Abstract
Activation of T lymphocytes requires the recognition of peptide–major histocompatibility complexes (MHCs) and costimulatory signals provided by antigen-presenting cells (APCs). It has been shown that T-cell activation without costimulation can lead to anergy. In this study, we developed a novel strategy to inhibit expression of B7 molecules (CD80/86) by transfecting APCs with a gene construct encoding a modified cytotoxic T lymphocyte antigen 4 (CTLA4) molecule (CTLA4-KDEL) that is targeted to the endoplasmic reticulum (ER). APCs expressing this construct failed to express CD80/86 on their surface, were unable to stimulate allogeneic and peptide-specific T-cell responses, and induced antigen-specific anergy of the responding T cells. Cells expressing CTLA4-KDEL do not up-regulate the indoleamine 2, 3-dioxygenase enzyme, unlike cells treated with soluble CTLA4-immunoglobin (Ig). This gene-based strategy to knock out surface receptors is an attractive alternative to using immature dendritic cells for preventing transplant rejection and treating of autoimmune diseases.
Introduction
Activation of T cells is a central feature in the immune response to antigens. Although the T-cell receptor (TCR)–mediated signals determine the specificity of the response, important costimulatory signals are required for optimal T-cell activation.1,2 The most characterized second signal is delivered by B7 family (CD80/86) molecules expressed on antigen-presenting cells (APCs) binding to CD28 which is expressed on CD4+ and CD8+ T cells.3 Not only is CD28 ligation required for T-cell activation, but TCR ligation without CD28 signaling can have the opposite effect, inducing T-cell anergy. Moreover, a second receptor for B7 molecules, cytotoxic T lymphocyte antigen 4 (CTLA4; CD152) is expressed on activated T cells,4 which serves to shut down T-cell activation, culminating in T-cell death or the induction of anergy.5
A variety of immunotherapeutic strategies aimed at modulating CD80/86-CD28 interactions have been developed. These include administration of soluble CTLA4-immunoglobulin (Ig), which, because of the high affinity of CTLA4 for CD80/86, serves to block the costimulatory signal.6,7 In addition, interest has been focused on the in vivo administration of dendritic cells (DCs) that have been treated with a variety of agents (such as dexamethasone) to “freeze” them in an immature (iDC) phenotype where they express low levels of CD80/86 and so induce anergy in antigen-specific T cells.8-10 Importantly, anergic cells have been shown to act as regulatory cells both in vitro and in vivo, and so tolerance induced to a limited number of epitopes can spread to regulate immunity against other determinants presented by same APCs.11,12 It has therefore been proposed that administration of iDCs expressing appropriate alloantigens and autoantigens could be used to prevent transplant rejection and to treat autoimmune diseases.13 However, neither approach is ideal because iDCs can become activated in vivo and they are less efficient than mature DCs at presenting antigen to T cells.
In this report we describe an alternative strategy in which we genetically modified DCs and other APCs to prevent the expression of CD80/86. Our approach was to express within the cells the extracellular part of CTLA4 fused to the endoplasmic reticulum (ER) retention or retrieval signal sequence, KDEL, because we reasoned that the CTLA4-KDEL fusion protein would bind to newly synthesized CD80/86 molecules in the ER and prevent them from reaching the cell surface.
Materials and methods
Subcloning of CTLA4-KDEL
The human CTLA4 gene from the pcDNA3-CTLA4 plasmid14 was amplified by polymerase chain reaction (PCR) with the primers, 5′-CTCCACAGGCGCGCACTCCATGGCTTGCCTTGGATTTCA-3′ and 5′-TTTTTGTTCTGCGGCCGCCAATTACATAAATCTGGGTTCCGT-3′ with BssHII and NotI restriction sequences at 5′ end and 3′ end, respectively. The gene was cloned into pCMV/myc/ER (Invitrogen, Paisley, United Kingdom).15 Approval for obtaining blood from healthy donors was obtained from the Hammersmith, Queen Charlotte's and Action Local Research Ethics Committee. Informed consent was provided according to the Declaration of Helsinki.
Cell culture
The human fibroblast M1 cell line transfected with human HLA-DR1 and CD80 genes were maintained as described.16,17 Epstein-Barr virus–transformed B-lymphoblastoid cell lines (B-LCLs) were from the 10th International Histocompatibility Workshop.12,18 DCs were isolated and differentiated in vitro.19 Prior to the use in the lymphocyte proliferation assay, the DCs were matured with cytokines (20 ng/mL interleukin 1β [IL-1β; PeproTech, London, United Kingdom], 20 ng/mL IL-6 [PeproTech], 20 ng/mL tumor necrosis factor α [TNF-α; PeproTech], 20 ng/mL lipopolysaccharide [LPS], 10 ng/mL prostaglandin E2 [PGE2], and 20 ng/mL interferon γ [IFN-γ] [PeproTech] for 24 hours). Adherent and nonadherent DCs were prepared as described.20 T-cell clone, HC3, specific for hemagglutinin (HA) amino acids 100 to 115 and restricted by DRB1*0101 was cultured and purified 10 days from the last stimulation to exclude any contamination by accessory cells as described.12,18
Transfection
Transfections were carried out using modified protocols.15,21,22 Following the transfection of monocytes, cells were cultured for 2 days before addition of G418 (Sigma, Poole, United Kingdom) and subsequent differentiation into DCs as previously described.15 B cells were transfected by standard electroporation methods.15 As a control the pCMV/EGFP (plasmid cytomegalovirus/enhanced green fluorescent protein) plasmid encoding enhanced green fluorescent protein was used (Clontech, Palo Alto, CA).
Flow cytometry
Flow cytometric analysis was performed10,22,23 using mouse monoclonal antibodies against human major histocompatibility complex (MHC) class II, CD11c, CD14, CD40, CD54, CD80, CD83, CD86, and inducible costimulator (ICOS) ligand (L) and 4G7 (anti-CD19) (Caltag, Silverstone, United Kingdom), and IgG (Sigma). Antibodies are described in Tan et al23,24 unless otherwise stated.
Western blotting
T cells were prepared from cultures by bead-selection as previously described.15 Cell lysates were prepared and Western blotting followed the electrophoresis on a reducing gel unless stated otherwise. The blots were probed with monoclonal antibodies against human CD80 (nonreducing gel), human CD86 (nonreducing gel), β-actin, the c-myc tag, p-PERK (protein kinase-like endoplasmic reticulum kinase; Cell Signaling Technology, Hitchin, United Kingdom)25 and p-eIF-2α (eukaryotic initiation factor 2α; Cell Signaling Technology).26 Antibodies are described in Tan et al10,23,24 unless otherwise indicated. When indicated, the DCs were treated with a cocktail of proteasome inhibitors (Type I-IV; 1 μM; Calbiochem, Nottingham, United Kingdom).27 Immunoprecipitation of cell lysates with anti-CTLA4 (Serotec, Oxford, United Kingdom) monoclonal antibody (mAb) and 9E10 was carried out.28,29 Cell-cycle analysis (including in vitro kinase reactions) and p27kip1 expression were determined as described.28,29
T-cell proliferation assays
Allogeneic T cells were isolated and purified,10,15,23 and accessory cell contamination was assessed by culture with 1 μg/mL phytohemagglutinin in a 48-hour assay. Purified cells (1 × 105/well) were cultured in the presence of 180-Gy x-irradiated B-LCL or irradiated DCs (except IDO [indoleamine 2,3-dioxygenase] experiments) or mitomycin-C–treated M1 transfectants in a mixed lymphocyte reaction (MLR) as described.12,18 When appropriate, the IDO inhibitor, 1-MT (1-methyl-tryptophan; Sigma) (concentrations up to 1 mM) was added to the cultures. T-cell clones (104 cell/well) were cultured in the presence of 180-Gy x-irradiated B-LCL (3 × 104/well) or mitomycin-C–treated M1 transfectants (3 × 104/well) pulsed with the appropriate peptide in flat-well microtiter plates in peptide-specific proliferation assays.12,18 Measurement of IL-4, IFN-γ, IL-12p70, and IL-10 in the supernatants was carried out by enzyme-linked immunosorbent assay (ELISA).10,15,23,30
Two-stage culture experiments were carried out as described.10 In brief, T cells were incubated with the appropriate antigen-presenting cells (APCs) for 5 days, then separated from APCs using bead selection and rested for a further 2 days. Recovered T cells were then incubated with fresh (unmodified) APCs, and the proliferation was determined on days 3, 5, and 7. In experiments to determine whether the cells had adopted a regulatory phenotype, a similar protocol was used, except that T cells were irradiated (60 Gy) prior to addition to the second culture and 1 × 105 fresh (nonirradiated) T cells were added to the same culture. The proliferation of the fresh T cells was determined on day 5.
RT-PCR assay and PCR–Southern blotting
DCs were transfected with mock-KDEL or CTLA4-KDEL, or treated with human Ig or CTLA4-Ig. Nonirradiated DCs were then used as stimulators in an allogeneic MLR (as described under “T-cell proliferation assays”). DCs were isolated from the MLR using negative bead selection (anti-CD4 beads) (Dynal, Wirral, United Kingdom). Reverse transcriptase (RT)–PCR assays were carried out using the paired primers, annealing temperatures, and protocols previously described.10,15,23 General hybridization protocols were carried out as described23 using the following probes: 5′-GATCATCTCACAGACCACAAATG-3′ and 5′-GCTATCCCTGTACGCCTCTG-3′ for IDO and β-actin, respectively.
Results
Transient transfection of artificial APCs with CTLA4-KDEL
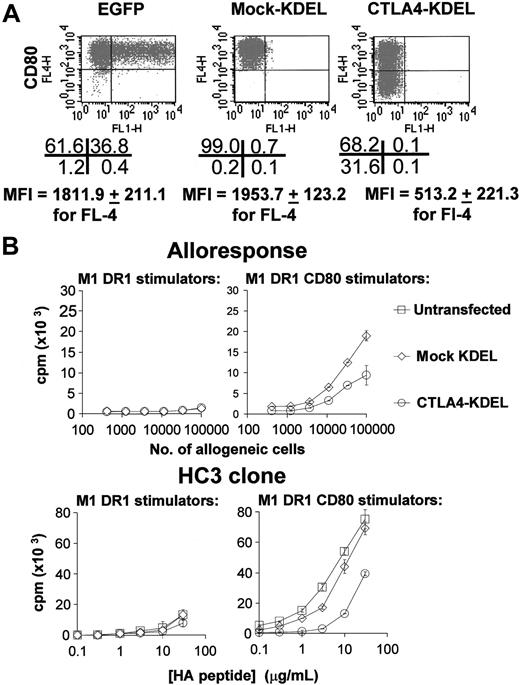
To investigate the effect of CTLA4-KDEL on expression of CD80 and CD86, M1 cells stably transfected with HLA-DR1 genes alone, or in combination with the CD80 gene (termed M1.DR1 and M1.DR1.CD80) were transiently transfected with CTLA4-KDEL. As shown in Figure 1A, M1.DR1.CD80 cells transfected with CTLA4-KDEL expressed less CD80 than did mock-transfected cells (cells transfected with pCMV/myc/ER with no insert; “mock-KDEL”), or cells transfected with pCMV/EGFP. CD80 expression was not totally inhibited, but this was expected given that only approximately one third of the M1.DR1.CD80 cells were transfected with CTLA4-KDEL (deduced from transfecting M1.DR1.CD80 cells with pEGFP). Furthermore, it can be seen in Figure 1B that when compared with untransfected cells or mock-transfected cells, these transfectants had a reduced ability to stimulate peripheral blood T cells in primary allogeneic MLRs and a reduced ability to stimulate HC3 T cells in peptide-specific proliferation assays. Similar data were seen with M1.DR1.cells transfected with CD86 or with a combination of CD80 and CD86 (data not shown).
Stable transfection of B cells with CTLA4-KDEL
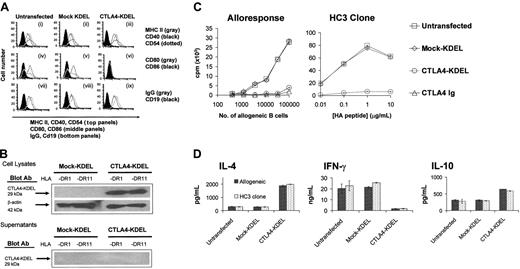
Having established the effect of the CTLA4-KDEL construct in a transient expression system, we went on to generate stably transfected B-cell lines (homozygous for either HLA-DR1 or -DR11). Transfection of HLA-DR1 B cells with CTLA4-KDEL did not affect the expression of MHC class II, CD40, CD54, CD19, and IgG. However, CD80 and CD86 expression was completely abolished (Figure 2A). Similar results were seen with HLA-DR11–expressing B cells (data not shown). Expression of the CTLA4-KDEL fusion protein in these cells was confirmed by Western blotting with mAb to the c-myc tag. No secreted CTLA4-KDEL was detected in culture supernatants (Figure 2B) or on the cell surface (data not shown).
Transient transfection of CTLA4-KDEL into artificial APCs reduces their ability to stimulate T cells. (A) M1 cells already transfected with DR1 and CD80 (M1 DR1 CD80) or cells transfected with DR1 alone (M1 DR1) were transiently transfected with mock-KDEL and CTLA4-KDEL constructs or with pCMV/EGFP encoding EGFP. CD80 (FL-4) or EGFP (FL-1) expression was determined by flow cytometry. The number of cells in each quadrant and mean fluorescence intensity (MFI) for CD80 expression are shown below (mean ± SD of 5 independent experiments). (B) The same transfected cells were used as stimulators in a MLR or peptide-specific T-cell response. For the mixed lymphocyte response, various numbers of stimulators were used, and proliferation was determined by 3H-thymidine incorporation after 5 days. The HC3 T-cell clone was used to test a peptide-specific response using the HA peptide at a range of concentrations with 3H-thymidine incorporation being assessed after 3 days. The results are representative of at least 5 independent experiments and show the mean ± SD of triplicate cultures.
Transient transfection of CTLA4-KDEL into artificial APCs reduces their ability to stimulate T cells. (A) M1 cells already transfected with DR1 and CD80 (M1 DR1 CD80) or cells transfected with DR1 alone (M1 DR1) were transiently transfected with mock-KDEL and CTLA4-KDEL constructs or with pCMV/EGFP encoding EGFP. CD80 (FL-4) or EGFP (FL-1) expression was determined by flow cytometry. The number of cells in each quadrant and mean fluorescence intensity (MFI) for CD80 expression are shown below (mean ± SD of 5 independent experiments). (B) The same transfected cells were used as stimulators in a MLR or peptide-specific T-cell response. For the mixed lymphocyte response, various numbers of stimulators were used, and proliferation was determined by 3H-thymidine incorporation after 5 days. The HC3 T-cell clone was used to test a peptide-specific response using the HA peptide at a range of concentrations with 3H-thymidine incorporation being assessed after 3 days. The results are representative of at least 5 independent experiments and show the mean ± SD of triplicate cultures.
In functional studies, CTLA4-KDEL–expressing B-cell lines were unable to stimulate a MLR (Figure 2C). This inhibition was comparable to that seen following addition of a saturating concentration (10 μg/mL) of soluble CTLA4-Ig from the start of the culture. Using 4 T-cell clones, HC3 (Figure 2C), NF4, MJ60, and MJ36 (data not shown), specific for HA peptides in the context of either HLA-DR1 or DR11, we demonstrated that stably transfected B-cell lines were also unable to stimulate antigen-specific T-cell proliferation.
Both allospecific and peptide-specific T cells stimulated with CTLA4-KDEL–expressing B cells showed a different pattern of cytokine expression to those stimulated with either unmodified or mock-KDEL–transfected B cells with increased levels of IL-4 and IL-10 and reduced IFN-γ, a profile typical of T helper 2 (Th2) skewing (Figure 2D).
Generation of anergic T cells after coculture with CTLA4-KDEL B cells
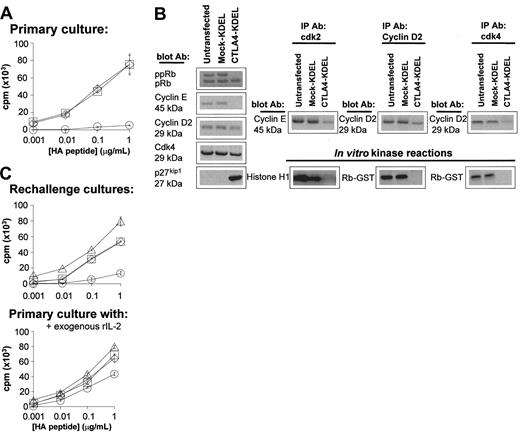
To determine whether the hyporesponsive T cells generated in this system had been rendered anergic, we cocultured the HC3 clone with stably transfected B cells in the presence of HA peptide. As expected, the clone was hyporesponsive on first encounter with HA antigen presented by CTLA4-KDEL–transfected B cells (Figure 3A). HC3 T cells exposed to CTLA4-KDEL–transfected B cells in the presence of HA peptide were arrested at the early G1 phase of the cell cycle as demonstrated by the lack of expression of phosphorylated retinoblastoma protein and Cyclin E, as well as lack of functional kinase activity by Cyclin D2, cdk2 and cdk4 and reduced association of cdk2 and cdk4 with Cyclins E and D2, respectively (Figure 3B). These cells also expressed a high level of p27kip1 typical of anergic cells. The HC3 T cells from the primary culture were then rested for 5 days, prior to rechallenge with untransfected B cells in the presence of HA peptide. The HC3 T cells remained unresponsive on rechallenge with untransfected B cells expressing costimulatory molecules, indicating that they had become anergic (Figure 3C). As expected from previous studies,12 this anergy could be reversed by addition of exogenous rIL-2 (Figure 3C).
CTLA4-KDEL transfection of DCs
To extend this approach to DCs, we transfected monocytes with immunolipoplexes.22 These were differentiated into DCs by culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 in the presence of G418. As previously reported,15 virtually all cells express the transduced gene (data not shown).
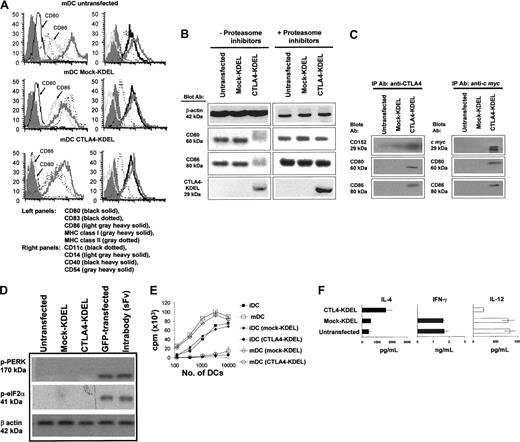
The phenotype of transfected DCs was analyzed either as iDC (data not shown) or following activation into mature DC (mDC) with a cocktail of proinflammatory cytokines (Figure 4A). The expression of MHC class I, class II, CD11c, CD14, CD40, and CD54 expression (Figure 4A) was identical in DCs that had been transfected with CTLA4-KDEL (drug-selected), mock-KDEL (drug-selected), or were untransfected (not drug selected), indicating that neither the transfection nor selection regimen had altered the phenotype of cells and that expression of CTLA4-KDEL had no effect on these molecules. However, both iDCs and mDCs that had been transfected with CTLA4-KDEL showed no expression of CD80 and CD86, suggesting that the CTLA4-KDEL construct was effective at reducing their expression.
RT-PCR showed that the mRNA levels of CD80/86 and other molecules were unaffected by CTLA4-KDEL expression (data not shown), whereas Western blotting (Figure 4B) and immunocytochemistry (data not shown) showed little expression of CD80/86, suggesting rapid degradation. To identify the likely degradation pathway, we treated CTLA4-KDEL–transfected DCs with a cocktail of proteasome inhibitors, this resulted in increased levels of CD80/86, indicating that degradation is a proteasome-dependent process (Figure 4B). By immunoprecipitating proteins from cell lysates using anti-CTLA4 or anti–c-myc, then Western blotting and probing with anti-CD80/86 antibodies, we showed that CTLA4-KDEL was colocalized with CD80 and CD86 proteins (Figure 4C), as was also suggested by deconvolution microscopy (data not shown).
We also determined whether expression of CTLA4-KDEL and the formation of the resulting complex in the ER induced a detectable stress response in the DCs. No marked increase in mRNA levels was seen for a range of heat shock proteins (hsp60, hsp70, mthsp75, grp78, hsc70, hps90α, and hsp90β) over that seen with cells transfected with GFP, as assessed by RT-PCR (data not shown), but more importantly there was no up-regulation of phosphorylation of the ER stress responsive enzyme PERK, or the molecule that it phosphorylates eIF-2α.31 In contrast, transfection of cells with either GFP or an intrabody (anti–VCAM-1 scFv fused to KDEL [unpublished observation]) did increase phosphorylation of PERK and eIF-2α, indicating induction of ER stress (Figure 4D).
Stable transfection of CTLA4-KDEL into B cells inhibits their ability to stimulate T cells. (A) HLA-DR1–expressing B cells were stably transfected with CTLA4-KDEL and mock-KDEL. The expression of CD40, CD54, and MHC II (i-iii), CD80 and CD86 (iv-vi), IgG and CD19 (vii-ix) was analyzed by flow cytometry. Staining with isotype matched control antibodies is shown in the solid profile. (B) Expression of the 29-kDa CTLA4-KDEL in cell lysates and supernatants of HLA DR1- or HLA DR11-expressing B cells transfected with either CTLA4-KDEL or mock-KDEL was determined by Western blotting using Ab against the c-myc protein tag incorporated in the construct. β-Actin was used as a housekeeping control. (C) HLA-DR1–expressing-B-cell lines that were either untransfected or transfected with the mock-KDEL or the CTLA4-KDEL plasmid were used as stimulators in a MLR and for a peptide-specific response. In the MLR, 10 μg/mL CTLA4-Ig was used as a control, and 3H-thymidine incorporation was determined after 5 days. The HC3 T-cell clone was used to test the peptide-specific response, and the B cells were incubated with different concentrations of HA peptide. 3H-thymidine incorporation was determined after 3 days. The results are representative of at least 3 experiments. (D) Culture supernatants from the HC3 clone and allogeneic T cells were collected on day 2 and 4, respectively, after coculture with HLA-DR1–expressing B cells that were either untransfected or stably transfected with CTLA4-KDEL or mock-KDEL. The levels of IL-4, IFN-γ, and IL-10 were measured using ELISA. The results are mean ± SD of 3 independent experiments.
Stable transfection of CTLA4-KDEL into B cells inhibits their ability to stimulate T cells. (A) HLA-DR1–expressing B cells were stably transfected with CTLA4-KDEL and mock-KDEL. The expression of CD40, CD54, and MHC II (i-iii), CD80 and CD86 (iv-vi), IgG and CD19 (vii-ix) was analyzed by flow cytometry. Staining with isotype matched control antibodies is shown in the solid profile. (B) Expression of the 29-kDa CTLA4-KDEL in cell lysates and supernatants of HLA DR1- or HLA DR11-expressing B cells transfected with either CTLA4-KDEL or mock-KDEL was determined by Western blotting using Ab against the c-myc protein tag incorporated in the construct. β-Actin was used as a housekeeping control. (C) HLA-DR1–expressing-B-cell lines that were either untransfected or transfected with the mock-KDEL or the CTLA4-KDEL plasmid were used as stimulators in a MLR and for a peptide-specific response. In the MLR, 10 μg/mL CTLA4-Ig was used as a control, and 3H-thymidine incorporation was determined after 5 days. The HC3 T-cell clone was used to test the peptide-specific response, and the B cells were incubated with different concentrations of HA peptide. 3H-thymidine incorporation was determined after 3 days. The results are representative of at least 3 experiments. (D) Culture supernatants from the HC3 clone and allogeneic T cells were collected on day 2 and 4, respectively, after coculture with HLA-DR1–expressing B cells that were either untransfected or stably transfected with CTLA4-KDEL or mock-KDEL. The levels of IL-4, IFN-γ, and IL-10 were measured using ELISA. The results are mean ± SD of 3 independent experiments.
Generation of anergic T cells after coculture with CTLA4-KDEL transfectants. The HC3 clone (106) was cocultured with an equal number of HLA-DR1–expressing B cells, which were nontransfected, CTLA4-KDEL transfected, or mock-KDEL transfected. (A) After 3 days, the proliferation of the clones was measured by 3H-thymidine incorporation. □ indicates untransfected; ⋄, Mock-KDEL; ○, CTLA4-KDEL. (B) After 2 days the clone was isolated, and the expression of phosphorylated retinoblastoma protein, cyclin E, cyclin D2, Cdk4, and p27kip1 proteins was analyzed by Western blotting. In addition, the coassociation of cdk2, Cyclin D2, cdk4 with other proteins was assessed by immunoprecipitation with appropriate Ab (IP Ab) followed by Western blotting and probing with anti–Cyclin E or Cyclin D2 Ab (blot Ab). The enzymatic activity of the proteins was determined using in vitro kinase reactions, with Histone H1 or a retinoblastoma–glutathione S transferase (Rb-GST) fusion protein. (C) From the primary coculture, the clones were then rested for 5 days before being stimulated with an equal number of unmodified HLA-DR1–expressing B cells prepulsed with HA peptide. The cultures were carried out in the absence (left) or presence (right) of 10 U/mL recombinant (r)IL-2. Proliferation of the T cells was determined by 3H-thymidine incorporation after 3 days. ▵ indicates no cells; □, untransfected; ⋄, Mock-KDEL; ○, CTLA-4 KDEL. The results are expressed as mean ± SD of triplicate wells of a single experiment. These data are representative of 3 independent experiments.
Generation of anergic T cells after coculture with CTLA4-KDEL transfectants. The HC3 clone (106) was cocultured with an equal number of HLA-DR1–expressing B cells, which were nontransfected, CTLA4-KDEL transfected, or mock-KDEL transfected. (A) After 3 days, the proliferation of the clones was measured by 3H-thymidine incorporation. □ indicates untransfected; ⋄, Mock-KDEL; ○, CTLA4-KDEL. (B) After 2 days the clone was isolated, and the expression of phosphorylated retinoblastoma protein, cyclin E, cyclin D2, Cdk4, and p27kip1 proteins was analyzed by Western blotting. In addition, the coassociation of cdk2, Cyclin D2, cdk4 with other proteins was assessed by immunoprecipitation with appropriate Ab (IP Ab) followed by Western blotting and probing with anti–Cyclin E or Cyclin D2 Ab (blot Ab). The enzymatic activity of the proteins was determined using in vitro kinase reactions, with Histone H1 or a retinoblastoma–glutathione S transferase (Rb-GST) fusion protein. (C) From the primary coculture, the clones were then rested for 5 days before being stimulated with an equal number of unmodified HLA-DR1–expressing B cells prepulsed with HA peptide. The cultures were carried out in the absence (left) or presence (right) of 10 U/mL recombinant (r)IL-2. Proliferation of the T cells was determined by 3H-thymidine incorporation after 3 days. ▵ indicates no cells; □, untransfected; ⋄, Mock-KDEL; ○, CTLA-4 KDEL. The results are expressed as mean ± SD of triplicate wells of a single experiment. These data are representative of 3 independent experiments.
Transfection of DCs with CTLA4-KDEL. Monocytes were transfected with CTLA4-KDEL, mock-KDEL, or left untransfected and then differentiated into DCs with GM-CSF and IL-4. On day 2 G418 was added to select for transfected cells. On day 8, the cells were matured with TNF-α, IL-1β, LPS, IFN-γ, and PGE2. (A) On day 10, the expression of MHC I, MHC II, CD80, CD86, CD83, CD54, CD40, CD11c, and CD14 was analyzed using flow cytometry. (B) Expression of CD80, CD86, CTLA4-KDEL, and β-actin was determined by Western blotting in the presence and absence of 1 μM proteasome inhibitors to determine the degradation pathway of CD80/86. (C) To demonstrate colocalization of CTLA4-KDEL and CD80/86, the cell lysates were immunoprecipitated with anti-CTLA4 (left) and anti–c myc (right) prior to Western blotting and probing with anti-CD80, CD86, or CTLA4 mAb. (D) To determine whether CTLA4-KDEL activated ER stress responses, Western blots were probed with antibodies against phosphorylated PERK and phosphorylated eIF-2α proteins. As a control in these experiments, DCs were also transfected with GFP or with an intrabody (scFv-KDEL) directed against vascular cell adhesion molecule 1 (VCAM-1). (E) The DCs were tested for their ability to stimulate an MLR by allogeneic T cells. The results are expressed as mean ± SD of triplicate wells from a representative experiment. (F) The supernatants from allogeneic T cells cocultured with either untransfected, CTLA4-KDEL, or mock-KDEL transfected DCs were collected on day 4, and the levels of IL-4, IL-12p70, and IFN-γ were measured by ELISA. The results are mean ± SD of 3 cocultures. The data are representative of 3 experiments.
Transfection of DCs with CTLA4-KDEL. Monocytes were transfected with CTLA4-KDEL, mock-KDEL, or left untransfected and then differentiated into DCs with GM-CSF and IL-4. On day 2 G418 was added to select for transfected cells. On day 8, the cells were matured with TNF-α, IL-1β, LPS, IFN-γ, and PGE2. (A) On day 10, the expression of MHC I, MHC II, CD80, CD86, CD83, CD54, CD40, CD11c, and CD14 was analyzed using flow cytometry. (B) Expression of CD80, CD86, CTLA4-KDEL, and β-actin was determined by Western blotting in the presence and absence of 1 μM proteasome inhibitors to determine the degradation pathway of CD80/86. (C) To demonstrate colocalization of CTLA4-KDEL and CD80/86, the cell lysates were immunoprecipitated with anti-CTLA4 (left) and anti–c myc (right) prior to Western blotting and probing with anti-CD80, CD86, or CTLA4 mAb. (D) To determine whether CTLA4-KDEL activated ER stress responses, Western blots were probed with antibodies against phosphorylated PERK and phosphorylated eIF-2α proteins. As a control in these experiments, DCs were also transfected with GFP or with an intrabody (scFv-KDEL) directed against vascular cell adhesion molecule 1 (VCAM-1). (E) The DCs were tested for their ability to stimulate an MLR by allogeneic T cells. The results are expressed as mean ± SD of triplicate wells from a representative experiment. (F) The supernatants from allogeneic T cells cocultured with either untransfected, CTLA4-KDEL, or mock-KDEL transfected DCs were collected on day 4, and the levels of IL-4, IL-12p70, and IFN-γ were measured by ELISA. The results are mean ± SD of 3 cocultures. The data are representative of 3 experiments.
Next, the functional ability of these DCs was tested. CTLA4-KDEL–expressing mDCs and iDCs failed to induce allospecific proliferation of freshly isolated T cells as shown in Figure 4E. However, supernatants of CTLA4-KDEL DC-stimulated T cells contained increased IL-4 and decreased IFN-γ and IL-12p70 when compared with those exposed to control DCs (Figure 4F). In contrast with T cells stimulated with B cells expressing CTLA4-KDEL, no IL-10 production was seen (data not shown).
Generation of anergic T cells after coculture with CTLA4-KDEL DCs
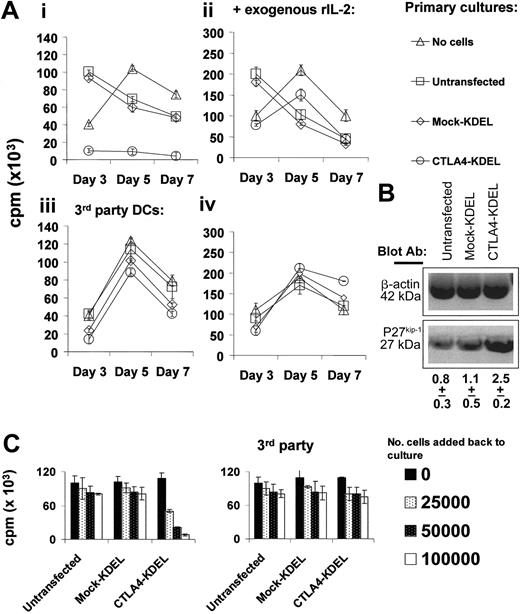
To determine whether T cells exposed to DCs expressing CTLA4-KDEL had become anergic, we carried out 2-step cultures. Naive T cells were cocultured with DCs (transfected with CTLA4-KDEL or mock-KDEL) for 5 days and rested for 5 days. These were then rechallenged with either mDCs from the same donor, or third-party DCs. Proliferation was assessed on days 3, 5, and 7 to differentiate between a primary and secondary response. T cells previously exposed to CTLA4-KDEL DCs failed to respond to mDCs from the same donor, although they did respond to third-party DCs with kinetics typical of a primary response (peak at day 5) (Figure 5A). T cells stimulated with control DCs in the first culture showed a subsequent rapid response to DCs from the same donor, typical of a secondary immune response (peak at day 3). The anergized T cells expressed elevated levels of p27kip1 (Figure 5B) and the majority of T cells were arrested at the early G1 phase of the cell cycle (data not shown), consistent with their anergic phenotype.28,29 As expected, the hyporesponsiveness of the T cells could be reversed by addition of exogenous rIL-2 (Figure 5A).
Generation of anergic and regulatory T cells after coculture with CTLA4-KDEL–transfected DCs. (A) Purified T cells (106) were cocultured with DCs (10:1 ratio), which were either untransfected or transfected with CTLA4-KDEL or mock-KDEL. After 5 days, T cells were rested in culture for another 5 days. The cells were then put into fresh culture with mDCs from either the same donor (i-ii) or third-party (iii-iv) mDCs at 5:1 ratio in the presence (ii,iv) or absence (i,iii) of 10 U/mL exogenous rIL-2. The proliferation of T cells was determined by 3H-thymidine incorporation on days 3, 5, and 7. The results are expressed as the mean ± SD of triplicate wells. (B) T cells exposed to untransfected DCs, mock-KDEL–transfected DCs, or CTLA4-KDEL–transfected DCs were analyzed for expression of p27kip1 and β-actin expression by Western blotting. The density of the p27kip1 bands (normalized to β-actin) are shown below, as the mean ± SD of 3 experiments. (C) The T cells generated following incubation with untransfected or with CTLA4-KDEL or mock-transfected DCs were added at varying numbers to an MLR between fresh T cells (from the same donor) and DCs from either the original stimulator or third-party DCs. 3H-thymidine incorporation was measured on day 5. All data are representative of 3 experiments and show the mean ± SD of triplicate cultures.
Generation of anergic and regulatory T cells after coculture with CTLA4-KDEL–transfected DCs. (A) Purified T cells (106) were cocultured with DCs (10:1 ratio), which were either untransfected or transfected with CTLA4-KDEL or mock-KDEL. After 5 days, T cells were rested in culture for another 5 days. The cells were then put into fresh culture with mDCs from either the same donor (i-ii) or third-party (iii-iv) mDCs at 5:1 ratio in the presence (ii,iv) or absence (i,iii) of 10 U/mL exogenous rIL-2. The proliferation of T cells was determined by 3H-thymidine incorporation on days 3, 5, and 7. The results are expressed as the mean ± SD of triplicate wells. (B) T cells exposed to untransfected DCs, mock-KDEL–transfected DCs, or CTLA4-KDEL–transfected DCs were analyzed for expression of p27kip1 and β-actin expression by Western blotting. The density of the p27kip1 bands (normalized to β-actin) are shown below, as the mean ± SD of 3 experiments. (C) The T cells generated following incubation with untransfected or with CTLA4-KDEL or mock-transfected DCs were added at varying numbers to an MLR between fresh T cells (from the same donor) and DCs from either the original stimulator or third-party DCs. 3H-thymidine incorporation was measured on day 5. All data are representative of 3 experiments and show the mean ± SD of triplicate cultures.
To determine whether the anergic cells had regulatory activity, we added them back into a MLR between unmanipulated T cells and DCs. As shown in Figure 5C, T cells that had been cocultured with CTLA4-KDEL–transfected DCs inhibited the second MLR in a dose-dependent manner when cocultured with DCs from the same donor as the original DCs. This was not seen when either third-party DCs were used or when the T cells had originally been incubated with control DCs.
Effect of CTLA4-KDEL on allogeneic T-cell proliferation is independent of IDO
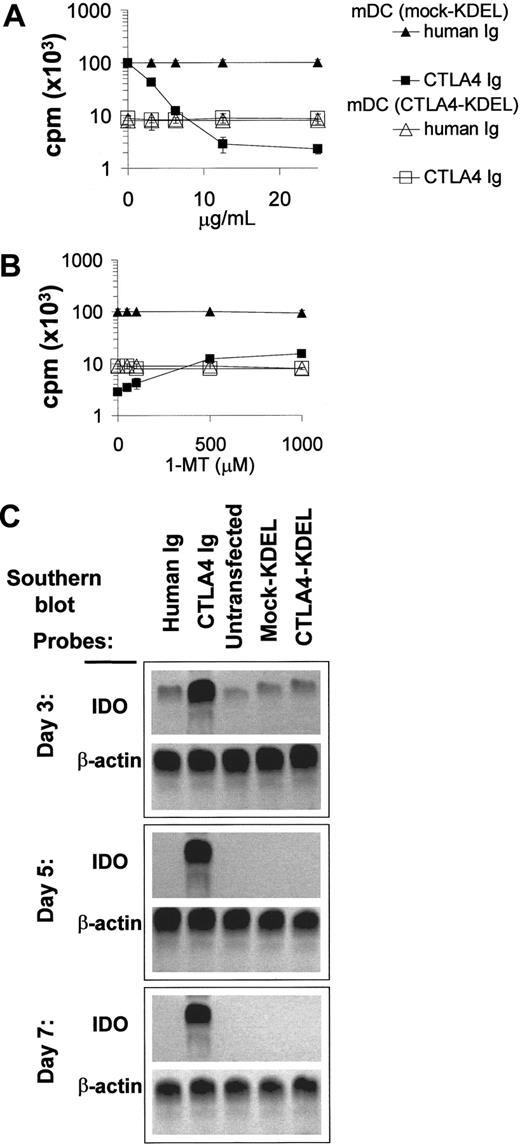
Soluble CTLA4-Ig can interact with CD80/86 on DCs to up-regulate IDO activity,32 which can contribute to T-cell unresponsiveness by increasing tryptophan catabolism. To determine whether this mechanism is important for the suppressive effects of CTLA4-KDEL–transfected DCs, we performed MLR in the presence of the IDO inhibitor 1-MT. This caused a slight reduction (< 10%) in the suppression of the MLR seen in the presence of saturating concentrations of CTLA4-Ig, but it had no effect on the response to CTLA4-KDEL DCs (Figure 6A-B). Furthermore, in MLRs using wild-type DCs in the presence of CTLA4-Ig there was up-regulation of IDO in the DCs, as determined by RT-PCR Southern blots; this which was not seen in cultures containing CTLA4-KDEL DCs (Figure 6C). These findings were also confirmed using the Western blots for IDO protein (data not shown) and the measurement of L-Kynurenine in supernatants (data not shown). These data indicate that the inhibition by CTLA4-KDEL is independent of IDO expression.
Discussion
In this study, we have used a fusion protein between CTLA4 and the ER-retention signal, KDEL,33,34 to prevent expression of CD80/86 by APCs. This strategy is similar to the use of intrabodies consisting of KDEL fused to antibody fragments to prevent cell-surface expression of target molecules35,36 or intrakines (chemokine-KDEL fusion), to block expression of chemokine receptors.37,38 The advantage of using a cell-surface molecule such as CTLA4, rather than antibody is that the construct is capable of blocking expression of all its ligands (in this case CD80 and CD86), rather than needing a separate antibody for each target. In addition, the immunogenicity of the construct would be low, as the sequence of CTLA4 is human. This is particularly important as we have shown that degradation of CD80/86 in CTLA4-KDEL–transfected DCs is blocked by proteasome inhibitors, suggesting that the molecules are likely to enter the antigen-processing pathway.
CTLA4-KDEL acts on DCs via an IDO-independent mechanism in affecting allogeneic T-cell proliferation. (A) CTLA4-KDEL or mock-KDEL–transfected DCs were cocultured with allogeneic T cells in the presence of a range of concentrations of human Ig or CTLA4-Ig. 3H-thymidine incorporation was measured on day 5. (B) The effect of the IDO antagonist, 1-MT, was determined in a parallel set of cultures using a fixed concentration of 10 μg/mL for both CTLA4-Ig and human Ig. 3H-thymidine incorporation was determined on day 5. The results shown on a log scale are expressed as the mean ± SD of triplicate wells from a representative experiment. (C) The up-regulation of IDO transcription in DCs following CTLA4-Ig crosslinking was studied. DCs were isolated from MLRs, which had been treated with human Ig (lane 1) or CTLA4-Ig (lane 2) or from MLRs set up with unmodified DCs (lane 3), mock-KDEL (lane 4), and CTLA4-KDEL (lane 5) transfected DCs. These were analyzed for expression of IDO mRNA by RT-PCR followed by probing with IDO-specific probe (β-actin was used as a control). These data are representative of 3 independent experiments.
CTLA4-KDEL acts on DCs via an IDO-independent mechanism in affecting allogeneic T-cell proliferation. (A) CTLA4-KDEL or mock-KDEL–transfected DCs were cocultured with allogeneic T cells in the presence of a range of concentrations of human Ig or CTLA4-Ig. 3H-thymidine incorporation was measured on day 5. (B) The effect of the IDO antagonist, 1-MT, was determined in a parallel set of cultures using a fixed concentration of 10 μg/mL for both CTLA4-Ig and human Ig. 3H-thymidine incorporation was determined on day 5. The results shown on a log scale are expressed as the mean ± SD of triplicate wells from a representative experiment. (C) The up-regulation of IDO transcription in DCs following CTLA4-Ig crosslinking was studied. DCs were isolated from MLRs, which had been treated with human Ig (lane 1) or CTLA4-Ig (lane 2) or from MLRs set up with unmodified DCs (lane 3), mock-KDEL (lane 4), and CTLA4-KDEL (lane 5) transfected DCs. These were analyzed for expression of IDO mRNA by RT-PCR followed by probing with IDO-specific probe (β-actin was used as a control). These data are representative of 3 independent experiments.
We have shown that APCs, including DCs, containing the construct do not express CD80/86 on the cell surface and are unable to stimulate either allogeneic or peptide-specific T cells. Indeed, antigen-reactive T cells are rendered anergic by encounter with CTLA4-KDEL–expressing B cells and DCs. Furthermore, these anergic cells are capable of inhibiting a MLR, indicating that they can act as regulatory cells. As far as we have observed CTLA4-KDEL expression does not alter the phenotype of APCs transfected with the construct, apart from preventing surface expression of CD80/86. Thus, although we have not ruled out that there is a nonspecific effect of CTLA4-KDEL expression on the function of the artificial APCs, B cells and DCs, the most probable explanation for the altered T-cell responses to CTLA4-KDEL transfected is the lack of CD80/86 expression.
The induction of regulatory T cells is of interest, both because of murine data showing that CD80/86 expression is needed for the generation and survival of regulatory cells,39-43 and because of the role of CTLA4 expression on T cells (and its presumed interaction with CD80/86) in anti-CD45RB–induced tolerance to islet allografts.44 However, in the human and mouse it is clear that under some conditions anergic T cells generated in the absence of costimulation can act as regulatory T cells.12,45-47 It may be that some CD80/86 expression will be required on APCs for the long-term maintenance of tolerance to an antigen. However, the induction of regulatory T cells suggests that this strategy will be capable of mediating linked suppression and may also regulate CD28– T cells that can be present in patients
Although T cells that had been cocultured with CTLA4-KDEL APCs were rendered anergic, (as determined by proliferation), these T cells did respond to CTLA4-KDEL–transfected APCs by an increase in IL-4 and IL-10 (in the case of B cells) secretion. Cultures contained a decreased amount of IFN-γ and IL-12p70, indicating a Th2 skewing. Similar Th2 skewing has been reported for T cells rendered anergic by incubation with nonprofessional antigen-presenting cells or T cells and has been reported in vivo following induction of anergy.48-50 The expression of ICOS L and programmed cell death ligand 1 (PD-L1) on DCs transfected with CTLA4-KDEL was unaltered (data not shown), and so this apparent Th2 bias may due to the unopposed (without CD28-CD80/86) costimulation through ICOS-ICOS L and PD-PD-L1 interactions, as has been previously described.51,52 This is in contrast with what was seen when CTLA4-Ig was added to cultures containing control DCs, which showed the expected reduction in IFN-γ, IL-4, and IL-10 (data not shown), which may in DCs be associated with up-regulation of IDO (not seen in CTLA4-KDEL–transfected cells).
Genetic modification of DCs as a strategy to induce tolerance has a number of advantages. DCs not only have the right antigen-processing capacities for efficient presentation to T cells, but also traffic to the appropriate sites to encounter T cells. Our CTLA4-KDEL–expressing DCs have the potential advantage over drug-treated iDCs (which have been shown to be actively tolerogenic8-10 ) in that the DCs can be activated to express high levels of MHC class II and will presumably show a normal homing pattern that allows efficient interaction with T cells. Furthermore, there is always the danger that drug-induced iDCs will become activated in vivo, especially in sites with high concentrations of inflammatory cytokines. This approach also has the advantage over the administration of soluble proteins, such as CTLA4-Ig,6,7 in that it is specific for the antigens expressed by the APCs and so will not induce generalized immunosuppression.
One potential problem with the use of KDEL-tagged fusion proteins to form complexes in the ER is that this might stress the cell, leading to nonspecific effects. In human DCs, we were unable to demonstrate any up-regulation of heat shock proteins, nor was any up-regulation of PERK (the ER stress response protein) and eIF-2α observed. This was in contrast to cells transfected with intrabody (scFv-KDEL fusion protein), which showed up-regulation of phosphorylated PERK and eIF-2α, possibly because of poor folding of the intrabody in the ER, with the subsequent activation of the ER stress pathway. The use of a surface ligand, which is normally expressed in the ER, may be an advantage in this respect.
Crosslinking of CD80/86 on DCs by CTLA4-Ig results in up-regulation of the IDO enzyme, resulting in increased tryptophan metabolism.32 However, the inhibition of proliferation by CTLA4-KDEL–transfected DCs was independent of IDO, as determined by the failure of 1-MT to block the inhibition and the lack of IDO mRNA in these cells during a MLR. This indicates that binding of CD80/86 by CTLA4-KDEL in the ER does not activate IDO transcription.
In summary, we have demonstrated in a human system a new strategy for inducing immunologic tolerance. Our findings raise the possibility of clinical therapy either by administration of ex-vivo CTLA4-KDEL–transfected APCs or by gene transfer of the construct to APCs in vivo. The ability of T cells anergized by contact with CTLA4-KDEL–transfected APCs to regulate the response of other T cells indicates that they may be effective in settings when only some of the antigens are expressed on DCs, or when only a proportion of the antigen-specific T cells has contact with the CTLA4-KDEL–expressing DCs. This approach could find application either in the context of transplantation, in which control of allospecific T cells is required or autoimmunity when tolerance to particular antigens is needed. The development of similar fusion proteins may also be used to block the expression of other costimulatory molecules, thus allowing a more subtle modulation of immune responses.
Prepublished online as Blood First Edition Paper, June 30, 2005; DOI 10.1182/blood-2005-05-1826.
Supported by research training fellowships and grants from the MRC (Medical Research Council), London, United Kingdom (A.J.T.G., P.H.T., and J.B.Y.); the Royal College of Surgeons Edinburgh, Edinburgh, United Kingdom (P.H.T.); the Leukemia Research Fund (LRF), London, United Kingdom (S.A.X.); the Wellcome Trust (M.P.W. and J.E.H.); the National Kidney Research Fund, Peterborough, United Kingdom (G.L.); and the Roche Research Foundation, Basel, Switzerland (A.J.T.G.). A.J.T.G. is a BBSRC (British Biotechnology and Biological Science Council, Swindon, United Kingdom) Research Development Fellow.
A.J.T.G. conceived this project; P.H.T., R.I.L., G.L., and A.J.T.G. designed and planned the research; P.H.T., J.B.Y., S.A.X., J.E.H., M.P.W., and W.J.J. performed the research. C.C. provided essential data analysis. J.B.Y., R.D., M.A.R., and G.L. provided essential reagents. All authors contributed to preparing the manuscript, but primary responsibility for this was taken by P.H.T. and A.J.T.G.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs C. Urch, S. Shaunak, and N. Rogers for critical review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal