Abstract
Recently, a Jak2V617F mutation has been described in the vast majority of patients with polycythemia vera (PV) as well as in subsets of patients with essential thrombocythemia (ET) and idiopathic myelofibrosis (IMF). The question arises whether this mutation is observed in those patients with ET and IMF who have also displayed previously described molecular markers, notably the ability to form endogenous erythroid colonies (EECs), overexpression of polycythemia rubra vera 1 (PRV-1), and decreased c-Mpl expression. We therefore analyzed the Janus kinase 2 (Jak2) DNA sequence, EEC growth, PRV-1 expression, and c-Mpl (myeloproliferative) levels in a cohort of 78 myeloproliferative disorder (MPD) patients (42 ET, 22 PV, and 14 IMF). Presence of the Jak2V617F mutation was very highly correlated with PRV-1 overexpression and the ability to form EECs in all 3 subtypes of MPDs (P < .001). (Blood. 2005;106:2862-2864)
Introduction
In 1951, Dameshek1 coined the term myeloproliferative disorders (MPDs) for a group of 4 clinically related diseases. At the time, this included polycythemia vera (PV), essential thrombocythemia (ET), idiopathic myelofibrosis (IMF), and chronic myelogenous leukemia (CML). Today, CML is regarded as a separate entity, defined by the t(9;22)(q34;q11) translocation, the “Philadelphia (Ph-) chromosome,” which results in production of the breakpoint cluster region/Abelson (Bcr/Abl) fusion protein and is not found in the remaining 3 MPD subtypes.2,3
Several aberrations have been described in patients with PV, ET, and IMF, but none appeared causally linked to the molecular pathogenesis.4-7 However, 2 alterations, the growth of endogenous erythroid colonies (EECs) and overexpression of the polycythemia rubra vera 1 (PRV-1) mRNA, are highly correlated in individual patients with all 3 subtypes of MPD.8,9 Therefore, we have proposed that EEC-positive, PRV-1-overexpressing MPD patients constitute a distinct molecular category.10 In our model, EEC/PRV-1-positive ET and IMF patients are molecularly and clinically more similar to PV patients than to other patients who carry the same clinically defined diagnosis.9-11
The recent description of a point mutation in the Janus kinase 2 (Jak2; Jak2V617F) in the majority of patients with PV as well as subgroups of patients with ET and IMF12-14 allows us to correlate the presence of this mutation with the occurrence of other markers in individual patients. This analysis can refute or prove the hypothesis that EEC/PRV-1-positive MPD patients share a common molecular determinant of disease etiology.
Study design
Patients
Peripheral blood samples were obtained from 42 patients with essential thrombocythemia (ET), 22 patients with polycythemia vera (PV), and 14 patients with idiopathic myelofibrosis (IMF). The diagnosis of ET, PV, and IMF were made according to the World Health Organization (WHO) criteria.15 Venous blood was anticoagulated with EDTA (ethylenediaminetetraacetic acid) or heparin and shipped by courier to the central laboratory without cooling. Maximum time interval between venipuncture and arrival in the laboratory was 24 hours. The study protocol was approved by the local ethics committee and the University Hospital Freiburg institutional review board, and informed consent was obtained from all patients. Each patient was assigned a unique patient number (UPN) for the protection of privacy.
Separation of cells
Peripheral blood granulocytes, mononuclear cells, and platelets were prepared as previously described.5,9,16
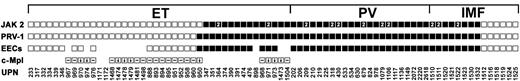
Molecular markers in MPDs. The indicated markers were evaluated in 42 ET, 22 PV, and 14 IMF patients. □ indicates that the marker is absent (ie, wild-type Jak2, normal expression of PRV-1, no Epo-independent growth, no EEC formation). ▪ indicates that the marker is present (ie, mutant Jak2V617F, overexpression of PRV-1, Epo-independent growth; EEC formation). For c-Mpl, ↓ indicates less than 40% of normal expression, whereas—indicates normal expression levels. Absence of a symbol indicates that the analysis was not performed. A “2” in the Jak2 analysis indicates that the patients were homozygous for the Jak2V617F mutant allele.
Molecular markers in MPDs. The indicated markers were evaluated in 42 ET, 22 PV, and 14 IMF patients. □ indicates that the marker is absent (ie, wild-type Jak2, normal expression of PRV-1, no Epo-independent growth, no EEC formation). ▪ indicates that the marker is present (ie, mutant Jak2V617F, overexpression of PRV-1, Epo-independent growth; EEC formation). For c-Mpl, ↓ indicates less than 40% of normal expression, whereas—indicates normal expression levels. Absence of a symbol indicates that the analysis was not performed. A “2” in the Jak2 analysis indicates that the patients were homozygous for the Jak2V617F mutant allele.
RNA preparation and PRV-1 quantification
Granulocyte RNA was isolated and PRV-1 mRNA levels were determined precisely as previously described.16
EEC assay
The EEC assay was conducted and scored precisely as previously described.9
c-Mpl immunoblotting and quantification
Platelets were isolated using Sepharose CL-2B columns (Amersham Pharmacia Biotech, Uppsala, Sweden) and the expression of c-Mpl (myeloproliferative) was analyzed by immunoblotting as previously described.5 For each patient, expression of both c-Mpl and the platelet glycoprotein IIIa (gpIIIa/CD 61) was quantitated by densitometric analysis of Western blots.
Jak2 cDNA sequence
Five hundred nanograms of RNA was reverse transcribed into complementary DNA (cDNA) using SUPERSCRIPT II RNase H-Reverse Transcriptase (Invitrogen, Karlsruhe, Germany) at the manufacturer's recommendation. One microliter of undiluted cDNA was amplified in a polymerase chain reaction (PCR) reaction using Pfu DNA polymerase (Stratagene, La Jolla, CA) and 10 pmol of forward and reverse primers each at an annealing temperature of 56°C: Jak2 forward, 5′ TAA AGG CGT ACG AAG AGA AGT AGG AGA CT 3′; Jak2 reverse, 5′ GGC CCA TGC CAA CTG TTT AGC 3′.
The PCR fragments were purified and sequenced using both the forward and the reverse primers.
Statistical analysis
Correlation of the markers was determined using the Spearman rank correlations coefficient (R).
Results and discussion
In the 78 MPD patients analyzed, the presence or absence of the Jak2V617F mutation correlated with the presence or absence of PRV-1 overexpression in 77 (98.7%) of 78 patients (R = 0.97; P < .001; Figure 1). Similarly, the Jak2 genotype correlated with the ability or inability to form EECs in 64 (98.4%) of 65 (R = 0.97; P < .001) patients for whom both analyses were conducted. As previously demonstrated in 2 independent cohorts, c-Mpl expression levels vary independent of EEC formation and PRV-1 expression.8,17,18 In addition, Jak2 genotype and c-Mpl expression are not correlated in this cohort (R = 0.25; P = .225; Figure 1).
In one ET patient, sequence analysis did not detect the G>T mutation at base pair 1849 resulting in the Jak2V617F allele. However, this patient overexpressed PRV-1 mRNA and displayed erythropoietin (Epo)-independent erythroid colony growth (UPN 950; Figure 1). It is possible that this patient is similar to those described by Baxter et al13 in that only a proportion of the peripheral granulocytes are clonal and hence sequence analysis does not detect the mutant allele. Alternatively, this patient may be similar to the 5 patients (11% of total) described by James et al12 who fulfill the Polycythemia Vera Study Group (PVSG) criteria for the diagnosis of PV but in whom the Jak2 mutation was not detected.
These data strongly support the hypothesis that 3 molecular markers, Jak2V617F, Epo-independent growth, and PRV-1 overexpression, define the same cohort of MPD patients, a subset that crosses the previously established, clinically defined categories of ET, PV, and IMF. This observation raises several questions. First, it is well established that the clinical classifications used to date stratify patients according to risk, for example, of thromboembolic complications. Patients diagnosed with ET have a lower risk of thrombotic complications than those diagnosed with PV.19-21 The question arises whether a reclassification of MPD patients according to molecular characteristics22 would be more or less accurate in staging individual patients for thromboembolic risk and/or subsequent disease evolution to myelofibrosis or acute leukemia than the current clinical categories. This can only be evaluated in the context of prospective treatment-controlled clinical trials.
Secondly, we have previously reported that PRV-1/EEC-positive ET patients, those now shown to also carry the Jak2V617F mutation, have a significantly higher risk of developing PV during their disease course than EEC/PRV-1-negative patients.9 Nonetheless, not all EEC/PRV-1-positive ET patients develop PV. Here we show that the Jak2V617F mutation is accompanied by similar secondary aberrations, EEC formation and PRV-1 overexpression, in all MPD subtypes irrespective of the clinical disease presentation. Which factors account for the differences in clinical phenotype? It has been suggested that more than one mutation is required for an MPD clone to develop.23 The second mutation may be heterogeneous among MPD patients. Alternatively, the MPD clone may arise in different progenitor cells, resulting in phenotypic variability. Finally, individual genetic backgrounds may modulate the effect of the Jak2V617F mutation, resulting in the heterogeneous clinical presentation.
These data underscore the remarkable clinical insight that led Dr Dameshek to coin the phrase “chronic myeloproliferative disorders” and to group these 3 diseases 55 years before their molecular similarity could be demonstrated.1
Prepublished online as Blood First Edition Paper, June 28, 2005; DOI 10.1182/blood-2005-04-1515.
Supported by a grant from the Else-Kröner-Fresenius-Stiftung (H.L.P.).
P.S.G. determined PRV-1 expression as well as the Jak2 genotype in the patients; C.S. conducted and scored the EEC assays; E.M. analyzed c-Mpl expression and aided in the Jak2 sequence analysis; P.L.J. provided blood samples of ET patients and critically reviewed the manuscript; B.A. provided blood samples of ET patients; M.G. contributed patient material (ET and PV patients) and critically reviewed the manuscript; H.G. provided blood samples of the IMF patients and critically reviewed the manuscript; H.H. was a major contributor to developing the idea of a unifying molecular change across clinically defined categories of MPDs, contributed patient material, and critically reviewed the manuscript; H.L.P. contributed pivotally to developing the idea of a unifying molecular change across clinically defined categories of MPDs, initiated this study, analyzed the data, and prepared the manuscript.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs A. Moliterno and J. Spivak for the generous gift of anti-c-Mpl antibody. Our sincere thanks to Prof Dr K. K. Geiger for his continued support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal