Abstract
Divalent metal transporter 1 (DMT1) mediates apical iron uptake in duodenal enterocytes and iron transfer from the transferrin receptor endosomal cycle into the cytosol in erythroid cells. Both mk mice and Belgrade rats, which carry an identical DMT1 mutation, exhibit severe microcytic anemia at birth and defective intestinal iron use and erythroid iron use. We report the hematologic phenotype of a child, compound heterozygote for 2 DMT1 mutations, who was affected by severe anemia since birth and showed hepatic iron overload. The novel mutations were a 3-bp deletion in intron 4 (c.310-3_5del CTT) resulting in a splicing abnormality and a C>T transition at nucleotide 1246(p. R416C). A striking reduction of DMT1 protein in peripheral blood mononuclear cells was demonstrated by Western blot analysis. The proband required blood transfusions until erythropoietin treatment allowed transfusion independence when hemoglobin levels between 75 and 95 g/L (7.5 and 9.5 g/dL) were achieved. Hematologic data of this patient at birth and in the first years of life strengthen the essential role of DMT1 in erythropoiesis. The early onset of iron overload indicates that, as in animal models, DMT1 is dispensable for liver iron uptake, whereas its deficiency in the gut is likely bypassed by the up-regulation of other pathways of iron use.
Introduction
Hypochromic microcytic anemia may result from inherited defects of globin chain synthesis or, more commonly, from acquired iron deficiency, usually because of low dietary iron intake, increased requests for growth or pregnancy, malabsorption, or chronic blood loss. Genetic models of iron deficiency have been identified in animals. Autosomal recessive defects of iron use were first characterized in rodents1,2 and were shown to be caused by inactivation of the iron transporter divalent metal transporter 1 (DMT1) or Nramp2/DCT1,3 also called SLC11A2. DMT1 is a transmembrane protein expressed at the brush border of duodenal enterocytes, where it is involved in dietary nonheme iron uptake from the intestinal lumen.4 DMT1 also functions in the transferrin endosomal cycle of the erythroid precursors, where it transfers iron from the site of uptake through transferrin receptor, to cytosol, and to mitochondria for use.2 A missense mutation of the DMT1 gene that results in the substitution of arginine for glycine at position 185 of the protein (G185R) leads to severe iron deficiency at birth in mk mouse1 and in Belgrade (b) rat.2 The mutation has multiple effects on folding, glycosylation, stability, and transport activity, globally reducing the function of the DMT1 protein.5-7
Recently, the first human model of the disease was reported in a woman who had a congenital defect of the erythroid pathway of iron use with resultant iron-deficient erythrocytes, but, in contrast to animal models, had increased hepatic iron stores.8 The patient carried a homozygous mutation of the last nucleotide of DMT1 exon 12, which caused a conservative amino acid change (E399D) but exaggerated the exon 12 skipping that occurs physiologically in minimal amounts.9 In the proband, a smaller RNA lacking exon 12 was the prevalent DMT1 variant in reticulocytes and in other blood cells. In addition, DMT1 protein reduction was documented by cytofluorimetric studies in patient reticulocytes compared with healthy controls.8 Given that abnormal DMT1 protein was not identified in the proband intestinal cells,9 it was concluded that the defect was a quantitative reduction of the DMT1 RNA.
Here we report the case of a 5-year-old child who was affected from birth by severe, unexplained microcytic hypochromic anemia and who was a compound heterozygote for 2 novel DMT1 mutations. Liver iron overload was documented in early life. The severe anemia was partially responsive to recombinant erythropoietin (rEpo) treatment.
Patient, materials, and methods
Patient
The propositus was born at term by cesarean section from nonconsanguineous parents living in southern Italy. Body weight at birth was 2.530 kg. At physical examination, the newborn was extremely pale, and the spleen and liver were mildly enlarged. Anemia was severe (hemoglobin [Hb] level, 40 g/L [g/dL]) and was accompanied by remarkable microcytosis (mean corpuscular volume [MCV], 71 fL) and hypochromia (mean corpuscular hemoglobin [MCH], 13 pg). The newborn underwent transfusions of 1 U red blood cells (RBCs) at birth and a second unit 2 days later (Table 1). At the age of 2 months the patient was referred to our department for persistent anemia. He was pale and had hepatosplenomegaly. Hematologic evaluation showed RBC count, 2.9 × 109/L (2.9 × 106/μL); Hb level, 74 g/L (7.4 g/dL); hematocrit [HCT], .25 (25%); MCV, 75 fL; MCH, 14 pg; reticulocyte count, 21 × 109/L (21 × 103 /μL); platelet count, 455 × 109/L (455 × 103/μL); white blood cell (WBC) count, 6.1 × 109/L (6.1 × 103/μL); and normal differential leukocyte count. Peripheral blood smear showed extreme anisocytosis and poikilocytosis. When the patient was 3 months of age, the degree of anemia was stable, transferrin saturation reached 100%, and serum ferritin level was remarkably elevated (864 μg/L [864 ng/mL]; Table 1). Other blood tests, including total and indirect bilirubin, lactate dehydrogenase (LDH), Coomb test, B12 and folate levels, Hb electrophoresis, and karyotype, were normal. Bone marrow aspirate showed remarkable erythroid hyperplasia. Free erythrocyte protoporphyrin (FEP) level was increased (4.7 μg/g Hb; normal value, less than 3 μg/g Hb). The α/non-α-globin chain synthetic ratio of peripheral blood reticulocytes was 0.85 (normal value, 1.0 ± 0.10).
Relevant clinical and laboratory data of the proband (at different ages) and parents
. | Father, I-1 . | Mother, I-2 . | . | . | Proband, II-1 . | . | . | . | . | Normal values (range) . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 35 y | 32 y | Birth | 2 mo | 3 mo | 6 mo | 1 y | 3 y | 5 y | 2-3 y | |
| Body weight, percentile | NA | NA | < 3rd | 3rd | 5th | 10th | 15th | 15th | 25th | NA | |
| Hb, g/L | 149 | 128 | 40 | 74 | 76 | 82 | 98 | 90 | 85 | 130 (120-150) | |
| MCV, fL | 84 | 79.6 | 71 | 75 | 69 | 50 | 50 | 48 | 51 | 80 | |
| MCH, pg | 28.8 | 27 | 14 | 14 | 15 | 15.3 | 14 | 13.5 | 15 | 26 | |
| Serum ion, μM | 14.3 | 12.9 | ND | 29.7 | 28.6 | 30.4 | 26.5 | 34.7 | 36.5 | 14.3 (10.6-21.5) | |
| Transferrin saturation, % | 28 | 35 | ND | 85 | 100 | 80 | 63 | 80 | 90 | 7-30 | |
| Ferritin, μg/L | 110 | 133 | ND | 256 | 864 | 110 | 70 | 26 | 34 | 7-140 | |
| FEP, μg/g Hb | ND | ND | ND | 4.7 | ND | ND | ND | ND | 5.3 | < 3 | |
| Treatment | None | None | 18 mL PRBCs | 25 mL PRBCs | 30 mL PRBCs | sc rEpo | sc rEpo | sc rEpo | sc rEpo | NA | |
. | Father, I-1 . | Mother, I-2 . | . | . | Proband, II-1 . | . | . | . | . | Normal values (range) . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 35 y | 32 y | Birth | 2 mo | 3 mo | 6 mo | 1 y | 3 y | 5 y | 2-3 y | |
| Body weight, percentile | NA | NA | < 3rd | 3rd | 5th | 10th | 15th | 15th | 25th | NA | |
| Hb, g/L | 149 | 128 | 40 | 74 | 76 | 82 | 98 | 90 | 85 | 130 (120-150) | |
| MCV, fL | 84 | 79.6 | 71 | 75 | 69 | 50 | 50 | 48 | 51 | 80 | |
| MCH, pg | 28.8 | 27 | 14 | 14 | 15 | 15.3 | 14 | 13.5 | 15 | 26 | |
| Serum ion, μM | 14.3 | 12.9 | ND | 29.7 | 28.6 | 30.4 | 26.5 | 34.7 | 36.5 | 14.3 (10.6-21.5) | |
| Transferrin saturation, % | 28 | 35 | ND | 85 | 100 | 80 | 63 | 80 | 90 | 7-30 | |
| Ferritin, μg/L | 110 | 133 | ND | 256 | 864 | 110 | 70 | 26 | 34 | 7-140 | |
| FEP, μg/g Hb | ND | ND | ND | 4.7 | ND | ND | ND | ND | 5.3 | < 3 | |
| Treatment | None | None | 18 mL PRBCs | 25 mL PRBCs | 30 mL PRBCs | sc rEpo | sc rEpo | sc rEpo | sc rEpo | NA | |
FEP indicates free erythrocyte protoporphyrin; NA, not applicable; ND, not detected; PRBCs, packed red blood cells; sc rEpo, recombinant erythropoietin at a starting dose of 800 IU/kg subcutaneously twice a week (see “Patient” for details).
Because of the presence of mild neutropenia (0.82 × 109 neutrophils/L [820/μL]) and slight increases in liver enzyme levels (GOP, GPT, and γ-GT), screening for viral infections was carried out that revealed positive anti-CMV IgM antibodies, positive CMV polymerase chain reaction (PCR) test results, positive pp65 Ag, and antineutrophil antibodies. A cycle of ganciclovir treatment was performed. Three months later results of tests, including PCR, to detect CMV infection were negative, but the anemia did not reverse.
Two more units of blood to reverse severe anemia were transfused when the patient was 2 and 3 months of age (Table 1). At this time, rEpo administration was started at the dose of 800 IU/kg subcutaneously twice a week, according to described protocols.10 The total weekly dosage was not adjusted for weight gain and resulted in a progressive tapering of the dosage. The treatment significantly ameliorated the degree of anemia and allowed transfusion independence (Table 1). A second bone marrow aspirate at 5 months of age showed hypercellular marrow and an increased proportion of immature erythroblasts with nonspecific dysplastic changes. A few erythroblasts had poorly hemoglobinized cytoplasm and rings of coarse basophilic granules. To exclude hereditary sideroblastic anemia, Perls staining was performed of the marrow smears but yielded no evidence of ringed sideroblasts.
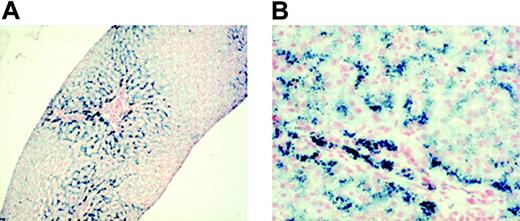
During the following years, the patient's Hb level was constantly maintained between 70 and 95 g/L (7 and 9.5 g/dL). Attempts to discontinue rEpo treatment, performed at 2 and 4 years of age, failed because the Hb level immediately dropped. When the patient was 5, his liver iron was measured noninvasively using superconductum quantum interference device (SQUID) biomagnetic susceptometry. This method quantifies the magnetic effect of the hemosiderin and ferritin iron complexes (paramagnetic properties) throughout a superconducting loop.11,12 The results are quantitatively equivalent to those obtained by chemical analysis of tissue biopsy.12 A remarkably increased liver iron concentration (LIC) (2536 ± 78 μg Fe/g hepatic wet weight) was observed that was equivalent to 14.2 mg/g liver dry weight and to a total body iron content of 150.5 mg/kg body weight.13 A 1.2-cm liver sample was obtained by needle biopsy. Six portal spaces were evaluable. Normal architecture with mild enlargement of portal spaces was observed, as were minimal focal inflammatory infiltrates. At Perl staining, severe iron overload was detected (grade 3 according to Sciot14 ), involving hepatocytes and Kupffer cells. The iron distribution was predominantly on zone 1 (Figure 1).
Family history was unremarkable. Both parents (father, I-1; mother, I-2) had normal iron parameters. I-2 had borderline low MCV values. Relevant clinical and laboratory data of the proband and parents are reported in Table 1.
Methods
After informed consent, blood was obtained for genetic analysis from the proband and his parents. Blood from healthy control subjects was obtained after informed consent, provided according to the Declaration of Helsinki. Approval for these studies was obtained from the Federico II University Medical School institutional review board.
Nucleotide sequence analysis of TFRC, GATA1, and DMT1 from genomic DNA. Anticoagulated (EDTA-treated) blood samples were obtained and stored at -20°C. DNA samples were extracted from 0.2 mL whole blood according to standard procedures.15
To screen for mutations of transferrin receptor 1 gene (TFRC) for the patient and his parents, each of the 18 exons with exon-intron boundaries was amplified by PCR using specific primers. PCR fragments were sequenced directly. A similar approach was used to analyze GATA-1: all coding exons, including splice junctions, and portions of the promoter region were amplified by PCR, and amplified fragments were directly sequenced. The GATA-1 and TFRC cDNA sequences from GenBank accession numbers NM_002049 and M_003234, respectively, were used as reference sequences.
Micrographs of liver biopsy sample showing the entity and the distribution of iron. Staining technique: Perl Prussian blue. (A) The liver has normal architecture. Iron is heavily distributed in zone 1, whereas zone 2 appears less loaded. Original magnification, × 25. (B) Most hepatocytes were massively loaded by hemosiderin (Sciot grading 3) and by Kupffer cells to a minor extent (Sciot grading 2). Original magnification, × 200. Images were observed using an Axioplan 2 microscope (Zeiss, Göttingen, Germany) and Plan-Apochromat 4×/0.10 numeric aperture (NA) and 20×/0.40 NA objectives. Images were captured using a Nikon Coolpix 950 (Nikon, Melville, NY), and processed with ACDsee version 6.0 software (ACD Systems, Saanichton, BC, Canada).
Micrographs of liver biopsy sample showing the entity and the distribution of iron. Staining technique: Perl Prussian blue. (A) The liver has normal architecture. Iron is heavily distributed in zone 1, whereas zone 2 appears less loaded. Original magnification, × 25. (B) Most hepatocytes were massively loaded by hemosiderin (Sciot grading 3) and by Kupffer cells to a minor extent (Sciot grading 2). Original magnification, × 200. Images were observed using an Axioplan 2 microscope (Zeiss, Göttingen, Germany) and Plan-Apochromat 4×/0.10 numeric aperture (NA) and 20×/0.40 NA objectives. Images were captured using a Nikon Coolpix 950 (Nikon, Melville, NY), and processed with ACDsee version 6.0 software (ACD Systems, Saanichton, BC, Canada).
To analyze DMT1, all 17 exons and the flanking intronic sequences were amplified by PCR using specific primers. Detailed protocols and primer sequences are available on request. The amplified products were isolated by electrophoresis on 1% agarose gel and purified using the QIAamp purification kit (Qiagen, Valencia, CA). Direct sequencing was performed using a fluorescence-tagged dideoxy chain terminator method in an ABI 310 automated sequencer (Applied Biosystem, Foster City, CA), according to the manufacturer's instructions. The DMT1 cDNA sequence from GenBank accession number NM_000617 was used as a reference sequence, where the A of the ATG translation initiation start site represents nucleotide +1.
DNA samples from 50 healthy white controls (100 chromosomes) were investigated for the identified DMT1 mutations using suitable restriction enzymes. Digestion of the appropriate PCR products by endonucleases MboII and HinfI (New England Biolabs, Beverly, MA) was conducted according to the manufacturer's instructions. Cleavage products were evaluated on 2% agarose gels.
Reverse transcription-PCR amplification of DMT1. Total RNA was prepared from the whole blood of healthy controls, the patient, and his parents using the blood RNA extraction kit (PreAnalitix; Qiagen) and following the manufacturer's instructions. Total RNA was reverse transcribed in a 20-μL reaction using Superscript III reverse transcriptase (Invitrogen, Life Technologies, Carlsbad, CA) and specific DMT1 reverse primer 5′-CCTAAGCCTGATAGAGCTAG-3′. Amplification of DMT1 cDNA was performed using 3 sets of primers encompassing specific coding exons. Segment 1 (exons 4-6) forward primer, 5′-CACCGGACCAGGTTTTCTTA-3′; reverse primer, 5′-GATAGCAATGGCTGAGCC-3′; segment 2 (exons 12-14) forward primer, 5′-TCATGGAGGGATTCCTGAAC-3′; reverse primer, 5′-ATGAGAGCAAAGGGAAGCTG-3′. Segment 3 (exons 9-13) was studied to evaluate exon 12 skipping: forward primer, 5′-CAGGTACTCAAGGGCATGTT 3′; reverse primer, 5′-GTTCAGGAATCCCTCCATGA-3′. Two microliters cDNA was used in each PCR reaction, 1 × PCR buffer, 2 mM dNTPs, and 2 U to 5 UTaq polymerase in a 50-μL reaction mixture. After initial denaturation at 95°C for 5 minutes, amplification was performed for 30 cycles at 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, then at 72°C for 10 minutes. PCR products were analyzed on 1% agarose gels then purified on a QIAquick PCR purification column (Qiagen). Sequencing was performed as described.
PBMC total protein lysate. Isolation of peripheral blood mononuclear cells (PBMCs) from the patient, his parents, and a healthy control was performed by density gradient centrifugation from heparinized blood. Total proteins were extracted by using a lysis buffer containing 10 mM Tris-HCl buffer (pH 7.5) and protease inhibitors (PIs; 2 μg/mL leupeptin, 2 μg/mL aprotinin, 1 μg/mL pepstatin, 100 μg/mL phenylmethylsulfonyl fluoride [PMSF], and 2 mM EDTA). All procedures were carried out at 4°C. Samples were stored frozen at -80°C. Protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA).
Immunoblotting. For DMT1 detection, 80 μg total protein lysate was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% polyacrylamide) and was transferred by electroblotting to polyvinylidene fluoride membranes. Because heat treatment of DMT1-containing samples was found to cause aggregation of the protein, samples were incubated for 30 minutes at room temperature in Laemmli buffer (with occasional vortexing) before SDS-PAGE. Loading and transfer of proteins were verified by staining the blots with Ponceau S (Sigma, St Louis, MO). Immunoblots were preincubated with blocking solution (0.02% Tween 20, 7% skim milk in PBS) for 3 hours at 20°C before incubation with primary antibodies for 16 hours at 4°C in blocking solution. For immunoblotting, the antibodies used were rabbit anti-DMT1 NT (1/200; a generous gift of Dr Philippe Gros, McGill University, Montreal, QC, Canada) and rabbit anti-β-actin (1/2000). After incubation with primary antibody, the blots were washed with TTBS and incubated with alkaline phosphatase-labeled antirabbit secondary antibody (1/2000) at room temperature for 90 minutes. Immunodetection was obtained using enhanced chemiluminescence reagents (Cell Signaling Technology; New England Biolabs). Densitometric analysis was performed using the Quantitative One 4.5.0. program (Bio-Rad).
Results
We identified an infant from nonconsanguineous parents who came to medical attention at birth because of severe hypochromic microcytic anemia. β-Thalassemia was not consistent with his presentation at birth or with the hemoglobin electrophoretic patterns of his parents. Globin chain synthesis suggested a mild α-thalassemia phenotype. However, the hypothesis of an α-thalassemia condition as the cause of the severe anemia was ruled out because no Hb H or Hb Bart was found at Hb electrophoresis (data not shown). In addition, analysis of the α-globin genes by means of Southern blot and sequencing of the entire α cluster showed normal findings. Sideroblastic anemia was excluded by bone marrow examination. Because of the patient's severe poikilocytosis, we considered the possibility of hereditary pyropoikilocytosis (HPP), but peripheral blood smears of the parents showed no evidence of elliptocytosis, and spectrin dimer percentages were normal (data not shown).
The proband's hematologic data suggested a condition related to an abnormality of iron metabolism, mimicking iron deficiency (hypochromia and FEP increase). However, serum iron, transferrin saturation, and serum ferritin levels were increased before treatment with rEpo. No mutations were found in the HFE gene. Altogether, the results suggested a defect of iron use.
Genomic analysis
In search of rare causes of microcytosis, we sequenced TFRC and GATA1 genes without finding mutations in either. Then we screened the DMT1 coding sequence and exon-intron junctions for mutations by direct sequencing.
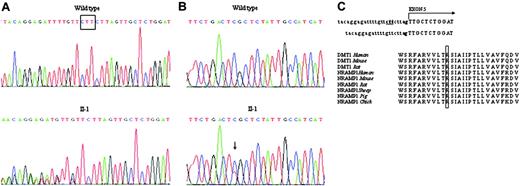
Two novel mutations were identified in the proband, both at the heterozygous state. The first was a 3-bp deletion in intron 4, c.310-3_5del CTT, that resulted in a splicing abnormality (Figure 2A). The second was a C>T transition at nucleotide 1246 in exon 13 (Figure 2B).
The 3-nucleotide deletion alters the acceptor site of intron 4, affecting the splicing process. This mutation was inherited by I-1. Mutant c.310-3_5del CTT abolishes a cleavage site for MboII endonuclease. We exploited this approach to investigate the sequence at this position in healthy controls. PCR products corresponding to intron 4-intron 5 of genomic DNA of family members and healthy controls were digested with MboII endonuclease. Wild-type PCR fragments (243 bp) generated 2 smaller fragments of 63 and 180 bp that were present in all 100 control chromosomes, excluding the presence of the 3-bp deletion.
c.1246C>T changes the CGC codon to TGC, causing the substitution of arginine 416 with cysteine (p. R416C) in the protein. This mutation was inherited from I-2. c.1246C>T mutation also abolishes a HinfI cleavage site. Restriction analysis performed using HinfI endonuclease on amplified exon 13 from family members and 50 controls revealed the absence of the site in I-2 and in the proband at the heterozygous state and the presence of the normal HinfI site in all control chromosomes (not shown). In addition, by using PROGRAMM blastn-SNP (http://www-btls.jst.go.jp/cgi-bin/Homology_Blast-SNP/submission_v3.cgi?PROGRAM=blastn-SNP), we excluded that this nucleotide change corresponded to a previously identified SNP.
The p. R416C mutation affects with a nonconservative substitution a residue that is highly conserved across species and in a DMT1 (or Nramp2) homologous protein, Nramp1 (Figure 2C). The mutation is localized in one of the putative DMT1 transmembrane domains (VVLTRSIAIIPTLLVAV), as identified by PSORT II analysis (K. Nakai, http://psort.nibb.ac.jp).
Identification of the DMT1 mutations in the proband. (A) Partial sequence of intron 4-exon 5 junction of the proband and wild-type (WT) DNA identifying the c.310-3_5del CTT mutation (underlined). The abnormality in the consensus sequence of the acceptor splice site is shown on the right. The dinucleotide acceptor site is in bold. (B) Sequence analysis of the DNA region encompassing exon 13 showing the heterozygous C>T transition at position 1246, causing a p. R416C mutation. (C) ClustalW alignment of the amino acid sequences of NRAMP2 (DMT1) and NRAMP1 orthologs from different species, showing complete conservativeness of the R416 residue (boxed).
Identification of the DMT1 mutations in the proband. (A) Partial sequence of intron 4-exon 5 junction of the proband and wild-type (WT) DNA identifying the c.310-3_5del CTT mutation (underlined). The abnormality in the consensus sequence of the acceptor splice site is shown on the right. The dinucleotide acceptor site is in bold. (B) Sequence analysis of the DNA region encompassing exon 13 showing the heterozygous C>T transition at position 1246, causing a p. R416C mutation. (C) ClustalW alignment of the amino acid sequences of NRAMP2 (DMT1) and NRAMP1 orthologs from different species, showing complete conservativeness of the R416 residue (boxed).
Analysis of DMT1 mRNA
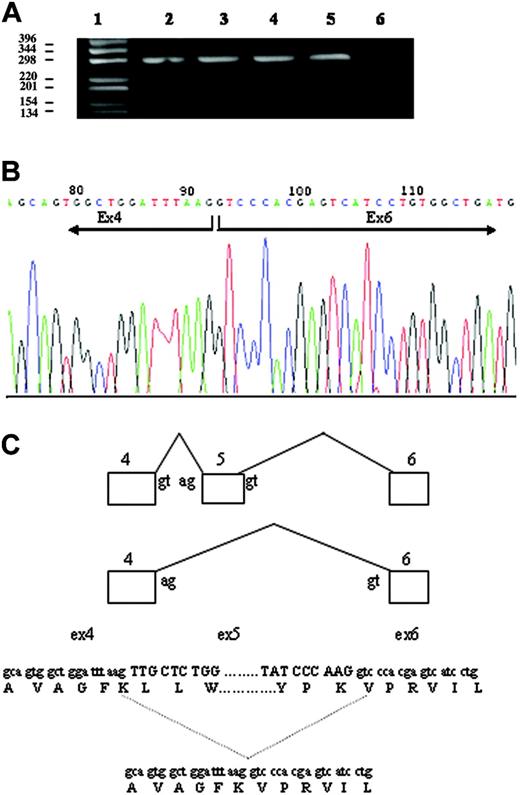
To determine the effect of both mutations, DMT1 cDNA was analyzed in the blood of the patient and his parents. Specific cDNA fragments that spanned regions encompassing the mutations (exons 4-6 and 12-14) were amplified by PCR, and the fragments were directly sequenced. Amplification of the exon 4-6 region of DMT1 from normal whole blood mRNA produced a single transcript of the expected size (298 bp). By contrast, 2 transcripts were obtained from cDNA of the patient and I-1, one corresponding to the expected size segment and a shorter one of approximately 180 bp (Figure 3A). Quantification of the 2 bands indicated that the smaller band, lacking exon 5, was approximately 30% to 35% of the total in both cases. Nucleotide sequencing showed that the 298-bp band contained the normal, full-size message, whereas the smaller band corresponded to a transcript with deleted exon 5. The improperly spliced RNA lacking exon 5 retained the correct reading frame, but the encoded protein lacked 40 amino acids (Figure 3B). The deleted region included a putative transmembrane domain, FKLLWILLLATLVGLLL, predicting a defect in the correct insertion of the protein into the plasma membrane or a defective iron transport. Sequencing of the amplified fragments corresponding to exons 12-14 confirmed the presence of the heterozygous c.1246C>T mutation in exon 13 in the proband and in I-2.
Given that exon 12 skipping had been previously observed in minimal amounts in hematopoietic cells of healthy subjects and was reported to be exaggerated in a patient with DMT1 homozygous mutation,9 we studied exon 12 skipping in all family members. Through study of the exon 9-13 regions of DMT1 mRNA, we demonstrated the presence of 2 transcripts whose sizes corresponded to variants with or without exon 12 in all family members. The smaller product was present in a low proportion (less than 10%), as described for healthy controls.
Protein expression
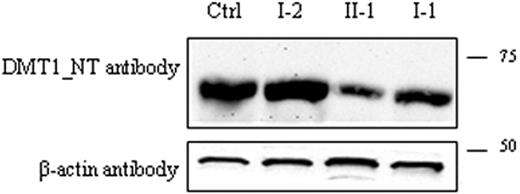
To evaluate the effect of the DMT1 mutations identified, DMT1 protein expression was then investigated in the PBMCs of the patient, his parents, and a healthy control. Immunoblots were analyzed using the anti-DMT1-NT antibody. A single band of approximately 65 kDa was present in all samples, and its expression was decreased in the proband (approximately 40% of the total) and in I-1. On the contrary, DMT1 protein in I-2 was similar to that observed in controls. These findings confirm that the paternal allele caused a quantitative defect of DMT1 protein, whereas the maternal allele with a missense mutation had no effect on the protein amount.
Analysis of the effect of c.310-3_5del CTT deletion. (A) Amplification of the exon 4-6 region of DMT1 mRNA from whole blood. cDNA was synthesized as described in “Patient, materials, and methods.” The exon 4-6 region of DMT1 cDNA was amplified by PCR, and the product was separated on 1% agarose gel. (lane 1) 1-kb DNA ladder. (lane 2) I-1. (lane 3) Proband. (lane 4) I-2. (lane 5) Healthy control. (lane 6) No template control. The top band measures 298 bp, and the bottom band in lanes 2 and 3 measures 178 bp. (B) Sequencing of PCR product of the 178-bp band (A) of II-1, resulting in exon 5 skipping and a 40 amino acid loss in the protein. (C) Schematic representation of DMT1 exon 5 skipping in the proband.
Analysis of the effect of c.310-3_5del CTT deletion. (A) Amplification of the exon 4-6 region of DMT1 mRNA from whole blood. cDNA was synthesized as described in “Patient, materials, and methods.” The exon 4-6 region of DMT1 cDNA was amplified by PCR, and the product was separated on 1% agarose gel. (lane 1) 1-kb DNA ladder. (lane 2) I-1. (lane 3) Proband. (lane 4) I-2. (lane 5) Healthy control. (lane 6) No template control. The top band measures 298 bp, and the bottom band in lanes 2 and 3 measures 178 bp. (B) Sequencing of PCR product of the 178-bp band (A) of II-1, resulting in exon 5 skipping and a 40 amino acid loss in the protein. (C) Schematic representation of DMT1 exon 5 skipping in the proband.
Detection of DMT1 protein in PBMCs. Total PBMC extracts were prepared as described: 80 μg total protein was loaded into each lane and was separated by SDS-PAGE on a 10% acrylamide gel followed by transfer to PVDF membranes. Membranes were incubated with anti-DMT1-NT antibody (A) and with β-actin antibody as an internal loading control (B). Samples in the different lanes are indicated. Sizes (in kDa) are on the right.
Detection of DMT1 protein in PBMCs. Total PBMC extracts were prepared as described: 80 μg total protein was loaded into each lane and was separated by SDS-PAGE on a 10% acrylamide gel followed by transfer to PVDF membranes. Membranes were incubated with anti-DMT1-NT antibody (A) and with β-actin antibody as an internal loading control (B). Samples in the different lanes are indicated. Sizes (in kDa) are on the right.
Discussion
Here we report the case of a child affected by compound heterozygosity for 2 different DMT1 mutations. He had severe microcytic-hypochromic anemia from birth. The diagnosis was reached after common causes of microcytosis were excluded. In animal models, heterozygosity for TFRC deletion may cause iron deficiency.16 Therefore, we sequenced the patient's TFRC gene but did not find mutations of the coding regions or exon-intron junctions. We also sequenced GATA1 because mutations of this gene were reported to cause microcytic anemia in males17 ; however, causal mutations again were not detected. The α-thalassemia-like picture and the high levels of FEP might have been induced by iron deficiency. However, high serum iron, transferrin saturation, and serum ferritin levels attested to a disorder of iron use.
Sequence analysis of DMT1 of the proband revealed compound heterozygosity for 2 novel mutations, one affecting the process of RNA maturation and the other introducing a nonconservative amino acid substitution in a putative transmembrane domain. The effect of the mutation in the consensus sequence of the acceptor splice site of intron 4 on the splicing process was demonstrated by the finding of consistent amounts of abnormal transcripts with deleted exon 5 in DMT1 cDNA from the blood cells of II-1 and I-1 (Figure 3).
Skipping of exons 10 and 12 may occur during DMT1 mRNA maturation.18 However, skipping of exon 5 as a physiologic process has never been reported. The predicted protein, lacking 40 amino acids of the first putative transmembrane domain, might be destabilized, nonfunctional, or degraded. A protein reduction was confirmed by immunoblotting studies in both carriers of the mutation (I-1 and II-1; Figure 4). As shown by the normal hematologic and iron parameters of I-1, the mutation was well tolerated at the heterozygous state, likely because a sufficient amount of DMT1 is provided by the normal trans allele.
The maternal mutation (p. R416C) predicted a nonconservative amino acid change in exon 13. In the absence of functional data, it is difficult to assess whether p. R416C represented a causal mutation. Studies in healthy controls and database searches ruled out a common polymorphic change. It is likely that residue 416 is relevant for the protein function because it is highly conserved across species and is localized in a putative (the 7th) transmembrane domain. In addition, the G185R mutation identified in mk mouse and b rat occurs in a predicted transmembrane domain (the 4th). Recently, in cell lines stably transfected with G185R DMT1, it was shown that only a proportion of the abnormal protein reaches the membrane and the endosomal compartment, whereas a large fraction is retained in the endoplasmic reticulum and is rapidly degraded by a proteasome-dependent mechanism.5 Low levels of exon 12 skipping were reported physiologically in blood cells,18 and exaggerated skipping of exon 12 was interpreted as causal in an E399D DMT1 mutation,9 reducing full-size RNA and producing a shorter protein lacking transmembrane domain 8 and unable to uptake iron.19 In our study dealing with different mutations, a transcript lacking exon 12 was present in minimal amounts in all family members, indicating that the missense p. R416C mutation does not interfere with the correct RNA processing. In addition, ESE analysis20 (http://genes.mit.edu/burgelab/rescue-ese/) excluded an enhancer role for the sequence encompassing the R416C mutation. In addition, the amount of the protein of I-2, a heterozygous carrier of the p. R416C mutation, was not reduced in PBMC Western blot analysis. I-2 had a slightly decreased MCV but did not have anemia or abnormalities of iron parameters.
The severity of the phenotype of the proband at birth is reminiscent of that observed in animal models and is consistent with a fundamental role for DMT1 in erythroid cell production during fetal life. A mouse with complete inactivation of DMT1 (DMT1-/-) was recently reported whose phenotype at birth was more severe than that of the mk mouse.21 The analysis of mice with selective DMT1 inactivation in the gut, bone marrow cells, and liver reinforced its essential role for intestinal absorption and erythroid use, but not for liver iron uptake, because hepatocyte iron storage was incompletely interrupted.21 The history of II-1 strengthens the DMT1 role in erythroid cells but clearly indicates that DMT1 is not essential for liver iron uptake in humans.
The patient's iron stores were elevated early in life, concomitant with severe anemia, as shown by ferritin levels. During follow-up, a decreased ferritin level was recorded after rEpo treatment and increased Hb level, likely reflecting iron use. Intriguingly, levels of serum ferritin were disproportionately low in the face of severe iron burden, as demonstrated through SQUID and at liver biopsy. The high liver iron content relative to the patient's age and the estimated high total body iron content13 were disproportionate to the iron received from transfusions or redistributed from the bone marrow, but it required a significant increase of intestinal absorption. We did not measure DMT1 protein levels in the gut; hence, in principle, sufficient functional DMT1 could be not rate limiting for basal iron use at this level. However, increased iron use might occur through DMT1-independent pathways, possibly involving the up-regulation of an iron-heme pathway of absorption.8
The proband's severe anemia required blood transfusions until rEpo treatment resulted in a hemoglobin level compatible with growth and development, as demonstrated by weight and height growth curves (Table 1 and data not shown). Spontaneous improvement in the degree of anemia was unlikely because attempts to reduce rEpo administration resulted in rapid worsening of anemia. The rEpo response of our patient was in agreement with what was observed in vitro by Priwizerova et al.8 The number and size of the erythroid colonies of their patient were significantly increased when grown in the presence of the Fe-SH iron donor chelate or high Epo levels.22 Epo might have a nonspecific effect on erythropoiesis, decreasing apoptosis of the erythroid precursors or of a specific one and inducing a DMT1-independent pathway of iron use. On the basis of the observation that the loss of TFRC produces more severe effects than the loss of DMT1, transporters other than DMT1 in the endosomal cycle have been hypothesized in animals.21 Because no variations were observed in MCV and MCH of the proband after partial correction of the anemia by Epo (Table 1), we favor the interpretation that Epo ameliorates total erythropoiesis. Because of the high iron content at liver biopsy and SQUID, iron chelation by deferoxamine was prescribed. The simultaneous administration of Epo and deferoxamine, apparently paradoxical, is justified by the dual nature of disorder, with features of both anemia and iron overload. However, this treatment requires careful monitoring of iron parameters and periodic noninvasive assessment of liver iron levels.
When DMT1 was initially identified as the gene encoding an intestinal iron transporter, it was predicted that a subset of human patients with congenital anemia might harbor mutations of it.1 The possibility of DMT1 defects, though rare, should be considered in patients with microcytosis, especially at birth, once thalassemia and HPP are ruled out or after the exclusion of sideroblastic anemia in patients with atypical microcytic anemia and elevated iron parameters.
Prepublished online as Blood First Edition Paper, September 13, 2005; DOI 10.1182/blood-2005-06-2477.
Supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC), Telethon Foundation Italy (project GP0202Y02 [A.I.] and grant GGP05024 [C.C.]), Fondo per gli Investimenti per la Ricerca di Base (FIRB), and Programmi di Ricerca di rilevante Interesse Nazionale (PRIN) 2004 of the Italian Ministry of University and Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Philippe Gros for the generous gift of the anti-DMT1 antibody. We thank Renzo Galanello for performing Southern blot analysis and sequencing the α-globin genes and Mario Cazzola for performing Perls reaction on the bone marrow of the proband. Furthermore, we thank Arturo Romondia for his clinical advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal