopenia‡ | NA | 3965.0 ± 971.7 | 617.2 ± 24.3 | 657.1 ± 54.8 | — | ||
| Patients with cytopenia§ | NA | 2085.0 ± 267.0 | 566.3 ± 65.5 | 580.8 ± 63.2 | — | ||
| AST, IU/L | |||||||
| All patients | 10-40 | 76.2 ± 16.0 | 26.2 ± 2.3 | 30.1 ± 3.2 | .02 | ||
| Patients without cytopenia | NA | 88.5 ± 28.3 | 24.4 ± 3.8 | 30.8 ± 5.7 | — | ||
| Patients with cytopenia | NA | 61.4 ± 9.8 | 28.4 ± 2.4 | 29.1 ± 2.3 | — | ||
| Haptoglobin, g/L | |||||||
| All patients | 0.5-2 | 0.06 ± 0 | 0.07 ± .01 | 0.14 ± 0.07 | NS | ||
| Patients without cytopenia | NA | 0.06 ± 0 | 0.08 ± .02 | 0.20 ± 0.12 | — | ||
| Patients with cytopenia | NA | 0.06 ± 0 | 0.06 ± 0 | 0.08 ± 0.01 | — | ||
| Hemoglobin, g/dL | |||||||
| All patients | 11.5-18 | 10.0 ± 0.4 | 10.3 ± 0.4 | 10.4 ± 0.4 | NS | ||
| Patients without cytopenia | NA | 10.4 ± 0.5 | 10.9 ± 0.6 | 10.8 ± 0.5 | — | ||
| Patients with cytopenia | NA | 9.5 ± 0.7 | 9.6 ± 0.4 | 9.9 ± 0.5 | — | ||
| Bilirubin, μM | |||||||
| All patients | 3-15 | 25.9 ± 4.3 | 28.2 ± 4.4 | 28.7 ± 4.0 | NS | ||
| Patients without cytopenia | NA | 30.6 ± 7.4 | 34.9 ± 6.6 | 34.6 ± 5.9 | — | ||
| Patients with cytopenia | NA | 20.2 ± 2.2 | 20.2 ± 3.6 | 21.7 ± 3.6 | — | ||
| Reticulocytes, × 10−3/mm3 | |||||||
| All patients | 20-80 | 161.4 ± 25.9 | 191.2 ± 23.6 | 189.6 ± 21.8 | NS | ||
| Patients without cytopenia | NA | 200.2 ± 38.4 | 243.4 ± 26.2 | 233.2 ± 27.4 | — | ||
| Patients with cytopenia | NA | 114.8 ± 21.9 | 128.5 ± 15.6 | 137.3 ± 15.2 | — | ||
| Platelets, × 109/L | |||||||
| All patients | 150-400 | 183.0 ± 35.3 | 183.6 ± 37.8 | 180.8 ± 35.8 | NS | ||
| Patients without cytopenia | NA | 250.2 ± 49.8 | 256.3 ± 51.8 | 251.5 ± 47.2 | — | ||
| Patients with cytopenia | NA | 102.2 ± 11.8 | 96.3 ± 18.7 | 95.9 ± 19.7 | — | ||
. | Normal range . | Time of analysis∥ . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Biochemical marker . | . | Before study† . | 12 wk . | 64 wk . | P* . | ||
| LDH, IU/L | |||||||
| All patients | 150-480 | 3110.7 ± 598.4 | 594.0 ± 31.7 | 622.4 ± 41.1 | .002 | ||
| Patients without cytopenia‡ | NA | 3965.0 ± 971.7 | 617.2 ± 24.3 | 657.1 ± 54.8 | — | ||
| Patients with cytopenia§ | NA | 2085.0 ± 267.0 | 566.3 ± 65.5 | 580.8 ± 63.2 | — | ||
| AST, IU/L | |||||||
| All patients | 10-40 | 76.2 ± 16.0 | 26.2 ± 2.3 | 30.1 ± 3.2 | .02 | ||
| Patients without cytopenia | NA | 88.5 ± 28.3 | 24.4 ± 3.8 | 30.8 ± 5.7 | — | ||
| Patients with cytopenia | NA | 61.4 ± 9.8 | 28.4 ± 2.4 | 29.1 ± 2.3 | — | ||
| Haptoglobin, g/L | |||||||
| All patients | 0.5-2 | 0.06 ± 0 | 0.07 ± .01 | 0.14 ± 0.07 | NS | ||
| Patients without cytopenia | NA | 0.06 ± 0 | 0.08 ± .02 | 0.20 ± 0.12 | — | ||
| Patients with cytopenia | NA | 0.06 ± 0 | 0.06 ± 0 | 0.08 ± 0.01 | — | ||
| Hemoglobin, g/dL | |||||||
| All patients | 11.5-18 | 10.0 ± 0.4 | 10.3 ± 0.4 | 10.4 ± 0.4 | NS | ||
| Patients without cytopenia | NA | 10.4 ± 0.5 | 10.9 ± 0.6 | 10.8 ± 0.5 | — | ||
| Patients with cytopenia | NA | 9.5 ± 0.7 | 9.6 ± 0.4 | 9.9 ± 0.5 | — | ||
| Bilirubin, μM | |||||||
| All patients | 3-15 | 25.9 ± 4.3 | 28.2 ± 4.4 | 28.7 ± 4.0 | NS | ||
| Patients without cytopenia | NA | 30.6 ± 7.4 | 34.9 ± 6.6 | 34.6 ± 5.9 | — | ||
| Patients with cytopenia | NA | 20.2 ± 2.2 | 20.2 ± 3.6 | 21.7 ± 3.6 | — | ||
| Reticulocytes, × 10−3/mm3 | |||||||
| All patients | 20-80 | 161.4 ± 25.9 | 191.2 ± 23.6 | 189.6 ± 21.8 | NS | ||
| Patients without cytopenia | NA | 200.2 ± 38.4 | 243.4 ± 26.2 | 233.2 ± 27.4 | — | ||
| Patients with cytopenia | NA | 114.8 ± 21.9 | 128.5 ± 15.6 | 137.3 ± 15.2 | — | ||
| Platelets, × 109/L | |||||||
| All patients | 150-400 | 183.0 ± 35.3 | 183.6 ± 37.8 | 180.8 ± 35.8 | NS | ||
| Patients without cytopenia | NA | 250.2 ± 49.8 | 256.3 ± 51.8 | 251.5 ± 47.2 | — | ||
| Patients with cytopenia | NA | 102.2 ± 11.8 | 96.3 ± 18.7 | 95.9 ± 19.7 | — | ||
— indicates not determined; NA, not applicable; and NS, not significant.
Comparisons of mean change from the prestudy period to the 64-week treatment period for all patients.
Mean values during 52-week period before treatment except for AST, which represents the baseline mean.
Platelet count ≥ 150 × 109/L; n = 6.
Platelet count < 150 × 109/L; n = 5.
Values are presented as ± standard error (SE).
LDH levels in patients with PNH before and during eculizumab treatment. Initiation of eculizumab dosing is indicated at time 0 on the x axis. LDH values are shown for 11 patients with PNH for 25 weeks before and 64 weeks during eculizumab treatment. (Normal) Upper limit of normal of the LDH range at the Leeds Teaching Hospital. The dashed line indicates off-scale points from one patient with a peak value of 12 100 IU/L. Two patients who experienced a return of serum hemolytic activity during treatment are identified (patients 1 and 2).
LDH levels in patients with PNH before and during eculizumab treatment. Initiation of eculizumab dosing is indicated at time 0 on the x axis. LDH values are shown for 11 patients with PNH for 25 weeks before and 64 weeks during eculizumab treatment. (Normal) Upper limit of normal of the LDH range at the Leeds Teaching Hospital. The dashed line indicates off-scale points from one patient with a peak value of 12 100 IU/L. Two patients who experienced a return of serum hemolytic activity during treatment are identified (patients 1 and 2).
Proportions of PNH blood cells
Clonal expansion of hematopoietic stem cells with a reduction in or an absence of GPI-linked membrane proteins (type II or type III cells, respectively) is a hallmark of PNH. The effect of eculizumab on the proportions of various PNH blood-cell types was assessed (Table 2). The proportion of PNH type III RBCs relative to the total RBC population increased from 36.7% ± 5.9% at baseline to 59.2% ± 8.0% and 58.4% ± 8.5% during the 12 and 64 weeks of treatment, respectively (P = .005 for 64-week comparison in all patients). The increase in the proportion of PNH type III RBCs was more pronounced in patients without cytopenia than in those with cytopenia. However, the lower proportion of type III RBCs in patients with cytopenia was a direct result of the dilutional effect from the increased administration of normal RBCs because of the higher transfusion requirement in this patient group. The proportion of PNH type II RBCs increased from 5.3% ± 1.4% before treatment to 13.2% ± 2.4% (P = .013) during the 64 weeks of treatment. Mean proportions of type III neutrophils and platelets were greater than 90% before eculizumab therapy and were stable during the study.
Changes in proportions of PNH blood-cell types during eculizumab treatment
. | Proportion of PNH cells, % . | . | . | . | ||
|---|---|---|---|---|---|---|
| PNH cell type . | Baseline§ . | 12 wk§ . | 64 wk§ . | P* . | ||
| Type III RBCs | ||||||
| All patients | 36.7 ± 5.9 | 59.2 ± 8.0 | 58.4 ± 8.5 | .005 | ||
| Patients without cytopenia† | 38.8 ± 3.7 | 73.5 ± 4.1 | 67.8 ± 7.0 | — | ||
| Patients with cytopenia‡ | 34.2 ± 12.9 | 42.1 ± 13.8 | 47.0 ± 16.3 | — | ||
| Type II RBCs | ||||||
| All patients | 5.3 ± 1.4 | 7.5 ± 2.1 | 13.2 ± 2.4 | .013 | ||
| Patients without cytopenia | 4.8 ± 1.8 | 6.8 ± 3.0 | 12.9 ± 1.9 | — | ||
| Patients with cytopenia | 5.8 ± 2.4 | 8.4 ± 3.2 | 13.5 ± 5.0 | — | ||
| Type III WBCs | ||||||
| All patients | 92.1 ± 4.6 | 89.9 ± 6.6 | 91.1 ± 5.8 | NS | ||
| Patients without cytopenia | 95.1 ± 2.3 | 94.9 ± 2.6 | 96.1 ± 1.1 | — | ||
| Patients with cytopenia | 88.4 ± 10.2 | 83.9 ± 14.5 | 85.2 ± 12.9 | — | ||
| Type III platelets | ||||||
| All patients | 92.4 ± 2.4 | 93.3 ± 2.8 | 92.8 ± 2.6 | NS | ||
| Patients without cytopenia | 93.2 ± 1.4 | 95.8 ± 2.1 | 95.0 ± 0.8 | — | ||
| Patients with cytopenia | 91.6 ± 5.4 | 90.2 ± 5.6 | 90.2 ± 5.8 | — | ||
. | Proportion of PNH cells, % . | . | . | . | ||
|---|---|---|---|---|---|---|
| PNH cell type . | Baseline§ . | 12 wk§ . | 64 wk§ . | P* . | ||
| Type III RBCs | ||||||
| All patients | 36.7 ± 5.9 | 59.2 ± 8.0 | 58.4 ± 8.5 | .005 | ||
| Patients without cytopenia† | 38.8 ± 3.7 | 73.5 ± 4.1 | 67.8 ± 7.0 | — | ||
| Patients with cytopenia‡ | 34.2 ± 12.9 | 42.1 ± 13.8 | 47.0 ± 16.3 | — | ||
| Type II RBCs | ||||||
| All patients | 5.3 ± 1.4 | 7.5 ± 2.1 | 13.2 ± 2.4 | .013 | ||
| Patients without cytopenia | 4.8 ± 1.8 | 6.8 ± 3.0 | 12.9 ± 1.9 | — | ||
| Patients with cytopenia | 5.8 ± 2.4 | 8.4 ± 3.2 | 13.5 ± 5.0 | — | ||
| Type III WBCs | ||||||
| All patients | 92.1 ± 4.6 | 89.9 ± 6.6 | 91.1 ± 5.8 | NS | ||
| Patients without cytopenia | 95.1 ± 2.3 | 94.9 ± 2.6 | 96.1 ± 1.1 | — | ||
| Patients with cytopenia | 88.4 ± 10.2 | 83.9 ± 14.5 | 85.2 ± 12.9 | — | ||
| Type III platelets | ||||||
| All patients | 92.4 ± 2.4 | 93.3 ± 2.8 | 92.8 ± 2.6 | NS | ||
| Patients without cytopenia | 93.2 ± 1.4 | 95.8 ± 2.1 | 95.0 ± 0.8 | — | ||
| Patients with cytopenia | 91.6 ± 5.4 | 90.2 ± 5.6 | 90.2 ± 5.8 | — | ||
— indicates not determined; WBCs, white blood cells; and NS, not significant.
Comparisons of mean change from baseline to the 64-week treatment period for all patients.
Platelet count ≥ 150 × 109/L; n = 6.
Platelet count < less than 150 × 109/L; n = 5.
Data are presented as ± SE.
Paroxysm and transfusion rates
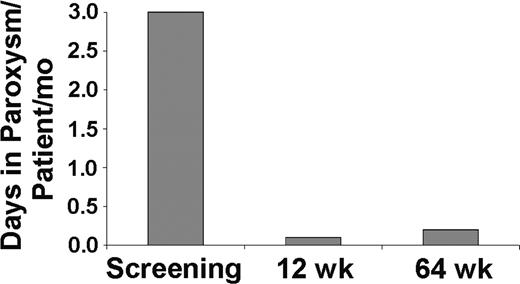
The presence of hemoglobin in the urine (hemoglobinuria) is characteristic of PNH and a central component of periodic exacerbations known as paroxysms. In the eculizumab study, paroxysm rates were defined as the number of days with the presence of gross hemoglobinuria, defined as a colorimetric urine score of 6 or greater on a scale of 1 to 10.2 In patients whose urine scores were assessed, the paroxysm rate decreased from 3.0 paroxysms per patient each month before eculizumab treatment to 0.1 paroxysms per patient each month during the initial 12 weeks and 0.2 paroxysms per patient each month during the total 64 weeks of treatment (Figure 3; P < .001).
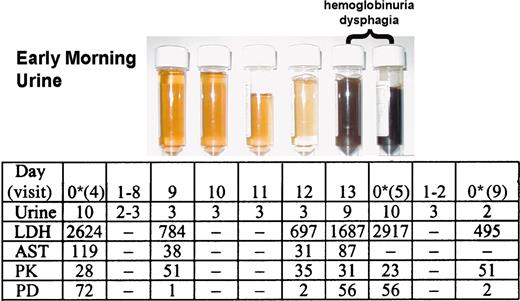
During the extension study, 2 patients did not sustain levels of eculizumab necessary to consistently block complement (Figure 1). This breakthrough in serum hemolytic activity occurred in the last 2 days of the 14-day dosing interval, a pattern that was repeated between multiple doses. A detailed analysis of the dosing interval between visits 4 and 5 in one of the breakthrough patients showed that sufficient levels of eculizumab (PK ≥ 35 μg/mL) were present to completely block serum hemolytic activity (PD ≤ 20% hemolytic activity) for the first 12 days (Figure 4). Effective complement blockade during these 12 days was reflected by normal urine color scores and low LDH and AST levels. On the 13th and 14th days of the dosing interval, a paroxysm occurred, as evidenced by severe hemoglobinuria (black urine), dysphagia, and dramatic increases in LDH and AST levels. These events correlated with insufficient levels of eculizumab (PK) and the return of serum hemolytic activity (PD). The patient was again dosed on day 14, which resulted in the resolution of hemoglobinuria and dysphagia by the next morning. A reduction in the dosing interval from 14 to 12 days between visits 5 and 9 was sufficient to maintain the levels of eculizumab necessary to effectively and consistently block serum hemolytic activity and, therefore, intravascular hemolysis in these patients (Figure 4, visit 9).
Paroxysm rate in patients with PNH before and during eculizumab treatment. A urine color scale2 was used to assess the incidence of paroxysms in 8 patients with PNH before and during treatment with eculizumab. Paroxysm was prospectively defined by a urine colorimetric score of 6 or more. Bars represent the paroxysm rates (number of paroxysms per patient per month) during the screening period, during the first 12 weeks, and over the total 64 weeks of eculizumab treatment. Three patients were not included in the analysis either because their pretreatment urine scores were inadvertently not collected (2 patients) or because an iron-chelating agent that resulted in artificially colored urine was administered during the extension study (1 patient).
Paroxysm rate in patients with PNH before and during eculizumab treatment. A urine color scale2 was used to assess the incidence of paroxysms in 8 patients with PNH before and during treatment with eculizumab. Paroxysm was prospectively defined by a urine colorimetric score of 6 or more. Bars represent the paroxysm rates (number of paroxysms per patient per month) during the screening period, during the first 12 weeks, and over the total 64 weeks of eculizumab treatment. Three patients were not included in the analysis either because their pretreatment urine scores were inadvertently not collected (2 patients) or because an iron-chelating agent that resulted in artificially colored urine was administered during the extension study (1 patient).
A statistically significant reduction in the rate of packed red blood cell (PBRC) transfusion was observed in the acute-phase study and was maintained during the extension study compared with the rate of transfusion before eculizumab therapy (Table 3). Mean transfusion rates decreased from 2.1 U per patient each month during the 1-year period before treatment to 0.6 U per patient each month during the initial 12-week and to 0.5 U per patient each month during the total 64-week treatment period. Similarly, median transfusion rates decreased from 1.8 U per patient each month before treatment to 0.0 U and 0.3 U per patient each month during the first 12 weeks and the total 64 weeks of therapy, respectively (P = .001 for 64-week comparison in all patients). The reduction in transfusions was more pronounced in patients without cytopenia; the mean rate in this population decreased from 2.4 U per patient each month during the prestudy period to 0.2 U per patient each month during the 12- and 64-week treatment periods. Transfusion reduction in patients with poor bone marrow reserve (patients with cytopenia) decreased from a mean rate of 1.8 U per patient each month during the 1-year pretreatment period to 1.2 and 0.8 U per patient each month during the 12- and 64-week treatment periods, respectively.
Changes in transfusion requirements during eculizumab treatment
. | Transfusion rates . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Before study* . | . | 12 wk . | . | 64 wk . | . | . | ||||||
| Patient group . | Mean . | Median . | Mean . | Median . | Mean . | Median . | P† . | ||||||
| All patients | 2.1 | 1.8 | 0.6 | 0.0 | 0.5 | 0.3 | .001 | ||||||
| Patients without cytopenia‡ | 2.4 | 2.0 | 0.2 | 0.0 | 0.2 | 0.1 | - | ||||||
| Patients with cytopenia§ | 1.8 | 1.8 | 1.2 | 0.7 | 0.8 | 0.7 | - | ||||||
. | Transfusion rates . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Before study* . | . | 12 wk . | . | 64 wk . | . | . | ||||||
| Patient group . | Mean . | Median . | Mean . | Median . | Mean . | Median . | P† . | ||||||
| All patients | 2.1 | 1.8 | 0.6 | 0.0 | 0.5 | 0.3 | .001 | ||||||
| Patients without cytopenia‡ | 2.4 | 2.0 | 0.2 | 0.0 | 0.2 | 0.1 | - | ||||||
| Patients with cytopenia§ | 1.8 | 1.8 | 1.2 | 0.7 | 0.8 | 0.7 | - | ||||||
Transfusion rates are units per patient per month.
- indicates not determined.
Values during 52-week period before treatment.
Comparisons of median change from before study to the 64-week treatment period.
Platelet count ≥ 150 × 109/L; n = 6.
Platelet count < 150 × 109/L; n = 5.
Relationship between complement inhibition with eculizumab and various hemolytic parameters and symptoms during a transient breakthrough in serum-complement activity. Urine color, symptoms, biochemical parameters of hemolysis, PK, and PD were assessed during a 14-day eculizumab-dosing interval in a patient with a transient breakthrough in serum hemolytic activity. Eculizumab was administered on day 0 after assay samples were collected. A urine colorimetric score of 6 or greater was considered abnormal (hemoglobinuria). Levels of the hemolytic markers LDH and AST are shown (IU/L). Eculizumab serum concentrations (PK, μg/mL) of ≥ 35 μg/mL were sufficient to maintain a serum hemolytic activity (PD, % serum hemolytic activity) of ≤ 20%, a value known to represent complete complement blockade. The eculizumab-dosing interval was reduced to 12 days between visits 5 and 9. Urine row presents colorimetric scores. — indicates not determined. *Dose of eculizumab.
Relationship between complement inhibition with eculizumab and various hemolytic parameters and symptoms during a transient breakthrough in serum-complement activity. Urine color, symptoms, biochemical parameters of hemolysis, PK, and PD were assessed during a 14-day eculizumab-dosing interval in a patient with a transient breakthrough in serum hemolytic activity. Eculizumab was administered on day 0 after assay samples were collected. A urine colorimetric score of 6 or greater was considered abnormal (hemoglobinuria). Levels of the hemolytic markers LDH and AST are shown (IU/L). Eculizumab serum concentrations (PK, μg/mL) of ≥ 35 μg/mL were sufficient to maintain a serum hemolytic activity (PD, % serum hemolytic activity) of ≤ 20%, a value known to represent complete complement blockade. The eculizumab-dosing interval was reduced to 12 days between visits 5 and 9. Urine row presents colorimetric scores. — indicates not determined. *Dose of eculizumab.
Quality-of-life measurements
Quality-of-life measurements were assessed using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 instrument during the total 64-week treatment period and were compared with baseline values (Table 4). Significant improvements in quality-of-life domains that were demonstrated in the acute-phase study were maintained in the extension study, including global health status (P = .009), physical functioning (P < .001), emotional functioning (P < .001), cognitive functioning (P = .001), fatigue (P < .001), dyspnea (P < .001), and insomnia (P = .031). In addition, changes in role functioning (P = .003) and pain domains (P = .023) achieved significance during the 64-week treatment period (P < .001). The constipation domain showed a significant increase over the treatment period. Taken together, these results indicate that various parameters of quality of life in patients with PNH rapidly improve with eculizumab therapy and that these changes are maintained for extended lengths of time.
Quality-of-life assessment during eculizumab treatment
Domain* . | Mean baseline score† . | 64-wk change from baseline score‡ . | P§ . |
|---|---|---|---|
| Global health status | 56.1 | 13.8 | .009 |
| Physical functioning | 70.9 | 14.3 | <.001 |
| Emotional functioning | 70.5 | 12.5 | <.001 |
| Role functioning | 66.7 | 14.5 | .003 |
| Cognitive functioning | 77.3 | 10.3 | .001 |
| Fatigue | 47.5 | −17.8 | <.001 |
| Dyspnea | 39.4 | −16.6 | <.001 |
| Insomnia | 30.3 | −8.2 | .031 |
| Pain | 21.2 | −8.2 | .023 |
| Constipation | 3.0 | 4.1 | <.001 |
Domain* . | Mean baseline score† . | 64-wk change from baseline score‡ . | P§ . |
|---|---|---|---|
| Global health status | 56.1 | 13.8 | .009 |
| Physical functioning | 70.9 | 14.3 | <.001 |
| Emotional functioning | 70.5 | 12.5 | <.001 |
| Role functioning | 66.7 | 14.5 | .003 |
| Cognitive functioning | 77.3 | 10.3 | .001 |
| Fatigue | 47.5 | −17.8 | <.001 |
| Dyspnea | 39.4 | −16.6 | <.001 |
| Insomnia | 30.3 | −8.2 | .031 |
| Pain | 21.2 | −8.2 | .023 |
| Constipation | 3.0 | 4.1 | <.001 |
Quality of life was assessed using the European Organization for Research and Treatment of Cancer QLQ-C30 instrument.
Mean values of linearly transformed scores.
Values represent least-square means. Positive change indicates improvement on Global Health Status and Functional scales, and negative change indicates improvement on Symptom scales.
From a mixed analysis-of-covariance model with visit as a fixed effect, patient as a random effect, and baseline as a covariate.
Safety
All patients completed the extension study. No deaths occurred and no thromboses developed during the 12-month treatment period. In no patients were antibodies against eculizumab detected. All patients had at least one adverse event (AE), and one patient had a serious adverse event (SAE). No patient withdrew because of an AE. The most common AEs were flulike symptoms (4 patients), sore throat (4 patients), pain (3 patients), nausea (3 patients), bruising (3 patients), cough (3 patients), and upper respiratory infection (3 patients).
One patient experienced an SAE during the extension study characterized by neutropenia with extravascular hemolysis, which was thought to have been caused by a viral syndrome. Briefly, the patient had a 5-year history of PNH with aplastic anemia (with a normal neutrophil count) and had been on eculizumab therapy for 14 months. A viral syndrome developed, and during the next several days hemoglobin levels dropped from 10.2 to 5.3 g/dL, whereas serum LDH levels increased only slightly. The patient received a transfusion of 4 U PBRCs; at the next study visit, the hemoglobin level had returned to 10.7 g/dL and the platelet count was normal, but the neutrophil count was abnormally low (260/μL). Two weeks later, the neutrophil count had recovered to almost-normal levels (1600/μL). The investigator did not think the SAE was caused by the study medication. The patient remained transfusion independent for the remainder of the extension study.
Discussion
We reported previously that eculizumab therapy in patients with PNH resulted in a dramatic reduction in hemolysis and transfusion requirements. The extension study was conducted to obtain long-term data regarding eculizumab use in this patient population. Here we report the sustained efficacy and safety of eculizumab in a 1-year, open-label extension study.
Serum hemolytic activity in 9 of 11 patients was completely blocked throughout the 64-week treatment period, with trough levels of eculizumab at equilibrium ranging from approximately 35 μg/mL to 350 μg/mL. One patient had a breakthrough of serum hemolytic activity during the acute-phase study, and another patient broke through early in the extension study. PK analysis of both breakthrough patients demonstrated a simultaneous decrease in eculizumab level below that required to completely block complement activity (≥ 35 μg/mL).9 Adjustment of the eculizumab dosing interval in these 2 patients from every 14 days to every 12 days successfully sustained trough levels of eculizumab higher than 35 μg/mL and consistently blocked serum hemolytic activity for the remainder of the extension study. The effective and consistent blockade of complement achieved with the 12-day dosing interval was supported by the resolution of symptoms, including hemoglobinuria and dysphagia, and lower levels of LDH and AST (Figure 4). Taken together, these data illustrate the tight relationship between complement blockade, hemolysis, and symptoms in PNH.
The dramatic reduction in LDH levels that was demonstrated immediately on the administration of eculizumab in the initial study was sustained during the extension study, providing strong evidence that eculizumab effectively and durably inhibits intravascular hemolysis in patients with PNH. LDH levels were reduced from a mean of 3110.7 IU/L during the 52-week period before treatment to a mean of 622.4 IU/L during the 64 weeks of eculizumab therapy. In addition, detailed analysis of PK and PD demonstrated a strong correlation between the return of complement activity, hemolysis, and an increase in LDH levels (Figure 4). These data confirm that LDH levels can be used as an accurate measure of intravascular hemolysis in PNH, and they provide evidence that effective complement blockade during eculizumab therapy can be determined by monitoring levels of this enzyme.
LDH levels remained slightly elevated in most patients during eculizumab treatment, suggesting low levels of ongoing hemolysis. Undetectable haptoglobin levels and elevated bilirubin levels also support residual hemolysis in the midst of terminal complement inhibition. This may be attributed to yet undefined, noncomplement-mediated mechanisms of PNH RBC clearance, as recently described.11 Alternatively, it is possible that a fraction of PNH RBCs is cleared through complement-mediated events before C5, such as a C3b coating and clearance. In this regard, slightly elevated levels of LDH have also been reported in a patient with coexistent PNH and C9 deficiency.12
The marked reduction in transfusions demonstrated in the initial eculizumab study was sustained in the extension study. Further, patients who had good bone marrow reserve (those without cytopenia) showed greater reductions in transfusion requirements than did patients who had hypoplasia (those with cytopenia). Importantly, 3 patients with hypoplasia who received transfusions during the acute-phase study were subsequently treated with erythropoietin during the extension study. Two of the 3 patients responded with an increase in reticulocyte counts and a further reduction in transfusion requirements (data not shown). These data suggest that erythropoietin therapy in PNH patients with hypoplasia increases erythropoiesis and that concomitant eculizumab therapy protects against increased hemolysis, with a resultant improvement in response. Three of 11 PNH patients remain transfusion independent after 64 weeks of eculizumab therapy.
Interestingly, eculizumab therapy resulted in dramatic reductions in transfusion requirements even though hemoglobin levels and reticulocyte counts frequently remained fairly constant. However, before eculizumab treatment, hemoglobin levels in patients were artificially maintained through regular transfusions of packed RBCs. Thus, stabilization of hemoglobin levels with a concomitant reduction in or cessation of transfusions likely represents a net increase in hemoglobin levels. These data suggest that the resolution of hemolysis in patients with PNH results in a new steady state hemoglobin level, determined by a combination of the extent of the underlying bone marrow dysfunction, the size of the type III RBC clone, and the new level (if any) of transfusion requirement.
There was a sustained improvement in quality of life for transfusion-dependent PNH patients administered eculizumab, as measured by the EORTC QLQ-C30. Quality-of-life parameters that showed significant improvement include global health status, physical functioning, emotional functioning, role functioning, cognitive functioning, fatigue, dyspnea, insomnia, and pain. Improvements in quality-of-life parameters in PNH patients taking eculizumab will be further investigated in subsequent placebo-controlled trials.
Clinical assessment of PNH symptoms not captured by the QLQ-C30 instrument, such as dysphagia, abdominal pain, and erectile failure, showed complete resolution, or at least dramatic improvement, during eculizumab treatment.13 These symptoms have been shown to correlate with a large PNH type III clone size, suggesting that they are related to excessive hemolysis.14 In addition, these symptoms have been attributed to smooth muscle dystonia caused by the scavenging of nitric oxide by free plasma hemoglobin.14-18 The capacity of free plasma hemoglobin to scavenge nitric oxide during intravascular hemolysis has been demonstrated in patients with sickle cell anemia.19 The relationship between levels of free plasma hemoglobin and nitric oxide in patients with PNH remains to be elucidated.
Thrombosis accounts for most deaths in patients with PNH. Studies have reported a strong correlation between a large PNH type III neutrophil clone and the occurrence of thrombosis.14,20 Hall et al20 reported that in approximately 44% of patients with large PNH clones, venous thrombosis developed in the first 10 years after diagnosis. The tendency toward thrombosis in patients with PNH is thought to be multifactorial in etiology, involving both the absence of GPI-anchored complement inhibitors on the surfaces of circulating platelets and the high levels of intravascular free plasma hemoglobin with the consequent scavenging of nitric oxide.18,21-25 It is reasonable to hypothesize that thrombosis resulting from either or both of these mechanisms should be reduced by terminal complement blockade. Although no thrombotic events occurred during eculizumab treatment, 6 of the 11 patients were on warfarin therapy before and during the study. Further analyses and studies are required to investigate whether eculizumab reduces the risk for thrombosis in patients with PNH.
Eculizumab continued to be safe and well tolerated in the PNH extension study. No deaths occurred and no thromboses developed in this study. Each patient had at least one AE; flulike symptoms and sore throat were the most common. Evaluation of AEs in other placebo-controlled eculizumab studies suggests that the reported AEs in the PNH extension study were similar in type and frequency to those reported in other placebo treatment groups. The single SAE, transient neutropenia, was not thought by the principal investigator to be related to study medication; rather, it was thought to be the result of a viral syndrome. All patients chose to continue treatment in a second extension study, and evaluation of eculizumab therapy in a broader population of PNH patients is under way.
Results of this 1-year extension study showed that eculizumab therapy continues to be safe and well tolerated in PNH patients. Additionally, long-term complement inhibition with eculizumab in this patient population has resulted in sustained reductions in hemolysis and blood transfusions and continued improvement in quality of life.
Prepublished online as Blood First Edition Paper, June 28, 2005; DOI 10.1182/blood-2005-02-0564.
Supported by Alexion Pharmaceuticals, Inc.
Several of the authors (J.C., C.F.M., R.P.R.) are employed by Alexion, whose potential product was studied in the present work. P.H. reports serving as a consultant to Alexion, and A.H. and P.H. have each received an educational grant from the company.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Leonard Bell and Stephen Squinto for critical review of the manuscript; Kathy Way and Karen Hannon for excellent technical support; and Louise Arnold, David Buchanan, Catherine Burton, Jan Farish, and the nurses of Healthcare-at-Home for their assistance in carrying out the study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal