Abstract
Several features of chronic lymphocytic leukemia (CLL) suggest that immune-based strategies may have therapeutic potential. A promising approach is provided by the transduction of CLL cells with CD40 ligand (CD40L) by viral vectors to enhance their immunogenicity. We compared the antigen-presenting capacity of CD40L-transduced CLL cells with mock-transduced or CD40L-stimulated CLL cells (CD40-CLL). A significantly higher number of T cells could be expanded using CD40L-transduced CLL cells as antigen-presenting cells (APCs) compared with the control group (P = .008). Using 5 different CLL-associated tumor antigens, including fibromodulin, MDM2 (murine double minute 2), survivin, p53, and KW-13, we show in interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT) assays after 35 days of in vitro culture that the number of antigen-specific autologous T cells was also significantly higher when CD40L-transduced CLL cells were used as APCs (P < .001). Thus, CD40L-transduced CLL cells are able to induce an antigen-specific T-cell response and might be superior to CD40-CLL cells for immune-based therapeutic strategies in CLL.
Introduction
Despite recent advances, chronic lymphocytic leukemia (CLL) as the most common leukemia remains a largely incurable disease. Several features of this disease suggest that immune-based strategies may have therapeutic potential.1 CLL cells express major histocompatibility complex (MHC) class 1 and 2 molecules and several tumor-associated antigens (TAAs).2-5 Although most CLL cells are ineffective antigen-presenting cells (APCs) with a low expression of costimulatory molecules, stimulation with CD40 ligand (CD40L) restores their poor antigen-presenting capacity.6,7 Transduction of CLL cells with CD40L by viral vectors presents an alternative strategy to enhance their immunogenicity with promising results in a first phase 1 clinical trial.1,8,9
Adeno-associated virus (AAV), a nonenveloped human parvovirus, has gained attention for human gene therapy because of its favorable safety features. High transfection rates of CLL cells are achieved using an adenovirus-free packaging system with recombinant AAV.10 Thus far, enhanced proliferation of T cells and interferon γ (IFN-γ) production was demonstrated using virally transduced CLL cells, but a CLL-specific (ie, TAA-specific) immune assessment has been lacking. In the present study we show that, based on several CLL-associated tumor antigens, CD40L-transduced CLL cells are indeed able to evoke an antigen-specific T-cell response in an autologous setting, and they seem to be more potent APCs than CD40-stimulated CLL cells. Therefore, we provide an in vitro model for the principle of action of a CD40L-based vaccine in CLL.
Study design
Patients and peptides
After informed consent was provided, peripheral blood was obtained from 5 HLA-A0201–positive patients who satisfied diagnostic criteria for B-cell CLL (B-CLL).11 Patients' characteristics were as follows: median age, 64 years (range, 48-84 years); Binet A, one patient; Binet B, one patient; Binet C, 3 patients; median leukocyte counts, 103 × 109/L (29.2-126 × 109/L); median hemoglobin level, 129 g/L (118-158 g/L); median platelet count, 99 × 109/L (57-235 × 109/L); median β2-microglobulin concentration, 312 nM (155-396 nM). Three of 5 patients were tested for CD38 expression, and all findings were negative. Cytogenetic analyses by interphase fluorescence in situ hybridization (FISH) revealed one patient with a normal karyotype and 3 patients with a deletion of 13q14. Two patients had received previous treatment with chlorambucil. All peptides used were HLA-A0201 binding, synthesized, purified by high-performance liquid chromatography (HPLC) by G.J. Arnold (Ludwig Maximilians University, Munich, Germany), and dissolved in dimethyl sulfoxide (DMSO). Four fibromodulin-derived peptides, one MDM2 (murine double minute 2)–derived peptide (81-88), 4 p53-derived peptides (p1(65-73), p2(264-272), p3(139-148), p4(149-157)), 2 survivin-derived peptides (S8, S9), and the SEREX (serologic identification by recombinant expression cloning)–derived peptide KW-13 were used.2,12-18 Approval for this study was obtained from the institutional review board of the Ludwig Maximilians University, Munich and the University of Cologne, Germany.
AAV transduction
Recombinant AAV (rAAV) vectors (AAV/mCD40L and AAV/EGFP) were produced as previously described.10 CLL cells (1.2 × 106 cells/well in 24-well plates) were cocultured with γ–irradiated CD40L-expressing mouse fibroblast L cells and were incubated in 500 μL basal Iscove medium (Biochrome, Berlin, Germany) supplemented with 10% fetal calf serum (FCS), and infectious AAV was added for 72 hours, resulting in a multiplicity of infection (MOI) between 20 and 50.19
Generation of effector T cells
CD40L-transduced, green fluorescence protein (GFP)–transduced, CD40L-stimulated or native CLL cells, which were all cultured in basal Iscove medium supplemented with 10% FCS, were used as APCs for T-cell stimulation. Autologous T cells were cultured as previously described.2 They were incubated in the presence of interleukin-2 (IL-2; 40 U/mL; Cellconcepts, Umkirch, Germany) and IL-7 (10 ng/mL; Cellconcepts). Stimulation with autologous APCs was performed on days 0, 7, 14, 21, and 28 without further adding of antigenic peptides.
ELISPOT assay
IFN-γ enzyme-linked immunospot (ELISPOT) assays were performed as described.20 On days 14 and 35, stimulator and effector cells were added in duplicate, together with the peptides (10 μg/mL) and, as indicated, a major histocompatibility complex (MHC) class 1–specific antibody (W6/32; Acris, Hiddenhausen, Germany), and incubated overnight. As stimulator cells, APCs corresponding to the APC used for T-cell culture were used. Spots were counted in a blinded manner. As a negative control, cells were incubated in the presence of DMSO, and the spots were subtracted from the experimental values to obtain the number of TAA-specific spots.
Statistical analysis
Because of nonnormality, we used the Kruskal-Wallis (more than 2 subgroups) and the Mann-Whitney U test (2 subgroups) for independent subgroups and the Wilcoxon test for dependent subgroups (version 11.5; SPSS, Chicago, IL). Statistical significance was accepted for P values below .05.
Results and discussion
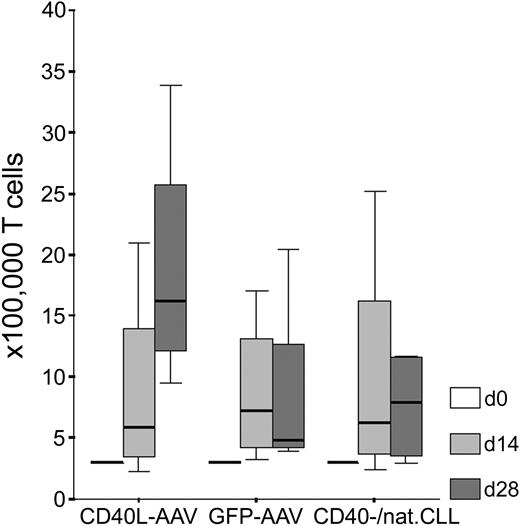
Fibromodulin, survivin, and KW-13 are known TAAs in CLL.2,3,5 MDM2 and p53 have recently been shown to serve as general TAAs in several types of malignancies, including leukemias.13-15,21-23 We wanted to test whether CD40L-transduced CLL cells are, in fact, better APCs than CD40-stimulated (CD40-CLL) or native CLL cells in terms of a higher specific recognition by autologous T cells. We generated antigen-specific T lymphocytes from 5 patients with CLL using different kinds of autologous APCs. A significantly higher number of T cells could be expanded using CD40L-transduced CLL cells as APCs compared with the control group consisting of GFP-transduced, CD40-CLL, or native CLL cells (Mann Whitney U test: z score, -2.593; P = .008). Starting with 3 × 105 T cells, we obtained a median number of 16.2 × 105 (range, 9.5-33.9 × 105) T cells with CD40L-transduced CLL cells as APCs after 28 days of in vitro culture. Using GFP-transduced CLL cells as APCs, we obtained between 3.9 and 20.5 × 105 T cells (median, 4.8 × 105 T cells), and with CD40-CLL or native CLL cells as APCs, we generated between 3.0 and 11.7 × 105 T cells (median, 7.9 × 105 T cells) after 4 weeks of in vitro culture (Figure 1). Notably, the rate of apoptotic CLL cells within these weekly restimulation assays was not different using different APCs (CD40L or GFP-transduced or CD40-CLL/native CLL cells; Kruskal-Wallis test: χ2, 0.642; P = .725) arguing that differences in T-cell proliferation are induced by different stimulatory capacities of APCs.
Proliferation of autologous T cells on stimulation with CLL cells in the presence of IL-2 and IL-7. Shown are T-cell numbers (× 100 000) after 0, 14, and 28 days of in vitro culture using CD40L-transduced (CD40L-AAV), GFP-transduced (GFP-AAV), or CD40-stimulated native CLL cells (CD40-/nat.CLL) as APCs without antigenic peptide pulsing.
Proliferation of autologous T cells on stimulation with CLL cells in the presence of IL-2 and IL-7. Shown are T-cell numbers (× 100 000) after 0, 14, and 28 days of in vitro culture using CD40L-transduced (CD40L-AAV), GFP-transduced (GFP-AAV), or CD40-stimulated native CLL cells (CD40-/nat.CLL) as APCs without antigenic peptide pulsing.
After 14 and 35 days of in vitro culture, IFN-γ ELISPOT assays were performed. We used the same kind of APCs as stimulator cells that we used for T-cell cultivation. In the assay, CLL cells were pulsed with different antigenic peptides derived from fibromodulin, survivin, KW-13, MDM2, and p53, respectively, to define the number of T cells that specifically recognize autologous CLL cells. After 14 days of in vitro culture with CD40L-transduced CLL cells, we detected 90 to 916 spots (median, 456 spots) of 100 000 T cells that were antigen specific. With APCs of the control group, we counted 0 to 584 spots (median, 258 spots) of 100 000 T cells, which was not different from results obtained with CD40L-transduced CLL cells (Mann-Whitney U test: z score, -1.339; P = .194).
After 35 days of in vitro culture with CD40L-AAV transduced CLL cells, we detected 198 to 8065 spots (median, 2280 spots) of 100 000 T cells that were antigen specific. With APCs of the control group, we determined only 0 to 1646 spots (median, 240 spots) of 100 000 T cells (GFP-AAV–transduced CLL cells: median, 485 spots [range, 0-1646 spots] of 100 000 T cells) (CD40-stimulated/native CLL cells: median, 120 spots [0-1455 spots] of 100 000 T cells). Taken together, after 35 days of in vitro culture, a significantly higher number of antigen-specific T cells was obtained using CD40L-transduced CLL cells as APCs compared with APCs from the control group, which is shown for different TAAs in CLL (Mann-Whitney U test: z score, -3.415; P < .001) (Table 1).
Higher immunogenicity of CD40L-transduced CLL cells compared with CD40-stimulated CLL cells (CD40-CLL) shown by IFN-γ ELISPOT assays
Sample, antigen specificity as tested in ELISPOT assay, and type of stimulation for T-cell expansion . | ELISPOT, d35 . |
|---|---|
| CLL-1 | |
| MDM2 | |
| CD40L-AAV | 8065 |
| GFP-AAV | 0 |
| CD40-CLL | 0 |
| p53 | |
| CD40L-AAV | 534 |
| GFP-AAV | 0 |
| CD40-CLL | 0 |
| CLL-2 | |
| Survivin | |
| CD40L-AAV | 3137 |
| GFP-AAV | 1646 |
| CD40-CLL | 1455 |
| KW-13 | |
| CD40L-AAV | 4750 |
| GFP-AAV | 984 |
| CD40-CLL | 616 |
| Fibromodulin | |
| CD40L-AAV | 4287 |
| GFP-AAV | 859 |
| CD40-CLL | 0 |
| CLL-3 | |
| MDM2 | |
| CD40L-AAV | 2280 |
| GFP-AAV | 680 |
| Native CLL | 840 |
| Fibromodulin | |
| CD40L-AAV | 1080 |
| GFP-AAV | 220 |
| Native CLL | 260 |
| p53 | |
| CD40L-AAV | 479 |
| GFP-AAV | 320 |
| Native CLL | 120 |
| CLL-4 | |
| MDM2 | |
| CD40L-AAV | 1800 |
| GFP-AAV | 485 |
| Native CLL | 0 |
| Fibromodulin | |
| CD40L-AAV | 2920 |
| GFP-AAV | 730 |
| Native CLL | 210 |
| CLL-5 | |
| MDM2 | |
| CD40L-AAV | 198 |
| GFP-AAV | 110 |
| Native CLL | 0 |
Sample, antigen specificity as tested in ELISPOT assay, and type of stimulation for T-cell expansion . | ELISPOT, d35 . |
|---|---|
| CLL-1 | |
| MDM2 | |
| CD40L-AAV | 8065 |
| GFP-AAV | 0 |
| CD40-CLL | 0 |
| p53 | |
| CD40L-AAV | 534 |
| GFP-AAV | 0 |
| CD40-CLL | 0 |
| CLL-2 | |
| Survivin | |
| CD40L-AAV | 3137 |
| GFP-AAV | 1646 |
| CD40-CLL | 1455 |
| KW-13 | |
| CD40L-AAV | 4750 |
| GFP-AAV | 984 |
| CD40-CLL | 616 |
| Fibromodulin | |
| CD40L-AAV | 4287 |
| GFP-AAV | 859 |
| CD40-CLL | 0 |
| CLL-3 | |
| MDM2 | |
| CD40L-AAV | 2280 |
| GFP-AAV | 680 |
| Native CLL | 840 |
| Fibromodulin | |
| CD40L-AAV | 1080 |
| GFP-AAV | 220 |
| Native CLL | 260 |
| p53 | |
| CD40L-AAV | 479 |
| GFP-AAV | 320 |
| Native CLL | 120 |
| CLL-4 | |
| MDM2 | |
| CD40L-AAV | 1800 |
| GFP-AAV | 485 |
| Native CLL | 0 |
| Fibromodulin | |
| CD40L-AAV | 2920 |
| GFP-AAV | 730 |
| Native CLL | 210 |
| CLL-5 | |
| MDM2 | |
| CD40L-AAV | 198 |
| GFP-AAV | 110 |
| Native CLL | 0 |
T cells from patients with CLL were expanded using autologous CD40L-transduced (CD40L-AAV), GFP-transduced (GFP-AAV), CD40-stimulated (CD40-CLL), and native CLL cells as APCs. IFN-γ ELISPOT assays were performed on day 35 (d35). Given are the numbers of TAA-specific spots per 100 000 T cells. APCs in the IFN-γ ELISPOT assay corresponded to the APCs used for the stimulation of T cells.
To test the antigen specificity, blocking experiments were performed with an MHC class 1–specific monoclonal antibody (W6/32) in 4 of 5 patients in both ELISPOT assays at day 14 and day 35. Adding the antibody resulted in a significant decrease in the number of spots detected (Wilcoxon test: day 14, z score, -2.666; P = .008; day 35, z score, -2.934; P = .003) (data not shown).
By using CD40L-transduced CLL cells as APCs, we observed a significant increase in the number of specific T cells detected in the ELISPOT assay at day 35 compared with day 14 (Wilcoxon test: z score, -2.666; P = .008), whereas APCs of the control group showed no significant increase (Wilcoxon test: z score, -1.799; P = .072). From previous studies we know that a certain threshold number of APCs must be reached to successfully expand autologous T cells in this experimental setting.2 For T-cell priming, an APC/T cell ratio of 7:1 was sufficient, which could then be decreased to a ratio of 1:1 in further T-cell stimulations. Here, in the described experiments, we used approximately half the number of APCs needed for adequate T-cell stimulation. Therefore, in most expansions using APCs of the control group, the critical threshold of activation was not reached and autologous T cells could not be further expanded later than day 21. In contrast, probably because of the activation of bystander CLL cells, the smaller number of APCs at the time of restimulation in the group with CD40L-transduced CLL cells was sufficient for T-cell expansion. We and other investigators could show that CD40L transduction of CLL cells has the unique potential to activate native bystander CLL cells regarding the expression of adhesion and costimulatory molecules, thus enhancing immunostimulatory effects, compared with CD40-activated CLL cells.8,10 In our view, this is at least one of the crucial processes that also results in a more potent antigen-specific T-cell response using CD40L-transduced CLL cells compared with CD40-CLL cells. In summary, we show that CD40L-transduced CLL cells are highly immunogenic and are able to induce an antigen-specific immune response superior to that of CD40-CLL cells and unstimulated native CLL cells. Besides a model system based on CD95-mediated apoptosis, we provide an antigen-specific, T cell–based explanation for the possible mode of action of a CD40L-based vaccine in vivo.24
Prepublished online as Blood First Edition Paper, July 12, 2005; DOI 10.1182/blood-2005-04-1742.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 455) (C.M., M.H., C.-M.W.), Wilhelm-Sander-Stiftung (95-056-2) (C.M., M.H., C.-M.W.), and Else-Kröner-Fresenius-Stiftung and Friedrich Baur-Stiftung, Germany (C.M., M.H., C.-M.W.).
C.M., M.H., and C.-M.W. were responsible for the conception and design of the presented work. C.M. performed the research. H.B., D.B., and D.M.K. contributed analytical tools. C.M. and C.-M.W. analyzed the data and wrote the paper.
D.M.K. and H.B. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal