Abstract
Dendritic cells (DCs) are able to open the tight junctions between adjacent epithelial cells (ECs) and to take up both invasive and noninvasive bacteria directly from the intestinal lumen. In this study, we describe a tight cross talk between ECs and human monocyte-derived DCs (MoDCs) in bacterial handling across epithelial monolayers. We show that the release of proinflammatory mediators by ECs in response to bacteria is dependent on bacterial invasiveness and on the presence of flagella. This correlates with the capacity of EC-derived factors to modulate MoDC function. MoDCs incubated with supernatants of bacteria-treated ECs are “noninflammatory” as they release interleukin-10 (IL-10) but not IL-12 and can drive only T helper (Th)-2 type T cells. Moreover, noninflammatory MoDCs release chemokines aimed at recruiting Th2 and T-regulatory cells. In contrast, when MoDCs are incubated with ECs and bacteria in a transwell coculture system, and can contact directly the bacteria across stimulated EC monolayers, they are more inflammatory as they release IL-12 and IL-10 and induce both Th1 and Th2 responses. These results suggest that ECs are not simply a barrier to bacteria entering via the oral route, but they actively influence the activating properties of DCs. (Blood. 2005;106:2818-2826)
Introduction
The intestinal wall is continuously exposed to myriad potentially harmful ingested bacteria; however, under physiologic conditions, we overreact only to pathogens. Entry of pathogens across the intestinal mucosa occurs mainly through specialized epithelial cells, called M cells, which are located in Peyer patches (PPs).1 In addition, we have recently described a new mechanism for bacterial entry that is mediated by dendritic cells (DCs).2 DCs are distributed as immature cells in nonlymphoid organs and in the blood, where they perform a sentinel function for incoming pathogens.3-8 Immature DCs are characterized by the capacity to take up antigens and to phagocytose macroparticles.9,10 During infection or inflammation, DCs are mobilized in and out of peripheral tissues11,12 and activated DCs are targeted to secondary lymphoid organs.13,14 Here, DCs have the unique function, among antigen-presenting cells, to activate naive T cells. Thus, DCs play an important role in the induction of immune responses.
Lamina propria DCs are able to open the tight junctions (TJs) between adjacent epithelial cells (ECs) and to capture bacteria directly across the mucosal epithelium.2,15 The epithelial barrier is preserved because DCs express TJ proteins whose level is regulated by bacteria or bacterial products, and establish TJ-like structures with neighboring epithelial cells.2 This mechanism is active both with invasive and noninvasive bacteria and is regulated by the expression of fractalkine receptor (CX3C chemokine receptor 1 [CX3CR1]) by DCs.16 Lamina propria DCs could be activated either by the direct contact with bacteria present in the intestinal lumen, or by ECs after their exposure to environmental bacteria. In fact, it is known that EC function is regulated by the type of encountered bacteria,17-20 which could be then translated into different signals to DCs. In this study, the interaction of human monocyte-derived DCs (MoDCs) with bacteria across epithelial monolayers was studied using an in vitro system established in our laboratory. This system allows simplifying the mucosal barrier to just 3 players: MoDCs, epithelial cells, and bacteria in a spatial distribution similar to that found in vivo. Two possible scenarios were considered that allowed us to study the interaction of MoDCs with bacteria either directly across the monolayer of activated ECs, or indirectly, through the response mediated by EC-derived factors. We found that MoDCs activated in these 2 ways were functionally different in their ability to release cytokines and to prime naive T cells, indicating that direct or indirect activation of MoDCs can lead to 2 distinct immunologic outcomes.
Materials and methods
Cells and reagents
DCs were derived from human peripheral blood monocytes according to a slightly modified protocol.21 Briefly, monocytes were purified by positive selection with anti-CD14 antibodies coupled to magnetic beads (Miltenyi, Bologna, Italy). CD14+ cells were incubated for 6 days in complete medium containing granulocyte-macrophage colony-stimulating factor (GM-CSF, 50 ng/mL; Peprotech) and interleukin-4 (IL-4, 20 ng/mL; Peprotech, Milan, Italy) in order to obtain immature MoDCs.
Bacterial strains
The following Salmonella enterica serovar typhimurium (SL) strains on SL1344 background were kindly provided by Dr G. Dougan (Imperial College, London, United Kingdom). Invasive strains: wild type, SL1344 WT; htrA; salmonella pathogenicity island (SPI)-II (ssaV), ompCompF, FliC; Noninvasive strains: msbB (lipid A mutant); SurA; SPI-I (InvA-). Attenuated S typhimurium strains were impaired either in invasiveness as SPI I (InvA-) deficient, in their capacity to survive inside the phagosome as SPII (ssaV) deficient, in porine expression as ompCompF, in the presence of flagellin as FliC, or in general toxicity as msbB, which lack productive lipopolysaccharide (LPS). Nonpathogenic bacterial strains were: Escherichia coli: DH5α, Lactobacillus plantarum (LP): NCIMB882 WT; and Bacillus subtilis (BS). All of the Salmonella strains were grown at 37°C in Lurian broth, supplemented with appropriate antibiotics to preserve carried mutations. LP was grown in de Man, Rogosa, and Sharpe (MRS) broth (DIFCO, Milan, Italy). BS was grown in Brain Heart Infusion (BHI; DIFCO).
Epithelial cell monolayers
In brief, Caco-2 cells were seeded in the upper chamber of a transwell filter (3-μm diameter of pores; Costar, Milan, Italy) for 7 to 10 days until a transepithelial resistance (TER) of 300 ohm/cm2 was achieved.
Direct system. Filters were turned upside down and MoDCs (4 × 105) were seeded on the filter facing the basolateral membrane of epithelial cells for 4 hours to let the cells attach to the filter. Alternatively, MoDCs (4 × 105) were first conditioned with supernatants of Caco-2 cell monolayers for 16 hours and then seeded on filters. Filters were then turned again upside down into 24-well plates. The transwells were either left untreated or were treated directly with bacteria (ratio of 10 bacteria to 1 MoDC, nearly 4 × 106 colony-forming units [CFUs]/transwell [TW]) from the apical surface (top chamber). One hour after incubation, bacteria were washed out and medium was changed with one containing antibiotics (gentamicine, 100 μg/mL). MoDCs and culture supernatants were collected after 16 hours from the bottom chamber. MoDCs were detached from filters by gentle centrifugation and analyzed by cytofluorometry for surface activation markers: CD83, CD80, and human leukocyte antigen-DR (HLA-DR) (all from Pharmingen, Milan, Italy). Cytokines were measured in culture supernatants by enzyme-linked immunosorbent assay (ELISA; IL-10, IL-12, IL-6, CXC chemokine ligand 8 [CXCL8], CC chemokine ligand 17 [CCL17], CCL22, CCL18, CCL19, CCL20, CCL3, CCL2, CXCL12; all from R&D Systems, Minneapolis, MN). Confocal microscopy on sample filters was performed to confirm that the MoDCs had direct access through tight junctions.
Indirect system. Epithelial cell monolayers were incubated with bacteria (5 × 107 CFU/TW) from the apical surface (top chamber). One hour after incubation, bacteria were washed out and medium was changed with one containing antibiotics (gentamicine, 100 μg/mL). Culture supernatants were collected 4 hours later from the bottom chamber (facing the basolateral membrane) and were used to activate MoDCs. Alternatively, EC monolayers were incubated with 4 × 106 CFU/TW bacteria and supernatants were collected 16 hours later (as in the direct activation). MoDCs were incubated for 24 hours in culture supernatant and then analyzed phenotypically for expression of activation markers (as in the direct activation). Analysis of cytokines released by epithelial cells or dendritic cells was performed by testing culture supernatants, as in the direct system, before and after MoDC incubation.
MoDC T-cell cocultures
MoDCs were collected after 24 hours of incubation with the different stimuli as for direct or indirect systems, and then incubated with allogeneic CD4+CD45RA+ purified T cells (Miltenyi) in 48-well plates (at a ratio of 10 T cells to 1 DC). After 5 days of culture, cells were restimulated with PMA + ionomycin for 4 hours and with Brefeldin A (Sigma, Milan, Italy) for an additional 2 hours. Cells were collected, fixed, and permeabilized with Cyto Fix/Perm (Becton Dickinson, Milan, Italy). Intracellular staining was performed with phycoerythrin (PE)-conjugated antibodies to IL-4, IL-10, and with fluorescein isothiocyanate (FITC)-conjugated anti-interferon (IFN)-γ antibody (all from Pharmingen). Stained cells were analyzed by fluorescence-activated cell-sorting (FACS) analysis.
Analysis of the ability of MoDCs to creep between epithelial cells in response to bacteria
Caco-2 cells were seeded in the upper chamber of a transwell filter (Costar 3-μm diameter of pores) facing the lower chamber for 7 to 10 days until a TER of 300 Ohm/cm2 was achieved. MoDCs (4 × 105) were seeded from the basolateral membrane. L plantarum or S typhimurium (107 CFU) were resuspended in RPMI medium containing 2% fetal calf serum (FCS) without antibiotics and were seeded from the apical face. Two hours later, filters were fixed in 3% paraformahaldehyde in phosphate-buffered saline (PBS) and were processed for immunofluorescence and laser confocal microscopy. Images were captured with a Leica TCS SP2 microscope (Leica, Milan, Italy) using a 40 ×/1.25 numeric aperture (NA) objective and were acquired and processed with Leica Power Scan software. Images were processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). To analyze the ability of MoDCs to intercalate between ECs and open tight-junction proteins, filters were stained for DCs (CD11c+) or tight-junction markers (occludin), as already described.2 Sections on the Z plane were collected.
Immunofluorescence for CCL20
Mice were anesthetized and ligated ileal loops were performed tying 2 knots 2 cm apart. CFUs (108) of bacteria resuspended in PBS were injected into the loops, and mice were killed at different time points ranging from 30 minutes to 4 hours. Intestinal pieces corresponding to the ligated loop area were snap-frozen in liquid nitrogen in optimum cutting temperature (OCT). Frozen sections (5-μm thick) were cut with a cryostat and affixed to poly-l-lysine-coated glass slides. Sections were fixed with 3% paraformaldehyde in PBS for 15 minutes at room temperature (RT), rinsed with PBS, and then blocked and permeabilized with 1% bovine serum albumin (BSA)/0.1% Triton in PBS. Sections were stained with goat anti-mouse CCL20 antibody (R&D Systems) and with carbocyanine 3 (Cy3) rabbit anti-goat antibody that was used as secondary antibody. Nuclear DNA was stained with TO-PRO-3 iodide (final 1 μM; Molecular Probes, Milan, Italy) according to manufacturer's instructions. Sections were observed under an Olympus BX61 fluorescence microscope (Olympus, Segrate, Italy), using an Olympus 60 ×/0.9 NA objective and equipped with a C5985 Hamamatsu black-and-white CCD camera (Hamamatsu, Milan, Italy), controlled by Olympus analysis software. Images were processed with Adobe Photoshop 7.0.
Statistical methods
Significance of difference between cytokine production by MoDCs treated as for direct or indirect activation was calculated by Student t test.
Results
EC monolayers are differently affected by the invasiveness of bacteria or by the presence of flagella
We first studied the response of EC monolayers to bacteria. Caco-2 monolayers were incubated apically with the following strains of bacteria: Salmonella typhimurium (SL) attenuated in different aspects of pathogenicity; Lactobacillus plantarum (LP); or laboratory strains of Escherichia coli (EC, DH5α) and Bacillus subtilis (BS). Attenuated S typhimurium strains were impaired either in invasiveness as SPI I deficient (SL-InvA), in their capacity to survive inside the phagosome as SPI II deficient, in the presence of flagellin as FliC (SL-FliC) or in general toxicity as msbB which lack productive lipopolysaccharide (LPS). Bacteria were seeded from the apical side, and supernatants were collected from the basolateral face 5 hours later and tested for cytokine or chemokine production by ELISA. This time point was chosen because at later times after incubation with invasive Salmonella strains, the integrity of the epithelial barrier was disrupted. As shown in Table 1, ECs responded differently according to the invasiveness and the presence of flagella of the bacterial strains used. In accordance with previous work, the production of CXCL8 (IL-8) was dependent on invasiveness and on the expression of flagellin as nonflagellated Salmonella (SL-FliC) was unable to induce CXCL8 release.20,22,23 We could not detect any production of tumor necrosis factor-α (TNF-α) or IL-1β in response also to invasive bacteria. The release of CCL18 (pulmonary and activation-regulated chemokine [PARC]), instead, was dependent on the invasiveness, but not on the presence of flagella as SL-FliC but not SL-InvA mutant was able to induce its production. CCL20 (macrophage inflammatory protein 3α [MIP-3α]) was released upon exposure to flagellated bacteria, regardless of their invasiveness. In fact, B subtilis, a noninvasive flagellated soil bacterium, induced levels of CCL20 comparable with invasive S typhimurium, whereas the SL-FliC mutant or the nonflagellated commensal L plantarum did not induce CCL20 expression (Table 1). We could not detect any production of CCL19 (MIP-3β), CCL3 (MIP-1α), CXCL12 (stromal cell-derived factor 1 [SDF-1]), CCL2 (monocyte chemoattractant protein 1 [MCP-1]), CCL22 (macrophage-derived chemokine [MDC]), or CCL17 (thymus and activation-regulated chemokine [TARC]). These results suggest that only invasive flagellated bacteria generate a strong inflammatory environment aimed at the recruitment of neutrophils in response to CXCL8. Bacterial invasiveness but not flagella is required for the release of CCL18, a chemoattractant of naive B24 and T cells25 as well as memory T cells,26 whereas flagellin but not invasiveness is required for the release of CCL20 that induces the recruitment of CCR6-expressing immature DCs.20,27 Finally, mature DCs probably are not recruited, as CCL19 is not produced.
Analysis of chemokine production by epithelial cell monolayers incubated with the indicated bacterial strains
Bacterial strains* . | CXCL8, ng/mL . | IL-10, ng/mL . | CCL20, ng/mL . | CCL22, ng/mL . | CCL18, ng/mL . | CCL19, ng/mL . | CCL2, ng/mL . | CCL17, ng/mL . | Invasive . | Flagellin . |
|---|---|---|---|---|---|---|---|---|---|---|
| SL 1344 WT | 1.55 | < DL | 0.96 | < DL | 17.0 | < DL | < DL | < DL | Yes | Yes |
| htrA | 1.13 | — | 1.08 | — | — | < DL | — | — | Yes | Yes |
| ssaV (SPI-II) | 1.31 | — | 1.13 | <DL | — | < DL | — | — | Yes | Yes |
| ompCompF | 0.72 | — | 0.65 | — | — | < DL | — | — | Yes | Yes |
| SL-FliC | < DL | < DL | 0.10 | < DL | 22.8 | — | < DL | < DL | Yes | No |
| msbB | 0.07 | — | 0.46 | — | — | < DL | — | — | No | Yes |
| SurA | 0.56 | — | 1.02 | — | — | < DL | — | — | No | Yes |
| SL-InvA (SPI-I) | 0.16 | < DL | 0.88 | < DL | 7.8 | < DL | < DL | < DL | No | Yes |
| BS | < DL | — | 1.10 | — | — | — | — | — | No | Yes |
| LP | < DL | < DL | 0.12 | < DL | 8.1 | — | < DL | < DL | No | No |
| DH5α | < DL | — | 0.08 | — | — | — | — | — | No | No |
| Untreated | < DL | < DL | 0.04 | < DL | 8.2 | < DL | < DL | < DL | — | — |
Bacterial strains* . | CXCL8, ng/mL . | IL-10, ng/mL . | CCL20, ng/mL . | CCL22, ng/mL . | CCL18, ng/mL . | CCL19, ng/mL . | CCL2, ng/mL . | CCL17, ng/mL . | Invasive . | Flagellin . |
|---|---|---|---|---|---|---|---|---|---|---|
| SL 1344 WT | 1.55 | < DL | 0.96 | < DL | 17.0 | < DL | < DL | < DL | Yes | Yes |
| htrA | 1.13 | — | 1.08 | — | — | < DL | — | — | Yes | Yes |
| ssaV (SPI-II) | 1.31 | — | 1.13 | <DL | — | < DL | — | — | Yes | Yes |
| ompCompF | 0.72 | — | 0.65 | — | — | < DL | — | — | Yes | Yes |
| SL-FliC | < DL | < DL | 0.10 | < DL | 22.8 | — | < DL | < DL | Yes | No |
| msbB | 0.07 | — | 0.46 | — | — | < DL | — | — | No | Yes |
| SurA | 0.56 | — | 1.02 | — | — | < DL | — | — | No | Yes |
| SL-InvA (SPI-I) | 0.16 | < DL | 0.88 | < DL | 7.8 | < DL | < DL | < DL | No | Yes |
| BS | < DL | — | 1.10 | — | — | — | — | — | No | Yes |
| LP | < DL | < DL | 0.12 | < DL | 8.1 | — | < DL | < DL | No | No |
| DH5α | < DL | — | 0.08 | — | — | — | — | — | No | No |
| Untreated | < DL | < DL | 0.04 | < DL | 8.2 | < DL | < DL | < DL | — | — |
The following cytokines were below the detection limit in culture supernatants from any conditions: CCL3, CXCL 12.
Bacterial strains: Invasive S typhimurium: wild type, SL 1344 WT; htrA; SPI-II (ssaV), ompComp, FliC. Noninvasive S typhimurium: msbB (lipid A mutant); SurA; SPI-I (InvA); E. coli: DH5α; B. subtilis: BS; L plantarum: LP.
< DL indicates below detection limit; —, not determined.
Flagellated noninvasive bacteria induce CCL20 expression in vivo in mice
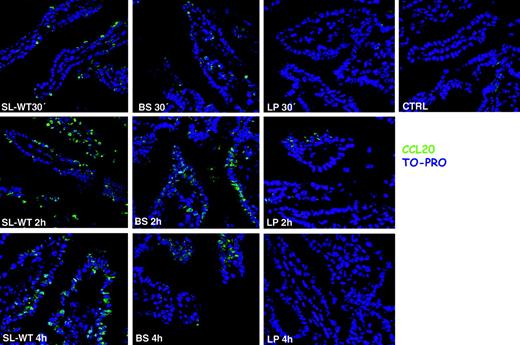
It has been shown that CCL20 is expressed by ECs in response to flagellin20,27 that binds to Toll-like receptor-5 (TLR-5).28 Recent reports suggest that TLR-5 is located only on the basolateral membrane of ECs,29,30 whereas others indicate that it might be present also apically.20,27 We have shown that both invasive and noninvasive bacteria that are unable to translocate flagellin to the basolateral membrane29,30 induce CCL20 expression by ECs, provided that they are flagellated. This suggests that TLR-5 is expressed also apically in the Caco-2 cell line. It is likely that different epithelial cell lines might have a pattern of TLR expression that is not physiological, showing a displacement of their expression from the apical to the basolateral membrane or vice versa, thus explaining these contrasting reports. Therefore, we decided to follow the expression of CCL20 directly in vivo, in mice, after injection into ileal ligated loops of different flagellated and nonflagellated, invasive and noninvasive, bacteria. CCL20 was detected by immunofluorescence on ileal sections of mice treated for 30 minutes, 2 hours, and 4 hours with different bacterial strains. Confirming the in vitro data, both the invasive SL-WT and the noninvasive flagellated B subtilis induced very high expression of CCL20 (Figure 1). By contrast, nonflagellated noninvasive L plantarum did not induce any significant induction of CCL20 expression (Figure 1). Altogether, these results confirm that flagellated bacteria induce CCL20 expression regardless of their invasiveness, suggesting that TLR-5, similarly to Caco-2 cells, is expressed also on the apical surface of ECs.
MoDCs do not discriminate between invasive and noninvasive pathogenic or commensal bacteria
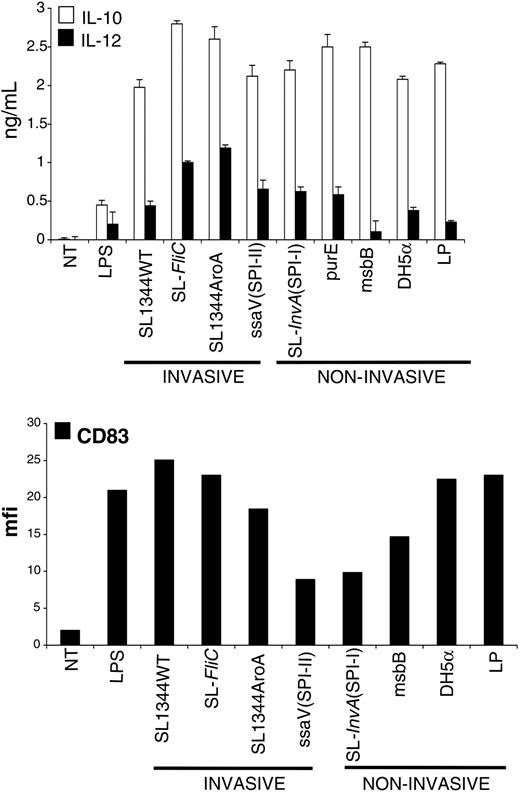
Having observed that ECs respond differently according to the nature of the bacteria encountered, we asked whether DCs also had the ability to discriminate between different sorts of bacteria. We derived DCs from CD14+ blood monocytes and evaluated the quality of our preparation by staining for acquisition of CD1a marker by cytofluorometry (Figure S1; see the Supplemental Materials link at the top of the online article, at the Blood website). MoDCs were incubated with the same bacterial strains as those used in the previous paragraphs. We found that MoDCs responded qualitatively very similarly to all of the tested bacterial strains (Figure 2), including pathogens and commensal bacteria. Bacteria-activated MoDCs up-regulated the expression of activation markers (CD83, Figure 2; DC-lysosomal-associated membrane glycoprotein [DC-LAMP], CD80, and HLA-DR, not shown) and released both IL-10 and IL-12p70 (Figure 2). Therefore, we can conclude that the qualitative response of MoDCs to the tested bacteria is very similar.
CCL20 (MIP-3α) is expressed in response to flagellated bacteria, regardless of their invasiveness. Ileal ligated loops were carried out in the intestine of C57BL/6 mice. 108 CFU of bacteria (S typhimurium: SL-WT; B subtilis: BS; L plantarum: LP) were injected into the loops. CTRL indicates control mice injected with PBS. Mice were killed at the indicated time points. Cryosections were stained with anti-CCL20 antibody (green) and with TO-PRO (blue) to detect nuclei. BS is a flagellated noninvasive bacterium and induces CCL20, whereas LP that is not flagellated does not. One of 2 independent experiments is shown.
CCL20 (MIP-3α) is expressed in response to flagellated bacteria, regardless of their invasiveness. Ileal ligated loops were carried out in the intestine of C57BL/6 mice. 108 CFU of bacteria (S typhimurium: SL-WT; B subtilis: BS; L plantarum: LP) were injected into the loops. CTRL indicates control mice injected with PBS. Mice were killed at the indicated time points. Cryosections were stained with anti-CCL20 antibody (green) and with TO-PRO (blue) to detect nuclei. BS is a flagellated noninvasive bacterium and induces CCL20, whereas LP that is not flagellated does not. One of 2 independent experiments is shown.
The ability of MoDCs to take up bacteria across EC monolayers is not restricted to pathogens
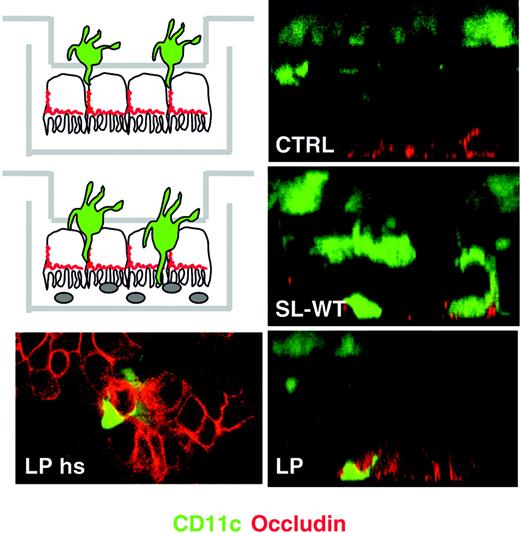
We have shown that the ability of DCs to take up bacteria across mucosal surfaces in mice kept in conventional animal houses is independent of the pathogenicity of the bacteria.2 Thus, we expected that DCs would creep between ECs and take up also the commensal bacterium L plantarum. We seeded MoDCs from the basolateral membrane of Caco-2 monolayers and evaluated their ability to cross the epithelial barrier upon apical incubation of either S typhimurium (SL-WT) or L plantarum (LP) by confocal microscopy. Both bacterial strains but not medium were able to induce MoDC migration across ECs all the way to the apical side (Figure 3).
MoDCs are differently activated if they can sense the bacteria across EC monolayers or if they are incubated with supernatants of bacteria-activated ECs
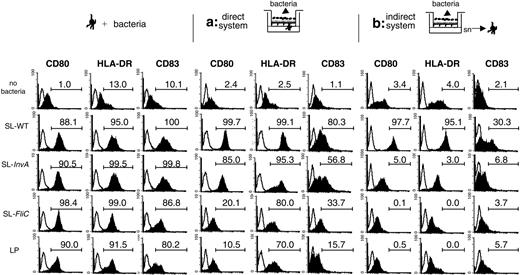
MoDCs are activated by the different tested bacterial strains and can translocate across EC monolayers also in response to the commensal LP; thus, we studied the activation of MoDCs across mucosal epithelia in an in vitro coculture system.31 In this model, Caco-2 cells were grown in a monolayer on a transwell filter and bacteria were incubated from the apical side. We compared 2 different situations. In the first situation (Figure 4, direct system), MoDCs were coincubated with ECs and were seeded facing the basolateral side of the monolayer, whereas in the second situation (Figure 4, indirect system), MoDCs were incubated only with supernatants from ECs activated or not with bacteria. In Figure 4, the response to 3 representative strains of S typhimurium (SL-WT, noninvasive SL-InvA, and nonflagellated SL-FliC) or to the commensal L plantarum is shown. In the direct system, MoDCs were induced to up-regulate activation markers (Figure 4). However, whereas SL-WT and SL-InvA induced maximal phenotypic activation of MoDCs, SL-FliC and LP induced a good up-regulation of HLA-DR molecules but a reduced increase of CD80 and CD83 (Figure 4). As we have shown that in the direct system MoDCs can creep between ECs and directly contact the bacteria (Figure 3), the observed up-regulation of MoDC activation markers could be due to the interaction of MoDCs with bacteria, but we cannot exclude that also the interaction of MoDCs with bacteria-stimulated ECs could participate in DC activation. By contrast, when MoDCs were incubated with supernatants of bacteria-treated ECs, they were activated only by supernatants of ECs previously incubated with SL-WT but not with SL-FliC, SL-InvA, or LP (Figure 4). As a control, up-regulation of activation markers in MoDCs after incubation only with the different bacteria strains is shown (Figure 4). In the indirect system, we could not detect any bacterial colonies in the supernatants collected from the basolateral face of ECs, suggesting that in the time frame of the experiment invasive bacteria were not translocating across the monolayer of ECs, or were disrupting the integrity of the epithelial barrier. This is confirmed by the observation that the transepithelial resistance was unchanged throughout the experiment (Figure S2). Further, the same supernatants were unable to activate murine DCs, suggesting that the activating factors were not of bacterial origin.32
Monocyte-derived DCs are activated by pathogenic (invasive and noninvasive) or nonpathogenic bacteria. MoDCs were incubated with WT or attenuated strains of S typhimurium (described in “Materials and methods”), or with a laboratory strain of E coli (DH-5α) or with L plantarum (LP), at a ratio of 1 DC to 10 bacteria for 1 hour in medium without antibiotics. Cells were washed and incubated for an additional 23 hours in medium containing 100 μg/mL gentamicine to kill extracellular and intracellular bacteria. Cell culture supernatants were collected for cytokine measurements (IL-10 and IL-12p70) by ELISA. Cells were harvested and processed for FACS analysis after staining for CD83 surface expression. As shown, all of the tested bacteria induced production of IL-12 and IL-10 and induced substantial activation of MoDCs as attested by increase of surface expression of CD83. Data are shown as means (± SD, top) and are representative of 3 independent experiments. NT indicates not treated.
Monocyte-derived DCs are activated by pathogenic (invasive and noninvasive) or nonpathogenic bacteria. MoDCs were incubated with WT or attenuated strains of S typhimurium (described in “Materials and methods”), or with a laboratory strain of E coli (DH-5α) or with L plantarum (LP), at a ratio of 1 DC to 10 bacteria for 1 hour in medium without antibiotics. Cells were washed and incubated for an additional 23 hours in medium containing 100 μg/mL gentamicine to kill extracellular and intracellular bacteria. Cell culture supernatants were collected for cytokine measurements (IL-10 and IL-12p70) by ELISA. Cells were harvested and processed for FACS analysis after staining for CD83 surface expression. As shown, all of the tested bacteria induced production of IL-12 and IL-10 and induced substantial activation of MoDCs as attested by increase of surface expression of CD83. Data are shown as means (± SD, top) and are representative of 3 independent experiments. NT indicates not treated.
Indirectly activated MoDCs release IL-10 but not IL-12p70
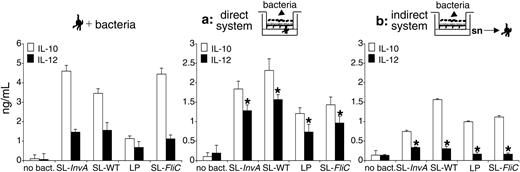
Next, we tested the ability of directly or indirectly activated MoDCs to release interleukins. We found that IL-10 was released by MoDCs activated either directly or indirectly by bacteria (Figure 5; Table 2). By contrast, only in the presence of a direct activation of the cells by the bacteria was IL-12 released (Figure 5). To confirm that in the indirect system the absence of IL-12 release by DCs was not due to EC-derived factors that were released later than the 4-hour experimental point, we performed the experiment at the same time point and bacterial amount as in the direct system. As shown in Figure S3, supernatants of bacteria-activated ECs supernatants collected 16 hours after bacterial activation still did not induce IL-12 release by DCs. At this time point, as opposed to the direct system, some bacterial colonies were detected in culture supernatants, suggesting that bacteria could gain access across the EC monolayer (Table S1); still, IL-12 was not released by DCs. This suggests that indirectly activated MoDCs are functionally different from those activated directly by bacteria and bacteria-stimulated ECs. As expected, MoDCs incubated with bacteria in the absence of ECs or EC supernatants released both IL-10 and IL-12 (Figure 5). Interestingly, in the indirect system, even though MoDCs were not phenotypically mature after treatment with EC supernatants previously incubated with noninvasive (SL-InvA or LP) or with nonflagellated (SL-FliC) bacteria (Figure 4), they acquired the ability to release IL-10, albeit at a lower amount compared with MoDCs incubated with SL-WT-activated ECs (Figure 5). As we recently showed that MoDCs conditioned with nontreated EC supernatant are refractory to IL-12 production after S typhimurium stimulation, we analyzed whether conditioned MoDCs were induced to release IL-12 when seeded with EC monolayers and apical bacteria. We found that EC-conditioned MoDCs were irreversibly blocked in their ability to release IL-12 even after direct contact with Caco-2 cells and apical bacteria (Figure S4).
Analysis of DC activation in terms of cytokine production and surface marker expression after incubation with supernatants of epithelial cells treated or not with the indicated bacteria
Bacterial strains . | IL-10, ng/mL . | IL-12p70, ng/mL . | CCL2, ng/mL . | CCL18, ng/mL . | CCL17, ng/mL . | CCL22, ng/mL . | HLADR+ cells, % . | DC-LAMPhi cells, % . | Invasive . | Flagellin . |
|---|---|---|---|---|---|---|---|---|---|---|
| SL 1344 WT | 2.46 | 0.3 | 5.1 | 61.8 | 0.36 | 12.0 | 95.1 | 90.6 | Yes | Yes |
| ssaV (SPI-II) | 2.99 | < DL | — | — | — | 13.0 | 92.8 | 96.0 | Yes | Yes |
| SL-FliC | 1.79 | 0.1 | < DL | 62.8 | 0.54 | 3.24 | 0.0 | — | Yes | No |
| msbB | 0.11 | < DL | 3.8 | — | — | — | — | 29.4 | No | Yes |
| SL-InvA (SPI-I) | 1.82 | 0.2 | 4.1 | 21.6 | 0.60 | 1.20 | 3.0 | 52.0 | No | Yes |
| DH5α | 0.392 | < DL | — | — | — | — | 2.0 | 33.4 | No | No |
| LP | 1.90 | 0.1 | < DL | 48.2 | 0.61 | 1.81 | 0.0 | — | No | No |
| Untreated EC supernatant | < DL | < DL | 1.4 | 36.2 | 0.24 | 1.14 | 4.0 | 28.0 | — | — |
| Untreated | < DL | < DL | 0.1 | 52.2 | 0.49 | 1.12 | 13.0 | 12.2 | — | — |
| DCs+SL 1344 WT | 2.263 | 1.3 | 3.2 | 48.0 | 0.54 | 27.29 | 95.0 | 98.8 | — | — |
Bacterial strains . | IL-10, ng/mL . | IL-12p70, ng/mL . | CCL2, ng/mL . | CCL18, ng/mL . | CCL17, ng/mL . | CCL22, ng/mL . | HLADR+ cells, % . | DC-LAMPhi cells, % . | Invasive . | Flagellin . |
|---|---|---|---|---|---|---|---|---|---|---|
| SL 1344 WT | 2.46 | 0.3 | 5.1 | 61.8 | 0.36 | 12.0 | 95.1 | 90.6 | Yes | Yes |
| ssaV (SPI-II) | 2.99 | < DL | — | — | — | 13.0 | 92.8 | 96.0 | Yes | Yes |
| SL-FliC | 1.79 | 0.1 | < DL | 62.8 | 0.54 | 3.24 | 0.0 | — | Yes | No |
| msbB | 0.11 | < DL | 3.8 | — | — | — | — | 29.4 | No | Yes |
| SL-InvA (SPI-I) | 1.82 | 0.2 | 4.1 | 21.6 | 0.60 | 1.20 | 3.0 | 52.0 | No | Yes |
| DH5α | 0.392 | < DL | — | — | — | — | 2.0 | 33.4 | No | No |
| LP | 1.90 | 0.1 | < DL | 48.2 | 0.61 | 1.81 | 0.0 | — | No | No |
| Untreated EC supernatant | < DL | < DL | 1.4 | 36.2 | 0.24 | 1.14 | 4.0 | 28.0 | — | — |
| Untreated | < DL | < DL | 0.1 | 52.2 | 0.49 | 1.12 | 13.0 | 12.2 | — | — |
| DCs+SL 1344 WT | 2.263 | 1.3 | 3.2 | 48.0 | 0.54 | 27.29 | 95.0 | 98.8 | — | — |
Bacterial strains: Invasive S typhimurium: wild type, SL 1344 WT; SPI-II (ssaV), FliC; noninvasive S typhimurium: msbB (lipid A mutant); SPI-I (InvA); E. coli: DH5a; L plantarum: LP.
< DL indicates below detection limit;—, not tested.
Lactobacillus plantarum induces the translocation of MoDCs across the epithelial monolayer. MoDCs were seeded on the upper face of transwell filters facing the basolateral membrane of Caco-2 cells (see schematics on the left). Bacteria were seeded apically. Transwell filters were fixed 2 hours after addition of L plantarum (LP) or S typhimurium (SL-WT) or medium (CTRL) and were processed for laser confocal microscopy analysis. Green stains DCs (CD11c); red stains occludin of tight junctions. Right panels: Z sections of the filters, left panel: horizontal section (hs) of the apical face of the filter illustrated on the right showing DC dendrites in close contact with epithelial occludin. For each situation, 1 of 6 transwells is shown.
Lactobacillus plantarum induces the translocation of MoDCs across the epithelial monolayer. MoDCs were seeded on the upper face of transwell filters facing the basolateral membrane of Caco-2 cells (see schematics on the left). Bacteria were seeded apically. Transwell filters were fixed 2 hours after addition of L plantarum (LP) or S typhimurium (SL-WT) or medium (CTRL) and were processed for laser confocal microscopy analysis. Green stains DCs (CD11c); red stains occludin of tight junctions. Right panels: Z sections of the filters, left panel: horizontal section (hs) of the apical face of the filter illustrated on the right showing DC dendrites in close contact with epithelial occludin. For each situation, 1 of 6 transwells is shown.
Indirectly activated MoDCs also showed a differential expression of chemokines according to the nature of the bacteria encountered by ECs (Table 2). MoDCs incubated with supernatants of ECs treated with invasive but not with noninvasive bacteria released CCL22 (MDC), a chemokine known to preferentially mediate recruitment of T helper 2 (Th2)33 and T regulatory cells.34,35 This was in part dependent on the expression of flagellin, as SL-FliC induced only a slight up-regulation of CCL22 (Table 2). The release of CCL2 (MCP-1) that attracts monocytes but not neutrophils36 was totally dependent on the presence of flagellin as it was released by MoDCs incubated with supernatants of ECs treated with SL-InvA but not SL-FliC. CCL17 (TARC) was released quite uniformly by treated and untreated MoDCs (Table 2). By contrast, we found that MoDCs activated with supernatants of invasive Salmonella-treated ECs up-regulated the expression of CCL18, a chemoattractant for naive B24 and T cells,25 independently on the expression of flagellin (Table 2). Altogether, these results suggest that when MoDCs are activated by EC supernatants they are prone to generate an anti-inflammatory mucosal environment through the release of IL-10 and the recruitment of Th2 and regulatory T cells. By contrast, MoDCs activated in the direct system are more inflammatory as they release IL-10 and IL-12.
MoDCs are phenotypically different when activated by bacteria directly across the EC monolayer or indirectly by bacteria-activated EC supernatants. MoDCs were treated for 24 hours as follows: (left) MoDCs were activated with S typhimurium (SL-WT, SL-InvA: noninvasive, FliC: nonflagellated) or L plantarum (LP). (Middle) Direct system (situation a), MoDCs were seeded facing the basolateral membrane of the epithelial cell monolayer. Bacteria were incubated from the apical face. (Right) Indirect system (situation b), MoDCs were incubated with supernatants (sn) of ECs incubated or not with bacteria from the apical face. Histogram plots show CD80, HLA-DR, and CD83 surface-marker staining of MoDCs treated as in Figure 2. Filled histograms indicate stained cells; open histograms, isotype controls. Numbers indicate the percentage of positive cells in the gate. One of 4 independent experiments is shown.
MoDCs are phenotypically different when activated by bacteria directly across the EC monolayer or indirectly by bacteria-activated EC supernatants. MoDCs were treated for 24 hours as follows: (left) MoDCs were activated with S typhimurium (SL-WT, SL-InvA: noninvasive, FliC: nonflagellated) or L plantarum (LP). (Middle) Direct system (situation a), MoDCs were seeded facing the basolateral membrane of the epithelial cell monolayer. Bacteria were incubated from the apical face. (Right) Indirect system (situation b), MoDCs were incubated with supernatants (sn) of ECs incubated or not with bacteria from the apical face. Histogram plots show CD80, HLA-DR, and CD83 surface-marker staining of MoDCs treated as in Figure 2. Filled histograms indicate stained cells; open histograms, isotype controls. Numbers indicate the percentage of positive cells in the gate. One of 4 independent experiments is shown.
Indirectly activated MoDCs release IL-10 but not IL-12p70. MoDCs were treated for 24 hours as follows: (left panel) MoDCs were activated with S typhimurium (SL-WT, noninvasive SL-InvA, nonflagellated SL-FliC), or L plantarum (LP). (Middle) Direct system (situation a), MoDCs were seeded facing the basolateral membrane of EC monolayer. Bacteria were incubated from the apical face. (Right) Indirect system (situation b), MoDCs were incubated with supernatants (sn) of ECs incubated or not with bacteria from the apical face. Cytokine release was measured in culture supernatants by ELISA. The difference of IL-12 production between directly and indirectly activated MoDCs is highly significant (*P < .01). Data are shown as means ± SD.
Indirectly activated MoDCs release IL-10 but not IL-12p70. MoDCs were treated for 24 hours as follows: (left panel) MoDCs were activated with S typhimurium (SL-WT, noninvasive SL-InvA, nonflagellated SL-FliC), or L plantarum (LP). (Middle) Direct system (situation a), MoDCs were seeded facing the basolateral membrane of EC monolayer. Bacteria were incubated from the apical face. (Right) Indirect system (situation b), MoDCs were incubated with supernatants (sn) of ECs incubated or not with bacteria from the apical face. Cytokine release was measured in culture supernatants by ELISA. The difference of IL-12 production between directly and indirectly activated MoDCs is highly significant (*P < .01). Data are shown as means ± SD.
Directly activated MoDCs induce both Th1 and Th2 responses, whereas indirectly activated MoDCs induce only Th2 responses
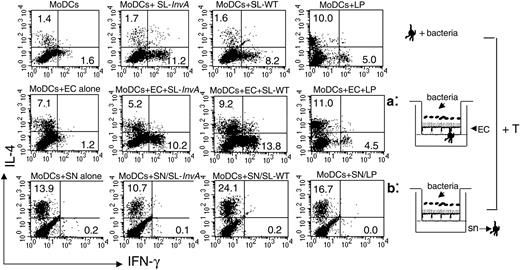
We next evaluated the ability of differentially activated MoDCs to polarize naive T cells toward a Th1 or a Th2 phenotype in a mixed allogeneic leukocyte reaction. We collected MoDCs from the basolateral side of the filters after bacterial activation in direct activation, or we collected MoDCs after their incubation with supernatants of ECs previously exposed to bacteria in indirect activation. MoDCs were seeded together with purified CD4+ CD45RA+ T cells for 5 days, and T cells were analyzed for intracellular staining of IL-4 and IFN-γ. Consistent with their ability to release IL-12, a strong inducer of Th1 differentiation,37 MoDCs collected from culture as in the direct system induced Th1 T-cell polarization regardless of the nature of the bacteria (both invasive and noninvasive, flagellated and nonflagellated, pathogenic and nonpathogenic). It is worth noting that MoDCs activated by LP were more prone to induce Th2 rather than Th1 responses (Figure 6). This most likely correlates with a reduced ability to produce IL-12p70 in response to LP (Figures 2 and 5). By contrast, EC supernatant-treated MoDCs were unable to drive Th1 differentiation and induced Th2 T cells. This ability was already conferred by EC-derived factors, independently on the presence of bacteria, as also MoDCs incubated with supernatants from untreated ECs drove a “default” Th2 polarization. Consistent with their activated phenotype, MoDCs incubated with supernatants of ECs treated with invasive flagellated Salmonella induced a higher percentage of Th2 T cells compared with MoDCs incubated with supernatants from SL-InvA- or LP-treated ECs (Figure 6). Altogether these results indicate that in the direct system, MoDCs are phenotypically activated, release both IL-10 and IL-12, and promote CD4+ Th1-dominated responses. By contrast, in the indirect system, MoDCs release IL-10 but not IL-12 and are unable to drive the differentiation of inflammatory Th1 T cells.
Directly activated MoDCs induce both Th1 and Th2 responses, regardless of the invasiveness of bacteria, whereas indirectly activated MoDCs induce only Th2 responses. Intracellular cytokine staining for IFN-γ and IL-4 of naive CD4+ allogeneic T cells incubated for 5 days with MoDCs, either nonconditioned and then incubated with bacteria (medium conditioned, top row), or treated as in situation a (middle row) or b (bottom row). Bacteria used: L plantarum LP, invasive SL-WT, and noninvasive SL-InvA. This is representative of 4 independent experiments. Numbers indicate the percentage of positive cells per quadrant. SN and sn indicate supernatant.
Directly activated MoDCs induce both Th1 and Th2 responses, regardless of the invasiveness of bacteria, whereas indirectly activated MoDCs induce only Th2 responses. Intracellular cytokine staining for IFN-γ and IL-4 of naive CD4+ allogeneic T cells incubated for 5 days with MoDCs, either nonconditioned and then incubated with bacteria (medium conditioned, top row), or treated as in situation a (middle row) or b (bottom row). Bacteria used: L plantarum LP, invasive SL-WT, and noninvasive SL-InvA. This is representative of 4 independent experiments. Numbers indicate the percentage of positive cells per quadrant. SN and sn indicate supernatant.
Discussion
The interaction of MoDCs with bacteria across epithelial monolayers was studied by using an in vitro system established in our laboratory. This system allowed us to simplify the mucosal barrier to just 3 players: MoDCs, epithelial cells, and bacteria in a spatial distribution similar to that found in vivo. Two systems were developed: the direct system in which MoDCs could contact bacteria directly across the monolayer of ECs (Figure 5, direct system) and the indirect system where MoDCs were incubated with supernatants of bacteria-stimulated ECs (Figure 5, indirect system). In the indirect system we found that the response of ECs in terms of release of proinflammatory mediators depended on the invasiveness of the bacteria and on the expression of flagellin. The release of CXCL8, a chemokine involved in the recruitment of neutrophils,38 was dependent on both invasiveness of bacteria and presence of flagella: bacteria lacking either of these 2 features were unable to induce CXCL8 release. By contrast, CCL20 that recruited immature CCR6-expressing DCs39 was released after exposure to both invasive and noninvasive flagellated bacteria, whereas CCL18, a chemoattractant for naive B24 and T cells,25 was up-regulated after an encounter with invasive bacteria independently on the expression of flagellin. CCL18 could also be involved in the recruitment of memory T cells, as a recent report shows its role in attracting memory T cells to the skin of atopic dermatitis patients.26 This indicates that ECs can discriminate between the various types of bacteria that they have encountered at their apical surface and dictate the type of induced immune response by recruiting different immune cells. Maximal alert of the immune system is achieved after encounter with invasive flagellated S typhimurium, which results in the recruitment of neutrophils, immature DCs, naive B and T cells, and probably also memory T cells.
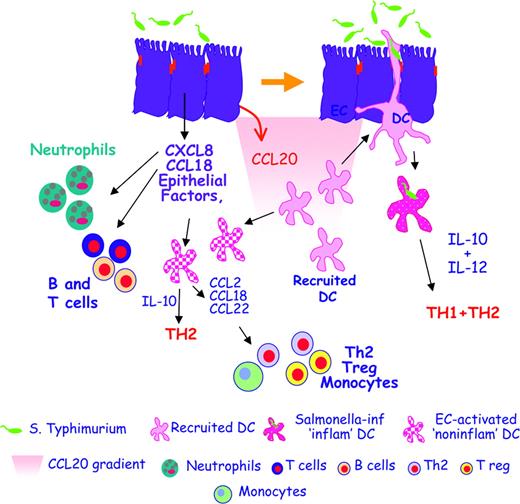
The cross talk between ECs and DCs helps maintain gut immune homeostasis. In the initial phases of infection, Salmonella typhimurium induces ECs to release proinflammatory chemokines like IL-8 (CXCL8) and PARC (CCL18), which attract neutrophils, granulocytes, and B and T cells that generate an inflamed site. Salmonella also induces the release of MIP-3α (CCL20), which recruits CCR6-expressing immature DCs. Newly recruited DCs creep between activated ECs, directly contact the bacteria, and release both IL-10 and IL-12, thus promoting Th1 and Th2 responses. This allows the establishment of protective anti-Salmonella responses. EC-derived factors can also activate “bystander” DCs that have not been in direct contact with the bacteria. DCs activated in this way are noninflammatory as they release IL-10 but not IL-12, and drive only Th2 T cells. Moreover, noninflammatory DCs release MCP-1 (CCL2), PARC (CCL18), and MDC (CCL22), thus recruiting monocytes, Th2, and T regulatory cells.
The cross talk between ECs and DCs helps maintain gut immune homeostasis. In the initial phases of infection, Salmonella typhimurium induces ECs to release proinflammatory chemokines like IL-8 (CXCL8) and PARC (CCL18), which attract neutrophils, granulocytes, and B and T cells that generate an inflamed site. Salmonella also induces the release of MIP-3α (CCL20), which recruits CCR6-expressing immature DCs. Newly recruited DCs creep between activated ECs, directly contact the bacteria, and release both IL-10 and IL-12, thus promoting Th1 and Th2 responses. This allows the establishment of protective anti-Salmonella responses. EC-derived factors can also activate “bystander” DCs that have not been in direct contact with the bacteria. DCs activated in this way are noninflammatory as they release IL-10 but not IL-12, and drive only Th2 T cells. Moreover, noninflammatory DCs release MCP-1 (CCL2), PARC (CCL18), and MDC (CCL22), thus recruiting monocytes, Th2, and T regulatory cells.
The ability of ECs to sense the presence of invasive flagellated Salmonella resulted also in the release of EC-derived factors that induced the phenotypic activation of MoDCs. This was dependent on both the presence of flagellin and the invasiveness of bacteria. EC-activated DCs were noninflammatory because they could release IL-10 but not IL-12, and drove Th2 but not Th1 T-cell polarization. Remarkably, MoDCs that were incubated with supernatants of ECs treated with nonflagellated SL-FliC, noninvasive SL-InvA, and LP, although not phenotypically mature, secreted IL-10. Whether this confers to MoDCs the ability to drive the differentiation of T regulatory cells remains to be established. Noninflammatory DCs also released chemokines aimed at recruiting noninflammatory immune cells, such as CCL22 and CCL2, that will attract Th2,33 T regulatory cells,34 and monocytes, but not neutrophils,36 respectively. The release of these chemokines was also dependent on the nature of the bacteria used for the indirect activation. We found that EC-activated MoDCs also up-regulated the release of CCL18, which has been recently shown to be down-regulated in DCs activated by several inflammatory stimuli, including bacteria.40 Down-regulation of CCL18 has been correlated with an inhibitory pathway devoted to limiting the generation of specific immune responses at peripheral sites. Our culture system could have mimicked an in vivo situation whereby intestinal DCs that are in close proximity to immune induction sites might retain their ability to release CCL18 and recruit naive B and T cells. Thus, noninflammatory MoDCs could recruit naive T cells and drive their polarization into Th2 T cells that could be involved in the differentiation of B cells into antibody-producing plasma cells. This is in agreement with a recent report showing that immunoglobulin A (IgA)-secreting cells can be generated directly in the lamina propria.41 Altogether, these results indicate that ECs can activate at the same time both inflammatory responses and noninflammatory responses by activating “bystander DCs” that are unable to release IL-12 and to activate Th1 T cells, and that will recruit Th2 as well as T regulatory cells. Whether the noninflammatory response is a way to avoid exaggerated inflammation, to initiate mucosal antibody responses, or an immune evasion mechanism induced by invasive Salmonella remain open questions.
On the other hand, in the direct system, activated MoDCs were more inflammatory as they produced IL-10 and IL-12 and polarized T cells toward both Th2 and Th1 types of response. The latter are necessary to kill intracellular organisms like S typhimurium42-44 or Toxoplasma gondii.45,46 However, whereas stimulation of MoDCs across EC monolayers with either SL-WT or noninvasive SL-InvA was very similar, stimulation with nonflagellated SL-FliC or with LP promoted MoDCs that were activated but not maximally and that released lower levels of IL-10 and IL-12. This suggests that also in the direct system the response to bacteria is somehow dependent on the pathogenicity of the strain. We cannot exclude that EC-DC cell-cell interactions together with EC-derived factors might play a role in the observed differential response. Further, although LP had a natural propensity to promote Th2-polarizing MoDCs, a good deal of potentially damaging inflammatory Th1 T cells were still induced in the direct system. This feature could be shared by other commensal bacteria. Considering that in the lower intestine the density of commensals reaches 1012 organisms per gram of intestinal content,47 this could lead to the generation of broad inflammation. Our recent findings suggest this is probably avoided because resident DCs are conditioned by intestinal ECs to inhibit the generation of inflammatory Th1 T-cell responses, even to pathogens.32
In conclusion, we propose a model describing EC-DC cross talk during Salmonella infection (Figure 7). During the initial phases of infection, Salmonella will induce ECs to release inflammatory mediators (CXCL8, CCL18, and CCL20) that will respectively recruit neutrophils, T and B cells, and immature DCs. The latter will not be conditioned by ECs and will be able to creep between bacteria-stimulated ECs and to contact the bacteria directly. Newly recruited bacteria-activated DCs will induce both Th1 and Th2 T cells. Some of the DCs will be unable to contact the bacteria directly and will be subjected to EC-mediated “bystander” activation. EC-activated DCs are noninflammatory: they will induce Th2 but not Th1 T cells, and will recruit Th2 and regulatory T cells as well as monocytes through the release of CCL22 and CCL2. The induction of a noninflammatory response could participate in re-establishing gut immune homeostasis.
Prepublished online as Blood First Edition Paper, July 19, 2005; DOI 10.1182/blood-2004-11-4321.
Supported by grants from the Crohn's and Colitis Foundation of America and Italian Association for Cancer Research to M. Rescigno, and European Union 6 FP (Mucosal Vaccines for Poverty Related Diseases [MUVAPRED] Project) LSHP-CT-2003-503240 to P.A. M.C. is a recipient of a Fondazione Italiana della Ricerca sul Cancro (FIRC) fellowship.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Gordon Dougan and Liljana Petrovska for providing the Salmonella typhimurium mutated strains.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal