Abstract
Heme released from heme-binding proteins on internal hemorrhage, hemolysis, myolysis, or other cell damage is highly toxic due to oxidative and proinflammatory effects. Complex formation with hemopexin, the high-affinity heme-binding protein in plasma and cerebrospinal fluid, dampens these effects and is suggested to facilitate cellular heme metabolism. Using a ligand-affinity approach, we purified the human hemopexin-heme receptor and identified it as the low-density lipoprotein receptor-related protein (LRP)/CD91, a receptor expressed in several cell types including macrophages, hepatocytes, neurons, and syncytiotrophoblasts. Binding experiments, including Biacore analysis, showed that hemopexin-heme complex formation elicits the high receptor affinity. Uptake studies of radio-labeled hemopexin-heme complex in LRP/CD91-expressing COS cells and confocal microscopy of the cellular processing of fluorescent hemopexin-heme complex established the ability of LRP/CD91 to mediate hemopexin-heme internalization resulting in cellular heme uptake and lysosomal hemopexin degradation. Uptake of hemopexin-heme complex induced LRP/CD91-dependent heme-oxygenase 1 mRNA transcription in cultured monocytes. In conclusion, hemopexin-heme complexes are removed by a receptor-mediated pathway showing striking similarities to the CD163-mediated haptoglobin-hemoglobin clearance in macrophages. Furthermore, the data indicate a hitherto unknown role of LRP/CD91 in inflammation. (Blood. 2005; 106:2572-2579)
Introduction
Hemopexin (Hx) is a 60-kDa acute-phase protein with the highest heme affinity of any known protein.1 The binding of extracellular heme to Hx prevents strong oxidative features and proinflammatory effects of free heme, which intercalates into cell membranes and other lipophilic structures, for example, low-density lipoprotein (LDL).2,3 Heme-mediated oxidative stress is suggested to contribute to various pathologic inflammatory conditions including neurodegenerative disorders.4 The intracellular heme proteins are hemoglobin, myoglobin, neuroglobin, cytoglobin, and enzymes including peroxidases, cytochromes, and catalase. Hemoglobin and myoglobin are the most abundant of these proteins, and Hx thus becomes increasingly important in conditions such as internal hemorrhage, hemolysis, and rhabdomyolysis. By sequestering heme from hemoglobin, Hx provides a backup mechanism for heme clearance via the hemoglobin-binding haptoglobin. The overlapping role of Hx and haptoglobin in relation to hemolysis is evident from gene knockout experiments showing that mice deficient in haptoglobin and Hx genes are much more sensitive to hemolytic stress when both genes are inactivated.5,6
In the cell, toxic effects of heme are prevented by the activity of the heme oxygenases (HOs), which cause breakdown of the porphyrin ring into biliverdin, carbon monoxide, and iron. Biliverdin is subsequently converted to bilirubin by the biliverdin reductase, whereas iron is bound to ferritin.7 Among the 3 HOs known (HO-1, HO-2, and HO-3), HO-1 is highly inducible by a great number of pro-oxidative and proinflammatory stimuli including its substrate heme.8 Interestingly, the heme metabolites overall have an antioxidative and anti-inflammatory effect,7 and the activity of HO-1 is suggested to play a major role in the inflammatory response of various anti-inflammatory mediators, for example, interleukin-10.9
Like haptoglobin-hemoglobin complexes, which undergo endocytosis in macrophages by means of the hemoglobin scavenger receptor CD163,10 Hx-heme complexes are endocytosed by a receptor-mediated mechanism in a variety of cells including hepatocytes, macrophages, and syncytiotrophoblasts.11-14 The primary receptor for uptake of Hx-heme complexes is hitherto not identified. The asialoform of Hx has been reported to recognize the asialoglycoprotein receptor, but this hepatocyte-specific receptor is suggested to represent a secondary receptor for catabolism of outdated Hx, which has lost the terminal sialic acids of the N-linked carbohydrates.15
To identify the primary receptor responsible for uptake of Hx-heme complexes in humans, we used a ligand-affinity purification approach similar to the one used for identification of the haptoglobin-hemoglobin receptor CD163.10 This attempt led to purification of the 600-kDa LDL receptor-related protein (LRP), alias CD91, and the α2-macroglobulin receptor, a multiligand receptor16 expressed in a variety of cell types including those shown to possess Hx-heme receptor activity. Subsequent functional analysis of purified LRP/CD91 and LRP/CD91-expressing cells confirmed LRP/CD91 as a novel Hx-heme receptor, thus linking heme metabolism to a known receptor with well-characterized endocytic properties and signaling functions.16
Materials and methods
Preparation of Hx-heme complexes and receptor-binding (activated) α2-macroglobulin
Hx was isolated from human serum by a heme affinity chromatography approach.17 Serum was dialyzed against phosphate-buffered saline (PBS), pH 7.4, overnight at 4°C, centrifuged 40 minutes at 48 000g at 4°C, filtered through a 0.2-μm filter, 0.1 mM phenyl methyl sulfonyl fluoride (PMSF) added, and loaded on a matrix of cross-linked 4% beaded heme-agarose (Sigma, Brøndby, Denmark). After washing with 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 0.14 M NaCl, pH 7.8, bound protein was eluted in 0.2 M CH3CO2Na, pH 4.0, in fractions of 1 mL and analyzed by 4% to 16% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Fractions containing Hx were pooled and further purified on a mono Q HR 5/5 column (Amersham, Hørsholm, Denmark) by high-performance liquid chromatography anion exchange. Purity of Hx was tested by SDS-gel electrophoresis showing more than 95% purity. Purified Hx was dialyzed against PBS, pH 7.4, and subsequently complexed to heme (Fluka, Buchs, Switzerland) or 55Fe-labeled heme (RI Consultants, Hudson, NH; 10.4 MBq/mg heme) in a molar ratio of 1:1.3 in PBS, pH 7.4, for 1 hour at 37°C. α2-Macroglobulin purified from human plasma was a kind gift from Dr Lars Sottrup-Jensen (University of Aarhus). Activated α2-macroglobulin, defined in the present report as the receptor-binding conformation of the protein, was obtained by treatment with methylamine as previously described.18 Hx-heme complexes and activated α2-macroglobulin were iodinated by the chloramine-T method. The specific activity was about 0.4 MBq/μg protein corresponding to 107 cpm/μg protein with the γ-counter used.
Purification of Hx-heme receptor
Hx-heme complex was coupled to cyanogen bromide (CNBr)-activated Sepharose 4B (Amersham) according to the instructions of the manufacturer and the Hx-heme Sepharose was loaded with Triton X-100-solubilized membranes from human liver or placenta prepared as previously described.18,19 After washing in PBS, 0.6% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid), pH 7.4, bound protein was eluted in PBS, 0.6% CHAPS, and 10 mM EDTA (ethylenediaminetetraacetic acid), pH 4.0, and collected in 1-mL fractions. The proteins in the eluted fractions were separated by 4% to 16% SDS-PAGE and by Western blotting using Sequi-blot polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Herlev, Denmark) and the murine monoclonal anti-LRP/CD91 antibodies (A2MRα-2 and A2MRβ-1)19 as primary antibodies. Alkaline phosphatase-conjugated polyclonal rabbit anti-mouse immunoglobulins (DakoCytomation, Glostrup, Denmark) were used as secondary antibody.
Mass spectrometry
Gel slices of proteins separated by SDS-PAGE were subjected to in-gel tryptic digestion followed by matrix-assisted laser desorption/ionization (MALDI) mass spectrometric peptide mapping as described.20
Analysis of the binding of Hx-heme to LRP/CD91
Binding of iodinated Hx-heme to LRP/CD91 was analyzed by a solid-phase assay with LRP/CD91 immobilized to microtiter wells (Nunc, Roskilde, Denmark) in 50 mM NaHCO3, pH 9.6, overnight at 4°C. Wells were blocked in 5% bovine serum albumin (BSA; 2 hours at room temperature) prior to washing in 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 0.14 M NaCl, pH 7.8, and incubated with 125I-Hx-heme (3000 cpm/well) in 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 0.14 M NaCl, pH 7.8, and 2% BSA in the presence of 0 to 10 μg/mL unlabeled Hx-heme, Hx or heme, 100 μg/mL receptor-associated protein (RAP), 100 μg/mL activated α2-macroglobulin, 300 μg/mL anti-LRP/CD91 IgG, 300 μg/mL nonimmune IgG, or 10 mM EDTA. After extensive washing with the same buffer, bound radioactivity was released by addition of 2 × 200 μL 10% SDS. Surface plasmon resonance analysis on a Biacore 3000 instrument was performed to analyze the importance of Hx-heme complex formation for receptor binding in wide range of ligand concentrations. For this analysis, type 5 Biacore sensor chips (Biacore, Uppsala, Sweden) were activated with a 1:1 mixture of 0.2 M N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide and 0.05 M N-hydroxysuccimide. LRP/CD91 was immobilized at a concentration 15 μg/mL in 10 mM sodium acetate, pH 3.0, and remaining binding sites were blocked with 1 M ethanolamine, pH 8.5. A control-flow cell was made by performing the activation and blocking procedure only. The resulting receptor density was 31 fmol receptor/mm2. Samples were dissolved in 10 mM HEPES, 150 mM NaCl, 1.5 mM CaCl2, 1.0 mM EGTA (ethylene glycol tetraacetic acid), and 0.005% Tween-20, pH 7.4. Sample and running buffers were identical. Regeneration of the sensor chip after each analysis cycle was performed with 1.6 M glycine-HCl buffer, pH 3.0. The Biacore response was expressed in mass-equivalent response units (RU), that is, the difference in response between protein and control flow channel.
Ligand uptake and degradation analysis
Cellular internalization of 125I-Hx, 125I-Hx-heme, and 125I-α2-macroglobulin complex was analyzed in LRP/CD91-expressing COS-1 cells (nontranfected) grown to confluence in 24-well plates (Nunc) in Dulbecco modified Eagle medium (DMEM; Invitrogen, Taastrup, Denmark) containing 10% fetal calf serum (FCS). After washing, cells were incubated with the radiolabeled protein (3000 cpm/well) in serum-free DMEM (Invitrogen) and 1% BSA was added in the presence of 0 to 100 μg/mL unlabeled Hx-heme. The inhibitory effect of RAP, anti-LRP/CD91 IgG, nonimmune IgG, Hx-heme, and Hx alone was analyzed by adding the incubation media prior to incubation with 125I-Hx-heme or 125I-Hx. Lysosomal degradation of radiolabeled protein was inhibited by addition of 300 μM leupeptin (Sigma) and chloroquine (Fluka). Cell-associated radioactivity was determined after washing and lysis of cells and degradation of labeled protein was measured as radioactivity in the trichloroacetic acid (TCA)-soluble fraction of the incubation medium.
Degradation of Hx-heme and activated α2-macroglobulin was further investigated by a pulse-chase assay in COS-1 cells grown. After washing, cells were incubated in serum-free medium containing 125I-labeled Hx-heme or activated α2-macroglobulin (100 000 cpm/well) for 1 hour. Excessive tracer was removed by washing the cell layer with PBS, pH 7.4. Cells incubated with Hx-heme and activated α2-macroglobulin tracer were added fresh medium containing 40 μg/mL RAP and activated α2-macroglobulin, respectively, to prevent reuptake of tracer while chasing. Degraded and cell-associated ligand was determined as described in the previous paragraph.
Confocal microscopy
COS-1 cells were grown on 2-chamber Permanox slides (Nunc) and washed in PBS, pH 7.4. Subsequently, 35 μg/mL Alexa 488 (Molecular Probes, Leiden, The Netherlands) was added to the labeled Hx-heme complex and incubated with or without 40 μg/mL unlabeled activated α2-macroglobulin for 1 hour in DMEM serum-free medium with 0.1% BSA, washed, and fixed in 4% formaldehyde for 1 hour at 4°C. After washing in PBS and 0.05% Triton X-100, pH 7.4, cells incubated with and without unlabeled activated α2-macroglobulin were incubated with 10 μg/mL anti-α2-macroglobulin (DakoCytomation) and anti-LRP/CD91 antibody,19 respectively, for 1 hour at room temperature in PBS, 0.05% Triton X-100, pH 7.4. Subsequently, cells were washed and incubated with Alexa 594-labeled secondary anti-rabbit IgG. In other experiments cells were incubated with 35 μg/mL Alexa 488-Hx-heme and monoclonal rhodamine-labeled anti-LRP/CD91 antibody (Serotec, Oxford, United Kingdom) for 2 hours at 4°C and subsequently 2 minutes at 37°C. To confirm uptake in early endosomes the cells were incubated with 35 μg/mL Alexa 488-Hx-heme for 2 hours at 4°C and 2 minutes at 37°C before the cells were fixed and stained with a mouse monoclonal antibody (catalog no. 610457; BD Biosciences, Milan, Italy) against the early endosome antigen 1 (EEA1). Nonimmune antibodies were used as negative controls. Slides were washed and mounted in Dako fluorescent mounting medium (DakoCytomation) and examined at 20°C with a Zeiss LSM-510 (Zeiss, Jena, Germany) confocal microscope using the Zeiss × 10 and × 63 lenses. The LSM-510 software was used for handling of pictures.
Measurement of HO-1 and HO-2 mRNA
EDTA-stabilized peripheral full blood was obtained from blood donors. Human peripheral-blood mononuclear cells were isolated from blood-donor buffy coats by gradient separation (Histopaque-1077; Sigma) and cultured for 3 days. Stimulation with Hx-heme, Hx, heme, and activated α2-macroglobin was carried out for 6 hours in serum-free medium with added 1% BSA. To examine whether stimulation of HO mRNA transcription by Hx-heme was related to LRP/CD91 activity, the effect of HO mRNA transcription was measured in the presence and absence of RAP, anti-LRP/CD91 IgG, and control IgG. Total RNA was isolated from the cells with the QIAamp RNA Blood Mini Kit (Qiagen, Albertslund, Denmark). Reverse transcriptase activity was obtained by adding 1 μL of the extracted RNA to a reaction mixture containing 6.3 mM MgCl2, 0.3 mM dNTP, 2.5 mM oligo dT primer, 20 U RNase inhibitor, and 50 U MuLV reverse transcriptase in a total volume of 20 μL (Applied Biosystems, Naerum, Denmark). cDNA was synthesized by incubation at 42°C for 30 minutes followed by 99°C for 5 minutes. Then, 2 μL of the cDNA sample was subsequently used as template for real-time quantitative polymerase chain reaction (qPCR) in a 10-μL reaction mixture containing 10 pmol of each primer (HO-1: forward primer 5′-GAA GAA GGC CAC CAA GGA GG-3′, reverse primer 5′-ACG TCA CCC AGG TAG CGG G-3′ or HO-2: forward primer 5′-AAG GAG CTG TTT AAG CTG GCC ACC-3′, reverse primer 5′-CAC CTG CTC CTC CAA GTT TTC ACC-3′), 1 μL FastStart SYBR Green I, FastStart Taq Polymerase, dNTP, SYBR Green I dye, and 10 mM MgCl2 (Roche Diagnostics, Hvidovre, Denmark). The qPCR was performed in a LightCycler System (Roche Diagnostics) at 95°C for 15 minutes followed by 40 cycles of 95°C for 10 seconds, 63°C/68°C (HO-1/HO-2 for 10 seconds and 72°C for 5 seconds). The PCR threshold cycle was determined by the LightCycler Software version 3.5. For each sample, the threshold cycle of unstimulated cells was used to estimate the level of up-regulation in stimulated cells, assuming a doubling of the DNA product in each cycle. Each experiment was carried out in triplicate. The specificity of all amplifications was checked by melting curve analysis and a representative of amplification products was verified by sequencing using ABI Prism 310 Genetic Analyzer (Applied Biosystems).
Results
Purification of the Hx-heme receptor
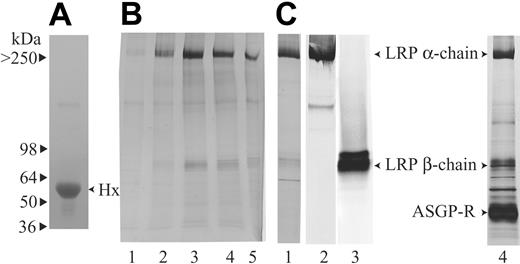
To purify the unknown Hx-heme receptor, Hx-heme affinity chromatography was carried out on solubilized membranes from human placenta and liver, tissues reported to possess receptor activity.12,21,22 Figure 1 shows SDS-gel electrophoresis of the eluted fractions from the Hx-heme column loaded with placenta membranes (Figure 1B lanes 1-5) and liver (Figure 1C lane 1). Two protein bands of approximately 500 and 85 kDa were predominant in both eluates. By mass spectrometry, these proteins were identified as the 515- and 85-kDa subunits of the LRP/CD9123 alias the α2-macroglobulin-receptor.24,25 The identity was confirmed by Western blotting with specific monoclonal antibodies19 against both subunits (Figure 1C lanes 2 and 3). Some less intensively stained proteins were also eluted from the column loaded with liver membranes. By mass spectrometry, a 60-kDa protein was identified as carboxylesterase and a 50-kDa protein was identified as the asialoglycoprotein receptor. The latter was not eluted from the freshly prepared Hx-heme column (Figure 1C lane 1) but became prominent (Figure 1C lane 4) after multiple receptor purification runs on the column. The binding of the asialoglycoprotein receptor, which is known to bind to asialohemopexin,14 might therefore reflect loss of terminal sialic acids of N-linked Hx carbohydrates on repeated use of the column.
Purification of the Hx-heme receptor from placenta and liver by Hx-heme affinity chromatography. (A) SDS-PAGE of the purified human Hx used for coupling to heme and the Sepharose matrix. (B) SDS-PAGE showing the elution profile of the eluate from the Hx-heme-Sepharose column loaded with solubilized human placenta membranes. (C) SDS-PAGE of the eluate (lane 1, peak fraction) from a Hx-heme-Sepharose column loaded with human liver membranes. Lanes 2 and 3 show Western blotting of liver Hx-heme affinity eluate using the monoclonal antibodies A2MRα-2 and A2MRβ-1, which recognize the LRP/CD91 α- and β-subunits, respectively. Lane 4 shows SDS-PAGE of the eluate from a Hx-heme column after multiple (> 10) loading-elution runs. Notice that the asialoglycoprotein receptor (ASGP-R) now is becoming a predominant band.
Purification of the Hx-heme receptor from placenta and liver by Hx-heme affinity chromatography. (A) SDS-PAGE of the purified human Hx used for coupling to heme and the Sepharose matrix. (B) SDS-PAGE showing the elution profile of the eluate from the Hx-heme-Sepharose column loaded with solubilized human placenta membranes. (C) SDS-PAGE of the eluate (lane 1, peak fraction) from a Hx-heme-Sepharose column loaded with human liver membranes. Lanes 2 and 3 show Western blotting of liver Hx-heme affinity eluate using the monoclonal antibodies A2MRα-2 and A2MRβ-1, which recognize the LRP/CD91 α- and β-subunits, respectively. Lane 4 shows SDS-PAGE of the eluate from a Hx-heme column after multiple (> 10) loading-elution runs. Notice that the asialoglycoprotein receptor (ASGP-R) now is becoming a predominant band.
Binding of Hx-heme to purified LRP/CD91
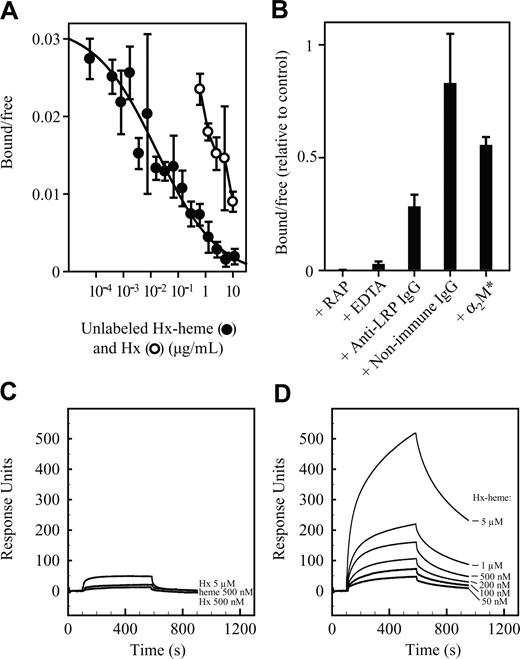
The binding of Hx-heme complexes to LRP/CD91 was analyzed in a solid-phase microtiter plate assay by incubation of purified and immobilized LRP/CD91 with labeled 125I-Hx-heme complex at 4°C. Figure 2A shows the concentration dependence of binding. Half-maximal binding (apparent Kd) was observed at 0.3 to 0.5 nM Hx-heme. Compared to Hx-heme the apoform of Hx was a far less potent inhibitor of the binding of 125I-Hx-heme complex. Half-maximal binding was measured at about 40 nM. No significant inhibition by heme in concentrations up to 15 μM was seen (not shown). As observed for other known LRP/CD91 ligands,16 binding of 125I-Hx-heme was completely inhibited by the LRP/CD91 inhibitor and the chaperone-like protein, RAP, and by complexing calcium with EDTA. Polyclonal anti-LRP/CD91 IgG but not nonimmune IgG inhibited binding. The receptor-binding conformation of α2-macroglobulin, a well-known LRP/CD91 ligand,19 only weakly inhibited binding (Figure 2B), thus indicating that it binds to a distinct site in LRP/CD91. 125I-Hx-heme complex was also tested for binding to megalin, a close homologue of LRP/CD91, which binds some LRP/CD91 ligands.26 However, like activated α2-macroglobulin,26 125I-Hx-heme did not exhibit measurable binding to megalin (data not shown). The binding to LRP/CD91 of the Hx-heme complex and the importance of the complex formation between Hx and heme for receptor recognition were verified by surface plasmon resonance analysis (Figure 2C-D), which allows for direct measuring of receptor binding at a wide range of ligand concentrations. This analysis showed a strong signal when Hx-heme (up to 5 μM) was exposed to a LRP/CD91 chip (Figure 2D). No significant signal was recorded for heme, and Hx showed at all concentrations (curves at 0.5 and 5 μM are shown in Figure 2C) more than a 10 times lower response compared to the identical concentrations of the Hx-heme complex (Figure 2D).
LRP/CD91-mediated endocytosis of Hx-heme complexes
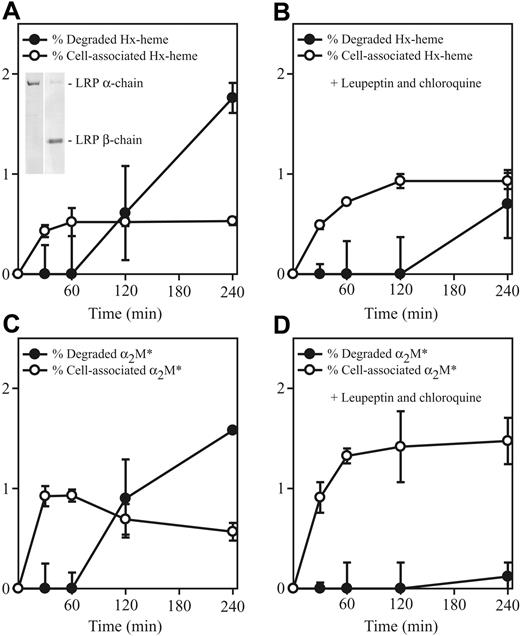
LRP/CD91-mediated uptake of Hx-heme was investigated in the LRP/CD91-expressing COS-1 cell line (Figure 3 inset) previously used for studying endocytic properties of this receptor.27 Figure 3A shows the time course for uptake of the 125I-Hx-heme complex. The complex was taken up and, after approximately 1 hour, radioactive degradation products were measurable in the medium. In accordance with degradation in lysosomes, the inhibitors of lysosomal activity, chloroquine and leupeptin, strongly inhibited degradation (Figure 3B). The curves for uptake and degradation of 125I-Hx-heme were almost indistinguishable from the similar curves of 125I-labeled activated α2-macroglobulin, except that this LRP/CD91 ligand exhibited a higher initial rate of uptake (Figure 3C-D).
Binding of Hx-heme to immobilized LRP/CD91. (A) Inhibition of binding of 125I-labeled Hx-heme by unlabeled Hx-heme complexes and noncomplexed Hx. (B) Effect on binding of 125I-labeled Hx-heme by RAP (100 μg/mL), EDTA (10 mM), rabbit anti-LRP/CD91 IgG (300 μg/mL), nonimmune rabbit IgG (300 μg/mL), and activated α2-macroglobulin (α2M*, 100 μg/mL). All values in panels A and B, representing the measured radioactivity in percentage of the added radioactivity, are the mean ± 1 SD of triplicate determinations. Each 200-μL well was incubated with 3000 cpm 125I-Hx-heme (∼0.5 ng). (C) Surface plasmon resonance analysis of the binding of Hx (500 nM and 5 μM) and heme (500 nM) to immobilized LRP/CD91. (D) Surface plasmon resonance analysis of the binding of Hx-heme (50 nM to 5 μM) to immobilized LRP/CD91.
Binding of Hx-heme to immobilized LRP/CD91. (A) Inhibition of binding of 125I-labeled Hx-heme by unlabeled Hx-heme complexes and noncomplexed Hx. (B) Effect on binding of 125I-labeled Hx-heme by RAP (100 μg/mL), EDTA (10 mM), rabbit anti-LRP/CD91 IgG (300 μg/mL), nonimmune rabbit IgG (300 μg/mL), and activated α2-macroglobulin (α2M*, 100 μg/mL). All values in panels A and B, representing the measured radioactivity in percentage of the added radioactivity, are the mean ± 1 SD of triplicate determinations. Each 200-μL well was incubated with 3000 cpm 125I-Hx-heme (∼0.5 ng). (C) Surface plasmon resonance analysis of the binding of Hx (500 nM and 5 μM) and heme (500 nM) to immobilized LRP/CD91. (D) Surface plasmon resonance analysis of the binding of Hx-heme (50 nM to 5 μM) to immobilized LRP/CD91.
LRP/CD91-mediated endocytosis of125I-labeled Hx-heme and activated α2-macroglobulin (α2M*) in COS-1 cells. (A) Time course of cell-associated radioactivity and degradation (measured as increase in TCA-soluble radioactivity) in cells incubated with 125I-labeled Hx-heme. The inset shows Western blot of solubilized COS-1 cells with the monoclonal antibodies (refer to legend for Figure 1) against the LRP/CD91 subunits. (B) Time course of uptake and degradation of 125I-labeled Hx-heme in the presence of the lysosomal inhibitors chloroquine and leupeptin (both 300 μM). (C-D) Similar experiments as in panels A and B using receptor-binding (methylamine-activated) 125I-α2-macroglobulin (α2M*) as radioligand instead. All values, which represent the measured radioactivity in percentage of the added radioactivity, are the mean ± 1 SD of triplicate determinations. Each well containing 300 μL medium was incubated with 3000 cpm 125I-Hx-heme (∼0.5 ng).
LRP/CD91-mediated endocytosis of125I-labeled Hx-heme and activated α2-macroglobulin (α2M*) in COS-1 cells. (A) Time course of cell-associated radioactivity and degradation (measured as increase in TCA-soluble radioactivity) in cells incubated with 125I-labeled Hx-heme. The inset shows Western blot of solubilized COS-1 cells with the monoclonal antibodies (refer to legend for Figure 1) against the LRP/CD91 subunits. (B) Time course of uptake and degradation of 125I-labeled Hx-heme in the presence of the lysosomal inhibitors chloroquine and leupeptin (both 300 μM). (C-D) Similar experiments as in panels A and B using receptor-binding (methylamine-activated) 125I-α2-macroglobulin (α2M*) as radioligand instead. All values, which represent the measured radioactivity in percentage of the added radioactivity, are the mean ± 1 SD of triplicate determinations. Each well containing 300 μL medium was incubated with 3000 cpm 125I-Hx-heme (∼0.5 ng).
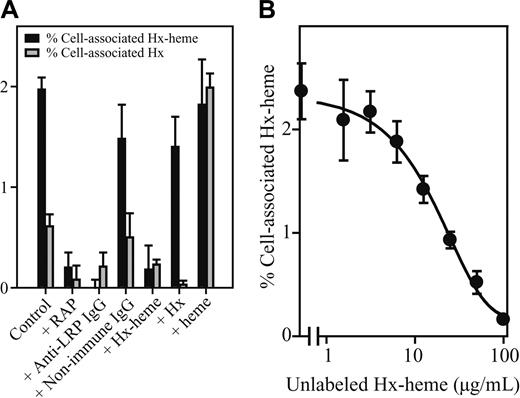
Internalization of 125I-Hx-heme and 125I-Hx in COS-1 cells was further analyzed over time with and without various inhibitors (Figure 4A). Both tracers were taken up in a RAP and anti-LRP antibody-inhibitable manner but the uptake of 125I-Hx was several fold less than the 125I-Hx-heme uptake. The importance of complex formation between Hx and heme was evident from the strong effect on uptake of 125I-Hx (25 pM) by adding heme to the medium. Furthermore, high concentrations (100 μg/mL) of Hx and heme had no or low effect on 125I-Hx-heme uptake, whereas 100 μg/mL Hx-heme completely inhibited uptake. Figure 4B shows that half-maximal inhibition of 125I-Hx-heme uptake was seen at about 10 μg/mL unlabeled Hx-heme complex. Because the cell incubation medium used for the uptake studies contained 1% bovine albumin, which binds heme with low affinity,28 the heme incubation experiment also indicates that albumin-heme does not compete for the receptor binding of Hx-heme.
Inhibition of LRP/CD91-mediated endocytosis of 125I-Hx-heme and 125I-Hx. (A) The effect on endocytosis 125I-Hx-heme and 125I-Hx by RAP (100 μg/mL), rabbit anti-LRP/CD91 IgG (300 μg/mL), nonimmune rabbit IgG (300 μg/mL), Hx-heme (100 μg/mL), Hx (100 μg/mL), and heme (50 μg/mL). The experimental conditions were otherwise as described in the legend for Figure 4. (B) The effect of various Hx-heme concentrations on 125I-Hx-heme endocytosis. Values shown in panels A and B represent the mean ± SD of triplicate determinations.
Inhibition of LRP/CD91-mediated endocytosis of 125I-Hx-heme and 125I-Hx. (A) The effect on endocytosis 125I-Hx-heme and 125I-Hx by RAP (100 μg/mL), rabbit anti-LRP/CD91 IgG (300 μg/mL), nonimmune rabbit IgG (300 μg/mL), Hx-heme (100 μg/mL), Hx (100 μg/mL), and heme (50 μg/mL). The experimental conditions were otherwise as described in the legend for Figure 4. (B) The effect of various Hx-heme concentrations on 125I-Hx-heme endocytosis. Values shown in panels A and B represent the mean ± SD of triplicate determinations.
RAP-inhibitable uptake was also observed when the heme moiety of the Hx-heme complex was labeled with 55Fe-labeled heme (not shown), confirming that the cells take up the entire complex.
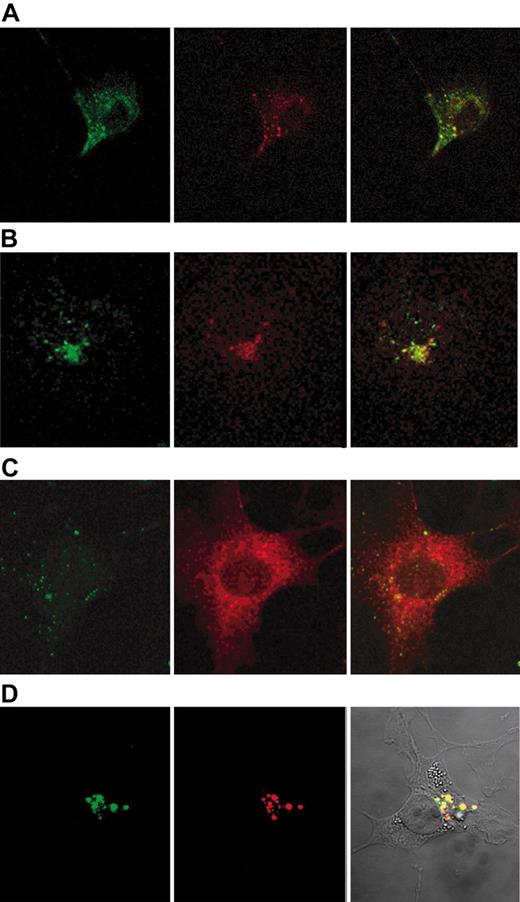
Confocal microscopy of COS-1 cells incubated for 2 minutes with fluorescent Hx-heme and fluorescent anti-LRP/CD91 monoclonal antibody showed similar staining of LRP/CD91 and Hx-heme (Figure 5A). Furthermore, the staining of Hx-heme showed overlapping staining with the early endosome protein EEA1 (Figure 5B). After 60 minutes the localization of the ligand fluorochrome was completely distinct from LRP/CD91 staining (Figure 5C) in accordance with classic receptor-mediated endocytosis, where the receptor segregates from the ligand in the early endosomes and recycles to the membrane. α2-macroglobulin, which ends up in late endosomes and lysosomes,29 showed, as expected, uptake in the same compartments as Hx-heme (Figure 5D).
Confocal microscopy of the uptake of Hx-heme and receptor-binding α2-macroglobulin in COS-1 cells. (A) Immunostaining of cells incubated 2 minutes at 37°C with Alexa 488-labeled Hx-heme (green color) and monoclonal rhodamine-labeled anti-LRP/CD91 antibody (red color). Notice the colocalization of the 2 fluorochromes. (B) Cells incubated 2 minutes at 37°C with Alexa 488-labeled Hx-heme (green color) and immunostaining for EEA1 using Alexa 594-labeled secondary antibody (red color). (C) Cells incubated for 60 minutes with Alexa 488-labeled Hx-heme (green color) and immunostained with LRP/CD91 using Alexa 594-labeled secondary antibody (red color). Notice the distinct staining of the 2 fluorochromes. (D) Vesicular staining of COS-1 cells incubated for 60 minutes with Alexa 488-labeled Hx-heme (green color) and receptor-binding (activated) α2-macroglobulin (α2M*), which was immunostained by an Alexa 594-labeled secondary antibody (red color). Notice the overlapping coloring in the overlay picture (right panel). Optic magnification was × 630 in all the figure displays.
Confocal microscopy of the uptake of Hx-heme and receptor-binding α2-macroglobulin in COS-1 cells. (A) Immunostaining of cells incubated 2 minutes at 37°C with Alexa 488-labeled Hx-heme (green color) and monoclonal rhodamine-labeled anti-LRP/CD91 antibody (red color). Notice the colocalization of the 2 fluorochromes. (B) Cells incubated 2 minutes at 37°C with Alexa 488-labeled Hx-heme (green color) and immunostaining for EEA1 using Alexa 594-labeled secondary antibody (red color). (C) Cells incubated for 60 minutes with Alexa 488-labeled Hx-heme (green color) and immunostained with LRP/CD91 using Alexa 594-labeled secondary antibody (red color). Notice the distinct staining of the 2 fluorochromes. (D) Vesicular staining of COS-1 cells incubated for 60 minutes with Alexa 488-labeled Hx-heme (green color) and receptor-binding (activated) α2-macroglobulin (α2M*), which was immunostained by an Alexa 594-labeled secondary antibody (red color). Notice the overlapping coloring in the overlay picture (right panel). Optic magnification was × 630 in all the figure displays.
Pulse-chase of COS-1 cells incubated with125I-labeled Hx-heme and α2-macroglobulin. The figure shows the time course of the appearance of TCA-precipitable and soluble radioactivity in the medium after pulse with 125I-Hx-heme (A) and methylamine-activated 125I-α2-macroglobulin (α2M*) for 60 minutes at 4°C followed by wash of the cells and incubation at 37°C (B). All values, which represent the measured radioactivity in percentage of the initial cell-associated radioactivity, are the mean ± 1 SD of triplicate determinations.
Pulse-chase of COS-1 cells incubated with125I-labeled Hx-heme and α2-macroglobulin. The figure shows the time course of the appearance of TCA-precipitable and soluble radioactivity in the medium after pulse with 125I-Hx-heme (A) and methylamine-activated 125I-α2-macroglobulin (α2M*) for 60 minutes at 4°C followed by wash of the cells and incubation at 37°C (B). All values, which represent the measured radioactivity in percentage of the initial cell-associated radioactivity, are the mean ± 1 SD of triplicate determinations.
The pathway for uptake of Hx-heme complexes has previously been reported30 to be similar to that of transferrin, which differs from α2-macroglobulin29 by returning intact to the cell surface after unloading its cargo in the endosome. To further explore the efficacy of the LRP/CD91-mediated degradation pathway for Hx-heme, we performed a pulse-chase experiment where degradation of Hx was measured in COS-1 cells preloaded with 125I-Hx-heme. This experiment showed that a large part of the endocytosed 125I-Hx-heme appeared as degraded ligand in the medium within 1 hour (Figure 6A). Compared to the receptor-binding form of α2-macroglobulin, no difference was observed (Figure 6B) except that Hx seems to undergo a faster degradation. In contrast, similar uptake studies with 125I-transferrin showed a completely different pattern with no degradation of the ligand (not shown).
To analyze whether the LRP/CD91-mediated endocytosis contributes to the activity of the heme-inducible HO-1 enzyme suggested to have an important role in inflammation, we investigated the effect of LRP/CD91 inhibitors on LRP/CD91- and HO-1-expressing human monocytes.31 In these cells the HO-1 transcription but not the HO-2 transcription was highly increased by heme and Hx-heme (Table 1). The strongest effect was seen with heme. Similar concentrations of Hx and activated α2-macroglobulin did not reveal any significant change in HO-1 activity (not shown). RAP and anti-LRP/CD91 IgG, but not control IgG, inhibited the Hx-heme-induced HO-1 transcription activity to approximately 50%.
The effect of Hx-heme on HO-1 and HO-2 mRNA transcription
Additive . | HO-1 . | HO-2 . |
|---|---|---|
| PBS | 1.2 ± 0.9 | 1.1 ± 0.9 |
| Heme + PBS | 174.5 ± 22.3 | 1.2 ± 0.3 |
| Hx-heme + PBS | 32.6 ± 9.5 | 0.9 ± 0.2 |
| Hx-heme + RAP | 17.4 ± 2.4 | 0.9 ± 0.3 |
| Hx-heme + anti-LRP/CD91 lgG | 11.6 ± 5.5 | 0.6 ± 0.4 |
| Hx-heme + nonimmune lgG | 25.9 ± 7.8 | 0.9 ± 0.1 |
Additive . | HO-1 . | HO-2 . |
|---|---|---|
| PBS | 1.2 ± 0.9 | 1.1 ± 0.9 |
| Heme + PBS | 174.5 ± 22.3 | 1.2 ± 0.3 |
| Hx-heme + PBS | 32.6 ± 9.5 | 0.9 ± 0.2 |
| Hx-heme + RAP | 17.4 ± 2.4 | 0.9 ± 0.3 |
| Hx-heme + anti-LRP/CD91 lgG | 11.6 ± 5.5 | 0.6 ± 0.4 |
| Hx-heme + nonimmune lgG | 25.9 ± 7.8 | 0.9 ± 0.1 |
The effect of Hx-heme on HO-1 and HO-2 mRNA levels in cultured monocytes as determined by reverse transcription-polymerase chain reaction. The cells were incubated for 6 hours at 37°C with 10 μM heme or with 10 μM Hx-heme in the absence or presence of 1 mg RAP, anti-LRP/CD91 lgG, and nonimmune lgG. The values shown are the mRNA levels relative to the levels before incubation. All values are the mean of triplicate determinations.
Discussion
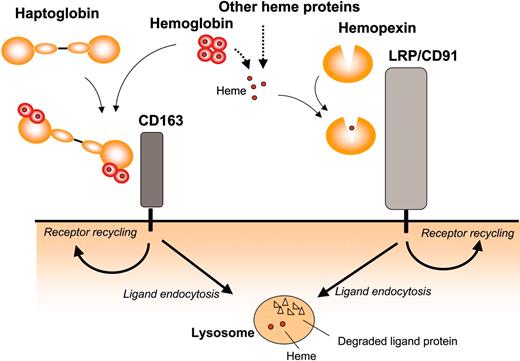
In the present study, we have purified LRP/CD91 as a receptor protein for Hx-heme complexes and shown that uptake by LRP/CD91 leads to endocytosis and lysosomal degradation of the protein component like the CD163-mediated endocytosis of haptoglobin-hemoglobin complexes10 and the LRP/CD91-mediated endocytosis of α2-macroglobin-proteinase complexes.16 These ligand-receptor systems have in common that their high-affinity ligands are formed when specific plasma proteins form complex with physiologic but potential toxic components to be removed from the circulation. Figure 7 shows the hemoglobin and heme uptake pathways, which in gene knock-out mouse models seem to have a concerted role in relation to hemolysis.6 The degradation pathways explain that high release of hemoglobin during excessive hemolysis causes substantial lowering of both haptoglobin and Hx in plasma.32
Whereas CD163 expression is restricted to monocytes and macrophages, LRP/CD91 is expressed in a wide spectrum of cell types including macrophages, hepatocytes, fibroblasts, adipocytes, neurons, and syncytiotrophoblasts.31 Hepatocytes and the macrophages in liver and spleen are the quantitatively most important cells for uptake of circulating LRP/CD91 ligands.16,33 In agreement with LRP/CD91-mediated endocytosis, injected Hx-heme predominantly ends up in the liver.32 The plasma lifetime of the LRP/CD91 ligands probably depends on the affinity for the hepatic receptors. α2-macroglobulin in its receptor-binding conformation (in complex with proteinases or activated in vitro by methylamine) has, for instance, a very high functional affinity for LRP/CD91 (apparent Kd = 40 pM)19 and a very short T1/2 (minutes) in rodent plasma,33 whereas the Hx-heme complexes have an approximately 10-fold lower affinity and a longer T1/2.32 During pregnancy the high expression of LRP/CD91 in the placenta34 may also contribute substantially to clearance of ligands from the circulation. HO-1 is expressed in the placenta35 and it is therefore likely that iron derived from uptake of Hx-heme by LRP/CD91 to some extent is delivered to the fetus.
Overview of the receptor pathways for endocytosis of extracellular heme and hemoglobin in complex with hemopexin and haptoglobin, respectively. LRP/CD91 and CD163 represent 2 pathways for uptake of extracellular heme incorporated in Hx-heme and haptoglobin-hemoglobin. Both receptors are highly expressed in phagocytic macrophages, which are known to metabolize heme into bilirubin, Fe, and carbon monoxide. In addition to the expression in macrophages, LRP/CD91 is highly expressed in several other cell types including hepatocytes, neurons, and syncytiotrophoblasts.31
Overview of the receptor pathways for endocytosis of extracellular heme and hemoglobin in complex with hemopexin and haptoglobin, respectively. LRP/CD91 and CD163 represent 2 pathways for uptake of extracellular heme incorporated in Hx-heme and haptoglobin-hemoglobin. Both receptors are highly expressed in phagocytic macrophages, which are known to metabolize heme into bilirubin, Fe, and carbon monoxide. In addition to the expression in macrophages, LRP/CD91 is highly expressed in several other cell types including hepatocytes, neurons, and syncytiotrophoblasts.31
Clearance of Hx-heme complexes from the interstitial fluids and other nonplasma tissue fluids is probably accounted for by LRP/CD91 in interstitial macrophages, fibroblasts, or organ-specific LRP/CD91-expressing cells. The heme metabolism in the central nervous system is particularly interesting because of the heme-mediated oxidative stress after cerebral tissue damage and haemorrhage.4,36 Hx is expressed in the brain37 and the Hx concentration in the cerebrospinal fluid is high compared to other proteins.38 This fact, combined with a high expression of LRP/CD91 and HO in neurons, suggests that the combined function of Hx, LRP/CD91, and HO protects the neural tissue against heme-mediated toxicity, which may be of particular importance in hemorrhagic events.
The clearance of Hx-heme in macrophages of the liver, spleen, and bone marrow may contribute to the recycling of iron as is the case for red-cell phagocytosis and uptake of haptoglobin-hemoglobin. Besides its phagocytic function, the macrophage is a multifaceted immune cell, and it is therefore intriguing to speculate that removal of the oxidative heme molecule by LRP/CD91 and CD163 (heme removed as a part of hemoglobin) contributes to the late anti-inflammatory response of interstitial macrophages in local areas of inflammation. Such an anti-inflammatory role of heme metabolism may be reinforced by the anti-inflammatory heme-oxygenase products, bilirubin and CO, generated in macrophages. In accordance with a potential anti-inflammatory aspect of heme metabolism in macrophages, LRP/CD91 and CD163 are predominantly expressed in phagocytic and anti-inflammatory (“alternatively activated”) macrophages.39,40
Previous attempts to identify the Hx-heme receptor have not revealed any protein structure besides the hepatic asialoglycoprotein receptor, which is suggested to represent a receptor for uptake of “outdated” asialo-Hx.15 However, several studies have published functional and structural aspects of cellular Hx-heme binding sites11,12,14,22,41 different from the asialoglycoprotein receptor. Various affinities are reported but in the most recent of these studies the affinity estimated (Kd = 0.34-0.85 nM) on human cytotrophoblasts is close to the estimated value for binding of Hx-heme to purified placental LRP/CD91. Size predictions of the Hx-heme-binding site by cross-linking experiments have estimated sizes of 80 to 90 kDa in mouse hepatoma cells41 and in placenta.12 The identity of this cross-linked component, which in size corresponds to LRP/CD91 β-chain (85 kDa), is yet unknown. One reason that LRP/CD91 might have escaped notification is the large size of LRP/CD91 α-chain, which only migrates in low percentage polyacrylamide (< 6%) SDS gels.16,18,19,24
LRP/CD91-mediated endocytosis is a degradation pathway for all known ligands of this receptor16 and, accordingly, the present data revealed that endocytosed Hx-heme follows this common issue. The transfer of the ligand to the late endosomes/lysosomes is probably controlled by the YWTD propeller repeats of LRP/CD91 because similar repeats in the LDL receptor have been shown to induce segregation of ligand and receptors when the pH decreases in the early endosomes.42 In a previous report Hx was suggested to recycle like transferrin.30 This suggestion was mainly based on the fact that pulse-chase of cultured hepatocyte cell lines incubated with Hx-heme resulted in recovery of some intact Hx (or Hx-heme) from the medium after cellular uptake of labeled ligand. The cellular release of intact Hx from the cells may be explained by the previous43 and present observation (Figure 5B) that LRP/CD91-mediated internalization, like that mediated by other endocytic receptors, is not fully effective in directing all receptor-bound ligands to the lysosomes.
Uptake of Hx-heme stimulates HO-1 transcription as shown here and previously.44,45 The stimulatory pathway is probably different from the strong HO-1 induction by free heme, which attacks the plasma membranes.2 The biologic effect of heme has been studied in endothelial cells, which are exposed and highly sensitive to heme-induced generation of reactive oxygen species and oxidized LDL.46,47 Hx has been shown to prevent the heme effects on endothelium including the induction of HO-1. The inhibition by Hx on heme-induced HO-1 mRNA transcription seems to be more pronounced in endothelium than in hepatic cells44,45 and in monocytes (data presented here). This difference in induction of HO-1 mRNA transcription by the Hx-heme complex might be due the fact that endothelial cells in contrast to monocytes and hepatocytes do not express LRP/CD91.31 A role of LRP/CD91 in HO-1 induction in monocytes was indicated by the approximate 50% inhibition of Hx-heme-induced HO-1 mRNA transcription by RAP and anti-LRP/CD91 antibody in cultured monocytes. The remaining stimulatory effect in the presence of LRP/C91 inhibitors seems not to be accounted for by an alternative Hx-heme receptor system because we were not able to measure specific uptake of Hx-heme in the presence of the inhibitors (data not shown). Instead, the noninhibitable HO-1 induction might be due to nonspecific fluid-phase uptake or some induction by the complexed heme in the medium. No induction of HO-1 was seen by activated α2-macroglobin, suggesting that the induction by Hx-heme is caused by the heme taken up in the cells rather than the ligand-receptor interaction per se. In accordance with such a heme-dependent mechanism of stimulation, a similar stimulation of HO-1 is seen in monocytes/macrophages after CD163-mediated endocytosis of haptoglobin-hemoglobin complexes.48
In conclusion, the identification of LRP/CD91 as the endocytic receptor for Hx-heme complexes now reveals a hitherto unidentified player in the cellular heme uptake, thereby providing a novel aspect in the research on the biologic consequences of heme metabolism. For instance, the present data imply that Hx-heme uptake occurs in a much wider spectrum of cell types than previously thought. Special attention for future investigation should be given to the potential importance of LRP/CD91 and Hx in dampening heme-mediated toxicity and inflammation after cerebral hemorrhage and other internal bleedings.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2005-03-1185.
Supported by grants from the Danish Research Council, Faculty of Health Science, Aarhus University, the Novo Nordisk Foundation and the Carlsberg Foundation.
V.H. performed research (major part) and participated in writing the paper; M.B.M. and H.J.M. performed research (RT-PCR analysis); C.J. performed research (Biacore analysis), P.H. performed research (MALDI mass spectrometry analysis); and S.K.M. designed the study and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are deeply indebted to Kirsten Lassen (deceased 2004) for her comprehensive and highly competent technical assistance in the present and previous receptor studies.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal