Abstract
Human herpesvirus 8 (HHV-8) is etiologically associated with Kaposi sarcoma (KS), the most common AIDS-associated malignancy. Previous results indicate that the HHV-8 viral transactivator ORF50 interacts synergistically with Tat protein in the transactivation of human immunodeficiency virus (HIV) long terminal repeat (LTR), leading to increased cell susceptibility to HIV infection. Here, we analyze the effect of HHV-8 infection on HIV replication in monocyte-macrophage and endothelial cells, as potential targets of coinfection. Primary or transformed monocytic and endothelial cells were infected with a cell-free HHV-8 inoculum and subsequently infected with lymphotropic or monocytotropic strains of HIV. The results show that HHV-8 coinfection markedly increases HIV replication in both cell types. HHV-8 infection induces also HIV reactivation in chronically infected cell lines and in peripheral blood mononuclear cells (PBMCs) from patients with asymptomatic HIV, suggesting the possibility that similar interactions might take place also in vivo. Furthermore, coinfection is not an essential condition, since contiguity of differently infected cells is sufficient for HIV reactivation. The results suggest that HHV-8 might be a cofactor for HIV progression and that HHV-8-infected endothelial cells might play a relevant role in transendothelial HIV spread. (Blood. 2005;106:2790-2797)
Introduction
Human herpesvirus 8 (HHV-8), also called Kaposi sarcoma-associated herpesvirus (KSHV), is a γ-2 herpesvirus originally identified in Kaposi sarcoma (KS) lesions.1 Since then, HHV-8 has been identified as the etiologic agent of all epidemiologic forms of KS, including classical, endemic African, and AIDS types.2 HHV-8 infection has also been implicated in the pathogenesis of other neoplastic disorders affecting immunocompromised hosts: primary effusion lymphomas (PELs),3 multicentric Castleman disease,4 lymphoproliferative disorders affecting patients infected with HIV,5 and neoplastic complications in patients after transplantation.6-8
HHV-8 DNA and transcripts have been detected in PEL-derived cells, lymph nodes, peripheral blood B cells, macrophages, keratinocytes, and endothelial and epithelial cells of patients with KS.3,9 In vitro, HHV-8 infects human B, endothelial, epithelial, and fibroblast cells, as well as animal cells, almost invariably with low yields of infectious progeny.10-12
The incidence of HHV-8 infection varies among different geographic areas,13-15 with higher values of prevalence in areas where HIV infection is widespread16,17 or in patients infected with HIV from areas where the virus is rarely detected in the healthy population.13,14,18-20
HHV-8 establishes latent infections in the healthy population, with high prevalence in sub-Saharan Africa, intermediate prevalence in the Mediterranean area, and very low prevalence in North Europe and North America.21 Also in KS and PELs most neoplastic cells support a latent HHV-8 infection. In most instances, lytic replication in neoplastic lesions is limited to a small number of cells, presumably reflecting spontaneous reactivation from latency.22-24 Nevertheless, latency alone is not sufficient to induce HHV-8 tumorigenesis, and preliminary evidence suggests that ongoing lytic replication is required for tumorigenesis, as indicated by clinical studies.25 Recent evidence shows that patients positive for HIV have circulating HHV-8 and shed virus even in the absence of disease.26 Therefore, it is likely that interactions between the 2 viruses might take place in patients positive for HIV.
Several studies indicate that HIV plays an important role in the pathogenesis of AIDS-related KS,27 possibly by promoting the initiation and progression of KS through the biologic effects of the Tat protein and inducing cytokine production.28,29 HIV infection stimulates HHV-8 replication30 and reactivation in latently infected cells,31 and HIV-1 Tat induces the lytic cycle replication of HHV-8.32 The effect of HHV-8 on HIV is still largely undetermined. Some, but not all, epidemiologic studies show that HHV-8 is associated to AIDS progression.33,34 From a molecular point of view, it has been shown that specific HHV-8 genes can activate HIV. The latency-associated nuclear antigen (LANA) activates the long terminal repeat (LTR) of HIV, stimulating p24 expression.35 We reported that ORF50, the major transactivator of HHV-8 lytic cycle, interacts synergistically with HIV-1 Tat, inducing HIV-1 LTR transactivation,36 leading to increased cell susceptibility to HIV infection.37,38 Furthermore, ORF50 activates several heterologous promoters, including interleukin 6 (IL-6),39,40 which has been shown to induce monocyte chemotactic protein 1 (MCP-1) production in macrophages, increasing susceptibility to HIV infection.41,42
To gain further insight into the biologic interactions between the 2 viruses in coinfected cells, we studied the effects of HHV-8 infection on HIV activation and replication in potential targets of coinfection, such as monocytes and endothelial cells. Primary or transformed cells of monocyte or endothelial origin were infected with a purified HHV-8 inoculum and subsequently infected with different lymphotropic or monocytotropic HIV strains. Retroviral infection was studied by polymerase chain reaction (PCR), reverse transcriptase-PCR (rtPCR), p24 analysis, and virus titration. The effect of HHV-8 was also evaluated on HIV reactivation in chronically infected cells, using both cell lines (U1) and primary peripheral blood mononuclear cells (PBMCs) isolated from patients with asymptomatic HIV. The results show that HHV-8 infection increases HIV replication in acutely infected cells and induces HIV reactivation in chronically infected cells, even when the cells are not simultaneously coinfected but simply cultured together.
Materials and methods
Cells
Human endothelial cells (HUVECs) were isolated from umbilical vein as previously described43 and cultured in EGM-2 complete medium (Cambrex; BioWhittaker, Verviers, Belgium), using collagen-coated flasks (Biocoat Collagen; BD Biosciences, Bedford, MA). Second to fifth passage HUVECs were used for all experiments. Approval was obtained from the University of Ferrara institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
The PEL-derived BC-3 cell line,44 used for HHV-8 production, was maintained in RPMI medium (Gibco, Grand Island, NY) supplemented with 10% inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (complete RPMI). Complete RPMI was also used for propagation of promonocytic U937 cell line,45 U1 cell line, chronically HIV infected and characterized by inducibility of HIV,46,47 H9 and C8166 T cell lines, used for HIV-1 IIIB production and titration.37
Primary monocyte-derived macrophages (MDMs) were obtained from buffy coats of healthy blood donors by Ficoll-Hypaque gradient, followed by plastic adherence of PBMCs for 3 days in complete RPMI supplemented with 20% FCS, at a concentration of 106/mL per cm2. Nonadherent cells (peripheral blood lymphocytes; PBLs) were removed, and adherent cells (MDMs) were extensively washed with medium and seeded in 12-well plates with fresh complete medium at a concentration of 0.5 × 106/mL. MDM preparation contained greater than 90% CD14+ cells, as assessed by acridine orange staining.48
Peripheral blood mononuclear cells were isolated from 14 patients with asymptomatic HIV (8 men and 6 women), who had not received any antiviral therapy and had low viral load (< 10 000 copies/mL) and high CD4+ T cells counts (> 400/μL). Mean age was 41 years (range, 34-51 years). Experiments of HIV reactivation were performed on total PBMCs, or on MDMs and PBLs separated by plastic adherence for 3 days. Positive controls of reactivation were obtained by stimulating cultured cells with 2 μg/mL anti-human CD3 monoclonal antibody plus 10 U/mL human IL-2 (Sigma, St Louis, MO), or with 20 ng/mL 12-O-tetradecanoyl phorbol 13-acetate (TPA).
Virus infection
HHV-8. Cell-free HHV-8 inoculum was prepared by stimulation of BC-3 cell line for 3 days with 20 ng/mL TPA (Sigma). The cells were collected by centrifugation and lysed by 3 cycles of rapid freezing and thawing followed by sonication (3 cycles of 5 seconds at medium power with 10-second intervals in a water bath sonicator). Cleared cellular content was added to culture supernatant, and virions were collected by centrifugation at 20 000g at 4°C. Virus particles were purified by density centrifugation using Optiprep self-forming gradients (Sentinel, Milan, Italy), at 58 000g for 3.5 hours at 4°C. Purified virions were washed in phosphate-buffered saline (PBS) and recovered by centrifugation at 20 000g for 30 minutes at 4°C. Collected virions were suspended in PBS containing 1% bovine serum albumin and stored at -80°C until use. Purified virus preparation (1 mL) was obtained from 4 × 108 stimulated BC-3 cells. The same ratio between cells and final suspension volume was maintained in all virus preparations to avoid variations in virus concentration between different stocks. Virus particles were morphologically intact, and the preparation was devoid of cell debris and membranes, as assessed by electron microscope observation. Infectivity of virus preparation was evaluated by specifically designed infection experiments performed in different cell types, using PCR, rtPCR and immunofluorescence assays to evaluate virus presence, transcription, and expression of antigens. More specifically, primers and conditions for PCR amplification are as follows: ORF50 amplification took place with primers 5′-TTGGTGCGCTATGTGGTCTG-3′ (forward) and 5′-GGAAGGTAGACCGGTTGGAA-3′ (reverse) for 30 cycles (1 minute at 94°C, 1 minute at 57°C, and 1 minute at 72°C plus 3 sec/cycle of extension); ORF73 amplification was performed with primers 5′-CTGCCTTTACTCCTCCAAAC-3′ (forward) and 5′-GCCACATCCAAGATACCAAC-3′ (reverse) for 35 cycles (1 minute at 94°C, 1 minute at 56°C, and 1 minute at 72°C plus 3 sec/cycle of extension); ORF26 was amplified with primers GCCGAAAGGATTCCACCAT and TCCGTGTTGTCTACGTCCAG for 45 cycles (1 minute at 94°C, 1 minute at 58°C, and 1 minute at 72°C). Prior to use, virus stock was treated with DNase-I and RNase-A, to eliminate free viral nucleic acids eventually present in the preparation.
For infection of primary endothelial cells (HUVECs), cells were seeded at 2 × 105 cells/well in 6-well plates. One day later, cells were washed once with PBS and infected with 10 μL/mL/well of purified virus preparation. After 3 hours of absorption at 37°C, the viral inoculum was removed, cells were washed twice with PBS and incubated in complete medium for a further 24 hours before HIV infection. Cell samples were harvested at specific time points and processed as described.
For infection, primary MDMs were harvested by scraping and plated into 12-well plates at 0.5 × 106 cells per well. Infection was performed 24 hours later as described for HUVECs.
Suspension cells (PBMCs, PBLs, U937, U1) were seeded at 0.5 × 106 cells/mL in 12-well plates and infected as already described. Control samples were mock-infected with UV-inactivated HHV-8, obtained by exposing the purified inoculum under UV light (200 mJ/cm2) for 30 minutes. This treatment completely inactivated the virus, as shown by the total absence of viral transcripts in infected cells, analyzed by rtPCR.
HIV. The lymphotropic strain HIV-1IIIB was propagated in CD4+ T H9 cell line and titrated in C8166 T-cell line as previously described.37 Virus stock contained 2 × 107 tissue culture 50% infective doses (TCID50) per mL. The prototypic monocytotropic strains HIVBaL and HIVADA were propagated and titrated in phytohemagglutinin-stimulated PBMCs.49,50 Virus stocks of HIVBaL and HIVADA contained, respectively, 1 × 106 TCID50/mL and 5 × 106 TCID50/mL.
HIV infection was performed 24 hours after HHV-8 infection. Cells were washed with complete medium and infected with HIV strains at multiplicity of infection (MOI) ranging from 1:10 to 1:1000 for the 3 virus subtypes. Following overnight absorption, all cultures were washed 3 times with PBS until no p24 antigen was detectable, seeded in 12-well plates, and refed with fresh medium. Cells and supernatant samples were collected for PCR, rtPCR, p24 analysis and HIV titration at 0, 4, 8, 12, 16, and 20 days after infection. Endothelial cells were passaged when reached complete confluence, every 7 days.
Cell transfection
All cells were seeded 24 hours before transfection to obtain optimal cellular density, transfected with plasmid pCR-50sp, expressing the mature transcript of HHV-8 ORF 50,36 and infected with HIV 24 hours after transfection. Control samples were transfected with pCR vector alone.
Promonocytic cell lines (U937, U1) were suspended in serum-free medium (107 cells/mL) and transfected by electroporation (960 μF, 250 mV) with 20 μg plasmid DNA using a Genepulser II (Bio-Rad, Hercules, CA). HUVECs or MDMs cells were seeded in 6-well plates, respectively, at 0.2 or 1 × 106 cells per well and transfected with 5 μg plasmid DNA per well using the GeneJuice transfection reagent (Novagen, Madison, WI). After 6 hours of incubation, the transfection mixture was replaced with complete growth medium, and cells were incubated for an additional 24 hours before HIV infection. Transfection and transcription of pCR-50sp was monitored in by PCR and rtPCR, using primers and conditions previously described.36 The efficiency of transfection for all cell types ranged between 16% and 30% and was assessed with the reporter plasmid pEGFP-C1 (BD Clontech, San Jose, CA), followed by fluorescence observation with a Nikon Eclipse TE2000-S microscope (Nikon, Tokyo, Japan). Images were processed with Photopaint (Corel, Ottawa, ON, Canada).
Cell cocultures
HUVEC/C8166 cocultures were used to detect the transmission of HIVIIIB from infected HUVECs to target C8166 T cells; 0.5 × 106 C8166 cells were cultured in complete RPMI with 0.5 × 106 HUVECs coinfected with HHV-8 and HIVIIIB. Culture supernatants were collected 7 days later, centrifuged, and analyzed for release of p24. To analyze HIV reactivation, 0.5 × 106 chronically HIV-infected U1 cells were cocultured with 0.5 × 106 resting, TPA-stimulated or HHV-8 infected HUVECs. Culture supernatants were collected at different time points and analyzed for p24 release. U1 cells were also cultured in the presence of 2-fold dilutions of supernatants obtained from resting, TPA-activated, or HHV-8-infected HUVEC monolayers.
HIV quantitation
HIV production was analyzed by measuring p24 antigen and virus release in culture supernatant, and by semiquantitative PCR for HIV proviral DNA in cell samples. Cells were collected by centrifugation and the recovered culture medium was tested separately. p24 analysis was performed by a commercial enzyme-linked immunosorbent assay (Perkin-Elmer, Boston, MA), as described.37 Release of infective virus was monitored by titration of cell supernatant on C8166 cells or mitogen-stimulated PBMCs, as already described.37,49 The presence of replication-competent HIV particles associated with cell preparation was evaluated by cocultures with sensitive C8166 T cells. The presence of proviral DNA was assessed by PCR, amplifying a 438-base pair (bp) fragment from the HIV-1 gag gene, as described.37 Semiquantitation of PCR products was performed by amplification of serial dilutions of template DNA. To normalize the amount of DNA contained in each sample, amplification of the housekeeping β-actin gene was used as a control.36 PCR products were run in 1.5% agarose gels and visualized by ethidium bromide staining. Acquisition and processing of images were performed by GelDoc (Bio-Rad) and PhotoPaint (Corel, Ottawa, ON, Canada).
Statistical analysis
Statistical analysis of collected data were performed by Student t test.
Results
Effect of HHV-8 on HIV infection in monocytes
To assess the effect of HHV-8 infection on HIV replication in monocytes, the promonocytic U937 cell line was infected with a purified cell-free HHV-8 inoculum and subsequently infected with HIV. To determine the effect of generic cellular activation and of ORF50 expression on HIV infection, cells were also stimulated with TPA or transfected with a plasmid encoding HHV-8 ORF 50 (pCR-50sp). Cells were mock-infected with UV-inactivated HHV-8 or transfected with pCR vector alone, as a negative control. After 24 hours, cells were infected with HIVIIIB (MOI, 1:10-1:1000), and HIV replication was evaluated in supernatant and in cell samples at days 0, 4, 8, 12, 16, and 20 after infection.
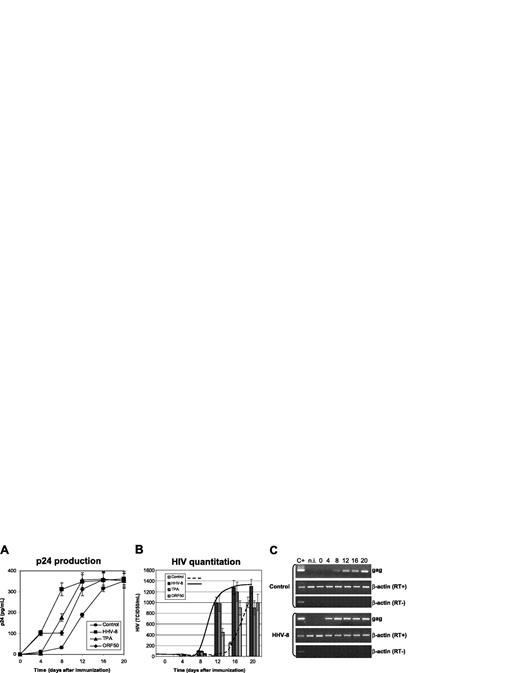
Effect of HHV-8 infection on HIV in monocytes. U937 cells were infected with HHV-8 or transfected with plasmid pCR-50sp and subsequently infected with HIVIIIB at a 1:1000 MOI. Control cells were cultured in the absence of TPA, or mock-infected with UV-inactivated virus, or transfected with the empty pCR vector; the results obtained were identical, and the values shown represent the mean of the 3 controls. p24 antigen release (A) and HIV virion production (B) were evaluated. Values represent the mean of duplicate samples of 2 separate experiments. Error bars indicate SD. (C) The results are shown of rtPCR amplification of HIV gag gene using 200 ng total RNA extracted from infected cells; β-actin amplification is shown as a control. Control for DNA contamination, performed by direct β-actin amplification of RNA without retrotranscription, is also shown.
Effect of HHV-8 infection on HIV in monocytes. U937 cells were infected with HHV-8 or transfected with plasmid pCR-50sp and subsequently infected with HIVIIIB at a 1:1000 MOI. Control cells were cultured in the absence of TPA, or mock-infected with UV-inactivated virus, or transfected with the empty pCR vector; the results obtained were identical, and the values shown represent the mean of the 3 controls. p24 antigen release (A) and HIV virion production (B) were evaluated. Values represent the mean of duplicate samples of 2 separate experiments. Error bars indicate SD. (C) The results are shown of rtPCR amplification of HIV gag gene using 200 ng total RNA extracted from infected cells; β-actin amplification is shown as a control. Control for DNA contamination, performed by direct β-actin amplification of RNA without retrotranscription, is also shown.
The results confirmed that U937 cell line supports replication of lymphocytotropic strains of HIV, because mock-infected or transfected control cells produced substantial amounts of p24 antigen and retroviral particles (Figure 1A). HHV-8 infection, as well as the expression of ORF50, resulted in a marked increase of HIV production (Figure 1B), particularly evident at early time points (4-12 days after infection). The increase of HIV titer was statistically significant at each time point tested (HHV-8 infected versus controls, P < .001) and was accompanied by earlier detection of HIV proviral DNA and mRNA (Figure 1C).
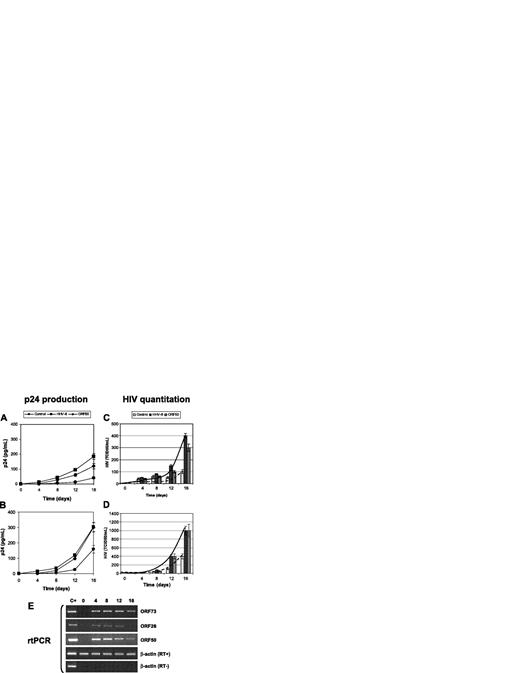
Effect of HHV-8 on HIV in primary macrophages. Primary MDMs from healthy donors were infected with HHV-8 or transfected with pCR-50sp and were subsequently infected with monotropic strains HIVBaL (A,C) and HIVADA (B,D) at a 1:1000 MOI; p24 antigen release (A-B) and HIV particles production (C-D) were evaluated. Values represent the mean of duplicate samples of 2 separate experiments. Error bars indicate SD. (E) The results are shown of rtPCR amplification of the indicated HHV-8 genes using 200 ng total RNA extracted from infected cells; β-actin amplification is shown as a control. Control for DNA contamination, performed by direct β-actin amplification of RNA without retrotranscription, is also shown.
Effect of HHV-8 on HIV in primary macrophages. Primary MDMs from healthy donors were infected with HHV-8 or transfected with pCR-50sp and were subsequently infected with monotropic strains HIVBaL (A,C) and HIVADA (B,D) at a 1:1000 MOI; p24 antigen release (A-B) and HIV particles production (C-D) were evaluated. Values represent the mean of duplicate samples of 2 separate experiments. Error bars indicate SD. (E) The results are shown of rtPCR amplification of the indicated HHV-8 genes using 200 ng total RNA extracted from infected cells; β-actin amplification is shown as a control. Control for DNA contamination, performed by direct β-actin amplification of RNA without retrotranscription, is also shown.
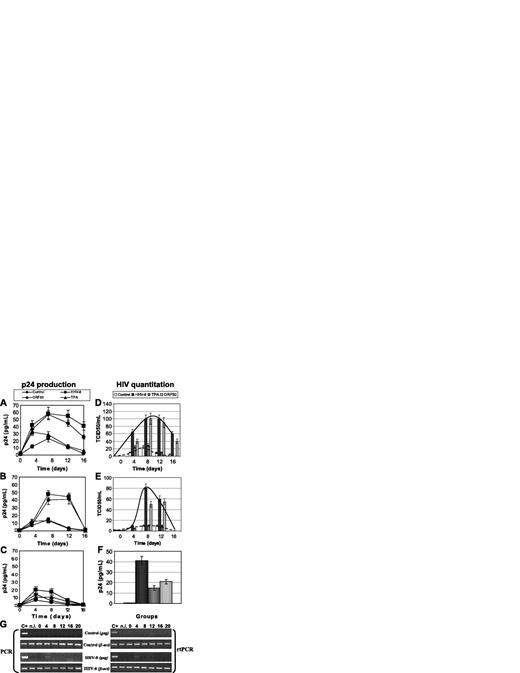
Effect of HHV-8 infection on HIV in primary endothelial cells. HUVEC cells were infected with HHV-8 or transfected with plasmid pCR-50sp, and 24 hours later were infected with HIV. Release of p24 antigen (A-C) and production of HIV infectious particles (D-F) were evaluated. Values represent the mean of duplicate samples of 2 separate experiments; (A,D) monotropic HIVBaL strain (MOI, 1:100), (B,E) monotropic HIVADA strain (MOI, 1:100), (C,F) lymphotropic HIVIIIB strain (MOI, 1:10). HIV production was measured by analyzing p24 release after coculturing infected HUVECs with C8166 target cells for 7 days. Error bars indicate SD. (G) Presence and transcription of HIVIIIB in untreated (Control) and infected (HHV-8) HUVECs. Results are of PCR and rtPCR analyses by amplification of HIV gag gene.
Effect of HHV-8 infection on HIV in primary endothelial cells. HUVEC cells were infected with HHV-8 or transfected with plasmid pCR-50sp, and 24 hours later were infected with HIV. Release of p24 antigen (A-C) and production of HIV infectious particles (D-F) were evaluated. Values represent the mean of duplicate samples of 2 separate experiments; (A,D) monotropic HIVBaL strain (MOI, 1:100), (B,E) monotropic HIVADA strain (MOI, 1:100), (C,F) lymphotropic HIVIIIB strain (MOI, 1:10). HIV production was measured by analyzing p24 release after coculturing infected HUVECs with C8166 target cells for 7 days. Error bars indicate SD. (G) Presence and transcription of HIVIIIB in untreated (Control) and infected (HHV-8) HUVECs. Results are of PCR and rtPCR analyses by amplification of HIV gag gene.
The study was extended to primary macrophages. MDMs were isolated from healthy blood donors negative for HIV and infected with HHV-8 or transfected with ORF50. Infection of MDMs with HHV-8 was checked by rtPCR detection of immediate early and late viral mRNAs (ORF 50, ORF 26, ORF 73) (Figure 2E). After 24 hours, cells were infected with the monocytotropic strains HIVBaL and HIVADA, and retrovirus replication was evaluated at 0, 4, 8, 12, and 16 days after infection.
The results show that the replication of monocytotropic HIV strains is significantly higher in the presence of HHV-8 infection (P < .001) (Figure 2), even if ADA was more efficient both in p24 release and in the production of infectious virus. Similar results were obtained also by transfection of ORF50.
Effects of HHV-8 on HIV infection in endothelial cells
Endothelial cells can be infected both by HHV-812 and by HIV51 and therefore represent a potential common target for coinfection. To examine possible cooperative effects between the 2 viruses, primary HUVECs were infected in sequence with HHV-8 and with lymphocytotropic or monocytotropic strains of HIV. TPA stimulation and ORF50 transfection were also performed, as previously described for monocytes. Negative controls were mock-infected or -transfected as described for monocytes.
HHV-8 entry and transcription in HUVECs was analyzed by PCR and rtPCR for 3 weeks after infection, showing that endothelial cells support lytic replication of HHV-8 for the first 7 days after infection, and afterward a latent infection is established. One day after HHV-8 infection, cells were infected with HIVIIIB, HIVBaL, or HIVADA, using MOI of 1:10 and 1:100. Supernatant and cells samples were collected at 0, 4, 8, 12, and 16 days after infection and analyzed for p24 production, virus particles release, and presence and transcription of HIV proviral DNA.
Analysis of p24 release showed that HUVECs support replication of monocytotropic strains of HIV (BaL, ADA) (Figure 3A-B), whereas they are not permissive for lymphocytotropic IIIB strain (Figure 3C). Compared with controls, HHV-8 infection induced a marked increase of p24 release and a 2- to 10-fold increase of production of infectious HIV particle release, as shown by direct virus titration on lymphoid blasts (Figure 3D-E). The difference was statistically significant at all time points (HHV-8 infected versus controls, P < .001). In addition, HHV-8 infection permitted partial replication of lymphocytotropic IIIB strain, as indicated by PCR and rtPCR results (Figure 3G). Nevertheless, it was not possible to detect virus release from HIVIIIB-infected cells; therefore, the production of HIV particles was analyzed by measuring p24 antigen release after 7 days of coculture with C8166 target cells (Figure 3F). The results showed that only coinfected cells were able to transmit infectious HIV particles to susceptible C8166 cells, confirming previous results obtained in other nonpermissive cellular systems.36,38
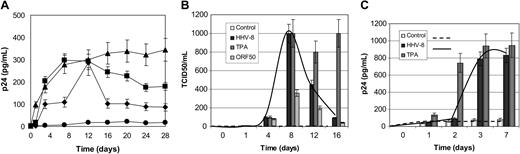
Effect of HHV-8 infection on HIV reactivation in chronically infected U1 monocytes. (A-B) U1 cells were infected with HHV-8 or transfected with plasmid pCR-50sp and analyzed for HIV reactivation by measuring p24 antigen release (A) and production of infectious particles in culture supernatant (B). (A) • indicates control; ▪, HHV-8 infection; ▴, TPA stimulation; and ♦, ORF 50 transfection. (C) HIV reactivation on contact with HHV-8-infected cells. U1 cells were cultured with HHV-8-infected or TPA-stimulated HUVECs or supernatant. HIV reactivation was analyzed by measuring the release in culture supernatants. Each value represents the mean of duplicate samples in 2 separate experiments. Error bars indicate SD.
Effect of HHV-8 infection on HIV reactivation in chronically infected U1 monocytes. (A-B) U1 cells were infected with HHV-8 or transfected with plasmid pCR-50sp and analyzed for HIV reactivation by measuring p24 antigen release (A) and production of infectious particles in culture supernatant (B). (A) • indicates control; ▪, HHV-8 infection; ▴, TPA stimulation; and ♦, ORF 50 transfection. (C) HIV reactivation on contact with HHV-8-infected cells. U1 cells were cultured with HHV-8-infected or TPA-stimulated HUVECs or supernatant. HIV reactivation was analyzed by measuring the release in culture supernatants. Each value represents the mean of duplicate samples in 2 separate experiments. Error bars indicate SD.
HIV reactivation in chronically infected cells
To investigate the effect of HHV-8 infection on HIV reactivation, the chronically HIV-infected U1 cell line was infected with HHV-8, and HIV replication was subsequently examined by analyzing antigen and virion release. As described for de novo HIV infection experiments, TPA stimulation and ORF50 transfection were also included in the study.
Analysis of p24 antigen release in culture medium showed that HHV-8 infection induced HIV reactivation as efficiently as TPA (Figure 4A).
Transfection of ORF50 induced a similar effect, although lower in magnitude, reaching the same amount of p24 production on day 12 after infection. At later times, p24 antigen release was decreased both in HHV-8-infected and ORF50-transfected cells, probably because of their transient expression, compared with TPA treatment.
Similarly, the results of direct HIV titration showed that the effect of HHV-8 on HIV replication was reversible and disappeared at 16 days after infection (Figure 4B). Microscopic observation of infected cells revealed that 3 weeks after infection HHV-8-infected U1 cells were still viable and actively proliferating, whereas TPA-treated cells showed a massive cytopathic effect.
To explore the possibility that the interaction between the 2 viruses was not necessarily caused by their simultaneous presence inside the same cell, we analyzed HIV reactivation in monocytes cocultured with HHV-8-infected endothelial cells. Previous studies demonstrated that contact with TPA-activated endothelial cells enhances HIV replication in in vitro-infected macrophages and chronically infected monocytic cell lines.52-54 We therefore evaluated the levels of p24 secreted by U1 cells cultured with resting, TPA-activated, or HHV-8-infected HUVECs.
The results showed that coculture with HHV-8-infected endothelial cells strongly enhanced p24 release from U1 monocytes, compared with U1 cultured alone or cocultured with resting HUVECs (Figure 4C). The increase was evident 3 days after coculture (9-fold increase; mean ± SD, 789 ± 46 pg/mL), persisted at 7 days (9.7-fold increase; mean ± SD, 830 ± 54 pg/mL), and was statistically significant (P < .001). As expected, also TPA-activated HUVECs increased p24 release from U1 cells. The increase was evident at day 2 and peaked at day 7 (18-fold increase; mean ± SD, 948 ± 97 pg/mL). Interestingly, a significant (P < .01) up-regulation of HIV replication was also observed when U1 were cultured in the presence of culture supernatant derived from HHV-8-infected HUVECs, suggesting that HHV-8 virions and/or other soluble factors capable of activating HIV are released by HUVECs in culture supernatant on infection with HHV-8 (not shown).
HIV reactivation in PBMCs from HIV+ patients
Experiments of HIV reactivation were performed on total PBMCs, or separated MDMs and PBLs, from patients positive for HIV. Cells were infected with purified HHV-8 and analyzed for HIV reactivation at days 0, 1, 3, 7, and 14 after infection. Positive controls of HIV reactivation were obtained by stimulating cells with TPA or anti-human CD3 plus IL-2. HIV reactivation was evaluated by PCR, rtPCR, and p24 antigen production analyses.
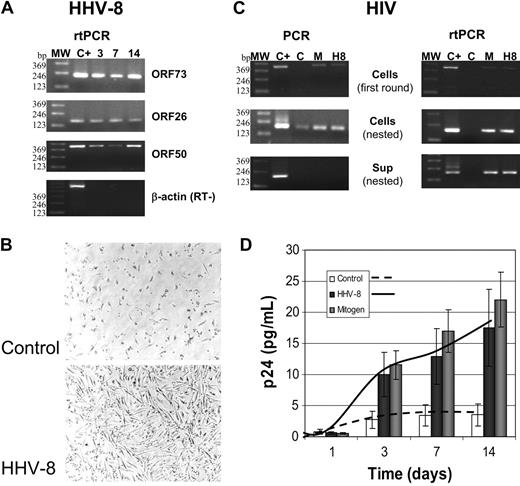
The results show that PBMCs derived from patients with HIV are readily infected by HHV-8, and virus was detectable by PCR and rtPCR until the end of experiments (14 days) (Figure 5A). HHV-8 infection did not induce any cytopathic effect in patient PBMCs. Rather, a morphologic change was seen only in monocyte cells (Figure 5B), likely resulting from cell activation because it was detected also in the presence of mitogens (not shown). HHV-8 infection induced HIV reactivation from latency in 12 of 14 samples (85.7%), without any appreciable difference between total PBMCs or enriched MDM and PBL cultures. As shown by PCR and rtPCR results (Figure 5C), the retrovirus was detected by first-round PCR amplification only in HHV-8-infected or mitogen-stimulated cells, whereas it was poorly visible by nested PCR in unstimulated cells. No HIV genomic RNA was detected in culture supernatant of unstimulated cells, whereas it was present in both mitogen-or HHV-8-treated cells, indicating that virus was actively released from these cells. Because of the low viral load in patient PBMCs, p24 antigen production was faintly detected, and the presence of reactivated virus was evaluated by coculturing infected cells with uninfected lymphoid blasts for 7 days. The procedure allowed the amplification of HIV infection, because of the de novo infection of actively proliferating target cells, and showed that all the samples infected with HHV-8 and positive by PCR and rtPCR produced infectious HIV (Figure 5D). By contrast, very low p24 release was detected in cocultures with uninfected PBMCs, indicating that there was almost no spontaneous reactivation of latent HIV following coculture with target cells, but rather that HHV-8 infection did induce HIV reactivation in latently infected cells. Alternatively, HHV-8 infection greatly enhanced HIV replication after a low-grade spontaneous reactivation.
Reactivation of HIV in PBMCs isolated from patients with asymptomatic HIV. PBMCs were infected with HHV-8 (H8) or treated with mitogens (M) and analyzed at the indicated times after infection. (A) rtPCR analysis for the presence and transcription of HHV-8; the results shown refer to one patient, but similar results were obtained for all the 14 subjects analyzed. MW indicates molecular weight marker. (B) Phase-contrast photomicrograph of HHV-8-infected PBMCs. A Nikon Eclipse TE2000-S microscope was used (with a 10 ×/2.8 numeric aperture objective), equipped with a DM100 digital camera (all from Nikon, Tokyo, Japan). (C) Results of PCR and rtPCR analyses of HIV load in cells and culture supernatant at 7 days after infection (same patient as A); similar results were obtained in 12 of 14 patients. (D) p24 antigen release in culture supernatant of control, HHV-8-, or mitogen-treated PBMCs from patients with HIV. Each value represents the mean of duplicate samples of the 12 responder patients. Error bars indicate SD.
Reactivation of HIV in PBMCs isolated from patients with asymptomatic HIV. PBMCs were infected with HHV-8 (H8) or treated with mitogens (M) and analyzed at the indicated times after infection. (A) rtPCR analysis for the presence and transcription of HHV-8; the results shown refer to one patient, but similar results were obtained for all the 14 subjects analyzed. MW indicates molecular weight marker. (B) Phase-contrast photomicrograph of HHV-8-infected PBMCs. A Nikon Eclipse TE2000-S microscope was used (with a 10 ×/2.8 numeric aperture objective), equipped with a DM100 digital camera (all from Nikon, Tokyo, Japan). (C) Results of PCR and rtPCR analyses of HIV load in cells and culture supernatant at 7 days after infection (same patient as A); similar results were obtained in 12 of 14 patients. (D) p24 antigen release in culture supernatant of control, HHV-8-, or mitogen-treated PBMCs from patients with HIV. Each value represents the mean of duplicate samples of the 12 responder patients. Error bars indicate SD.
Discussion
HHV-8 plays a major pathogenetic role in individuals infected with HIV, and Kaposi sarcoma, uncommon in the immunocompetent population, is the most frequent neoplasia in patients with AIDS. The pathogenic role of HHV-8 is enhanced both by HIV-induced immunosuppression and by induction of cytokine production. In addition, HIV has direct effects on HHV-8, inducing reactivation and lytic replication of latent HHV-8.31,32,55 Recent reports suggest that also HHV-8 might have an effect on HIV infection. In vitro, cocultivation of HHV-8-infected B-cell lines with HIV-infected CD4+ T cells results in a significant increase of HIV replication.56 The simultaneous intracellular presence of HHV-8 and HIV-1 up-regulates reciprocal gene expression57 ; expression of HHV-8 LANA (ORF73) activates HIV LTR,35 and HHV-8 viral G protein-coupled receptor cooperates with HIV Tat in NF-AT (nuclear factor of activated T cells) and NF-κB activation.58 Recently, we showed that the main HHV-8 transactivator, ORF50, increases Tat activation of LTR with a synergistic effect, resulting in significant biologic effects of small amounts of Tat, not active per se.36 Both constitutive and transient expression of ORF50 increases susceptibility to HIV infection in natural target cells (T lymphocytes) and induces transient susceptibility in nonpermissive cells (B lymphocytes, glial cells),37,38 which acquire the ability to transmit HIV infection to not infected susceptible T cells. These results suggest that HHV-8 infection might expand the range of cell types acting as a reservoir for HIV infection, inducing retrovirus reactivation in latently infected cells, and increasing its replication in actively infected cells.
KS is a multifocal vascular neoplasm that involves the skin, visceral organs, and lymph nodes, containing distinctive proliferating spindle cells, activated endothelial cells, fibroblasts, smooth muscle cells, and infiltrating inflammatory cells59 ; therefore, it is possible that, in coinfected patients with HIV, infected cells might come in contact with cells supporting active HHV-8 infection and replication or even be coinfected by both viruses. Endothelial cells and monocytes represent potential sites of interaction between the 2 viruses. Both cell types are infected by HHV-8, either abortively (endothelial cells) or acutely (monocytes). Monocytes, together with T helper cells, are the most common HIV-infected cells in the bloodstream, and tissue macrophages are thought to be responsible for HIV dissemination in tissues and organs. Likewise, HIV infection of endothelial cells has been repeatedly described, even if the success of infection depends on the endothelial type.51 Also the metabolic state plays an important role. In fact, HIV does not replicate in resting HUVECs, but it establishes a transient productive infection with release of infectious virions in proliferating and cytokine-stimulated HUVECs.60
To study the effects of HHV-8 on HIV replication, we infected monocytes and endothelial cells. In both cell types HHV-8 infection is characterized by an initial productive infection followed by establishment of latency, as shown by the detection of latency transcripts alone at the later stages of infection. On infection, endothelial cells assumed the typical “spindle” pheno-type, accordingly with what was already observed by others.61-63 No cytopathic effect was observed in endothelial- and monocytic-infected cells, although viral DNA and RNA persisted in monocytes until 30 days after infection.
Primary monocytes produce significantly higher amounts of HIV in the presence of HHV-8, with different efficiencies for the 2 monocytotropic HIV strains (Figure 2). Also HUVECs show significant increase of HIV production when coinfected by HHV-8, with transient productive replication of monocytotropic HIV strains (Figure 3). This effect was still present, but less relevant, in the case of HIV IIIB. Infected endothelial cells acquired the ability to transmit HIV-infecting particles to susceptible target T cells by direct contact.
The same enhancing effect of HHV-8 was detected also in chronically infected U1 cells and, more importantly, in macrophages and lymphocytes derived from asymptomatic patients with A1 phase HIV, suggesting that an undergoing HHV-8 active infection in coinfected patients might act as a cofactor for HIV replication. Furthermore, HIV reactivation was also observed when HHV-8-infected endothelial cells were cocultured with HIV-infected monocytes. This suggests that the cellular colocalization of both viruses is not an essential requisite for interaction, but the contact of differently infected cells is sufficient to induce HIV replication. At the present we do not know the mechanisms of HHV-8 enhancement on HIV. It has been reported that HHV-8 encodes viral products partially homologous to cellular chemokines, which can induce inflammatory cytokine production in monocytes, macrophages, and dendritic64 and endothelial cells,65 which in turn might influence HIV replication. However, the HIV-enhancing effects were observed in part with the transfection of ORF50; therefore, transactivation of cellular or retroviral functions could play a determinant role.
In conclusion, HHV-8 enhances HIV replication and induces HIV reactivation in latently infected monocytic cells; furthermore, coinfected endothelial cells might act as a significant reservoir for HIV spread. These observations suggest the possibility that HHV-8 might play a role in the initial stages of AIDS progression, and that the presence of HHV-8 might activate HIV during macrophage passage through the endothelial barrier. It has been reported that patients with KS with increasing HHV-8 viral loads have also increasing HIV loads,66 and recent epidemiologic analysis showed that the presence of antibodies to HHV-8 lytic antigens is significantly associated to the development of AIDS.33 It is important to consider that HHV-8 viremia in patients infected with HIV is significantly associated to increased KS risk.67 Because the development of KS is a defining condition of AIDS, in clinical studies it is difficult to discriminate whether HHV-8 replication is associated just to KS development or whether it also contributes to AIDS progression. Nevertheless, the evidence provided by our studies suggests the possibility that HHV-8 might increase HIV load and spread, therefore accelerating the progression to AIDS.
Prepublished online as Blood First Edition Paper, June 23, 2005; DOI 10.1182/blood-2005-04-1390.
Supported by grants from the Italian Ministry of Health (Istituto Superiore di Sanità, AIDS project) and the Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Linda M. Sartor for English revision of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal