Comment on Zimring et al, page 187
A mouse model is used to show that RBC surface proteins recognized as foreign can trigger recipient CD8+ T-cell responses even in the absence of donor leukocytes.
Blood transfusion is not an immunologically inert event. In a relatively short time frame, the recipient is exposed to a large quantity of polymorphic antigens, and antigen-specific immune responses can and do develop. The most well-recognized of these is the development of alloantibodies against polymorphic epitopes located on red blood cell (RBC) surface proteins. Alloantibodies are responsible for delayed hemolytic transfusion reactions, but when alloantibodies are detected, clinical problems are, for the most part, fairly easily avoided through the selection of appropriate, cross-match compatible units.
A less well appreciated immunologic effect of blood transfusion is the development of cell-mediated immunity and the induction of CD8+ cytotoxic T lymphocytes (CTLs) with specificity against polymorphic peptides derived from allelic variants of normal proteins, also known as minor histocompatibility antigens (MiHAs). This type of immune response may contribute to the unusually high rates of rejection of allogeneic bone marrow observed among patients with sickle cell disease who undergo HLA-matched bone marrow transplantation (BMT).1 Such patients typically have received previously numerous blood transfusions, and may therefore have developed CTLs with specificity to MiHAs.
The mechanisms involved in the development of anti-MiHA CTLs following transfusion are not well understood, but it has been generally assumed that donor white cells are important. This belief stems in part from the demonstrated beneficial effects of leukoreduction in preventing humoral alloimmune responses in other transfusion situations,2 as well as from the ameliorating effects of leukoreduction in transfusion-associated BMT graft rejection in experimental dog models.3
The current study by Zimring and colleagues challenges the assumption that a CD8+ T-cell response to RBC-associated MiHAs cannot occur in the absence of donor leukocytes. The authors use a well-characterized mouse model in which both the antigen and the responding T cells are known in advance. The authors prepared highly leuko-reduced mouse RBCs and then chemically modified them to express on their cell surface a xenoantigen—chick ovalbumin (OVA). These modified RBCs (now expressing a “MiHA” in the form of the cross-linked OVA) were transfused into mice that had been preloaded with CD8+ T cells capable of recognizing a peptide from OVA. In response, this T-cell population greatly expanded. The transfusion of RBCs modified with an irrelevant antigen had no such effect; neither did the transfusion of soluble OVA. Together, the results suggest that antigen-specific CD8+ T cells in the transfusion recipient can respond to a “polymorphic protein” present on the surface of RBCs. Furthermore, the data imply that the RBC MiHA protein gains access to the recipient's own antigen presentation machinery through cross-priming, a mechanism by which exogenous protein may be delivered to the class I presentation pathway.4
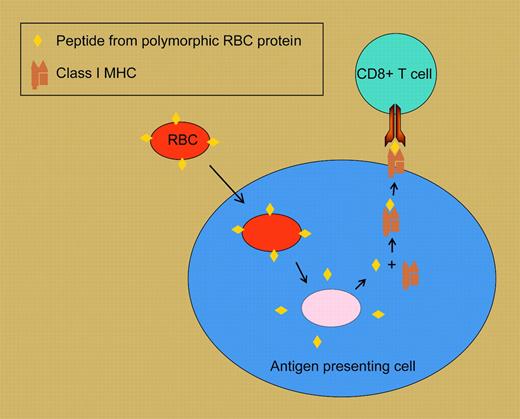
Cross-priming of CD8+ T-cell responses to RBC surface antigens. In this highly schematized model, a tranfused RBC expressing a polymorphic variant of a cell-surface protein is taken up by a recipient antigen presenting cell. Peptides derived from RBC proteins are loaded onto class I MHC molecules, then shuttled to the cell surface, where they can stimulate responses in recipient cognate CD8+ T cells.
Cross-priming of CD8+ T-cell responses to RBC surface antigens. In this highly schematized model, a tranfused RBC expressing a polymorphic variant of a cell-surface protein is taken up by a recipient antigen presenting cell. Peptides derived from RBC proteins are loaded onto class I MHC molecules, then shuttled to the cell surface, where they can stimulate responses in recipient cognate CD8+ T cells.
These results need to be confirmed in other systems: for example, using mice that have not been preloaded with antigen-specific T cells, to see if leuko-reduced RBCs bearing an alloantigen can initiate a CD8+ T-cell response de novo. In addition, it's not clear which particular RBC alloantigens may be relevant in BMT graft rejection. These notwithstanding, the findings have implications for allogeneic BMT in the treatment of hemoglobinopathies, typically associated with heavy transfusion use. That is, the use of leuko-reduced blood may be relatively ineffective in preventing the development of anti-MiHA CTLs and reducing the frequency of subsequent BMT graft rejection. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal