The chemokine receptor CXCR3 is predominantly expressed on activated T and natural killer (NK) cells. CXCR3 and its ligands, CXCL11, CXCL10, and CXCL9, play a major role in T-helper 1 (Th1)–dependent inflammatory responses. CXCL11 is the most dominant physiological inducer of adhesion, migration, and internalization of CXCR3. To study the role of CXCR3 carboxyl-terminus and the third intracellular (3i) loop in chemokine-mediated migration, adhesion, and CXCR3 internalization, we generated CXCR3 receptors mutated in their distal (Ser-Thr domain) or proximal (trileucine domain) membrane carboxyl terminus, and/or the third intracellular loop. We found that migration of CXCR3-expressing HEK 293 cells toward CXCL11 was pertussis toxin–dependent and required the membrane proximal carboxyl terminus of CXCR3. Internalization induced by CXCL11 and protein kinase C (PKC) activation was also regulated by the membrane proximal carboxyl terminus; however, only CXCL11-induced internalization required the LLL motif of this region. Internalization and Ca2+ flux induced by CXCL11 were independent of the 3i loop S245, whereas migration at high CXCL11 concentrations, integrin-dependent adhesion, and actin polymerization were S245 dependent. Our findings indicate that CXCL11-dependent CXCR3 internalization and cell migration are regulated by the CXCR3 membrane proximal carboxyl terminus, whereas adhesion is regulated by the 3i loop S245. Thus, distinct conformational changes induced by a given CXCR3 ligand trigger different downstream effectors of adhesion, motility, and CXCR3 desensitization.
Introduction
Chemokine receptors (CKRs) are expressed widely on leukocytes, and have been implicated in their chemotactic recruitment to sites of inflammation.1 CXCR3 is an inflammatory, inducible type of chemokine receptor. It is expressed on activated effector and memory T cells, on the T helper (Th) subset, preferentially of the Th1 phenotype, on the subset of B cells, and on natural killer (NK) cells.2,3 CXCR3 was also shown to be functionally expressed on endothelial cells and on some tumors such as human melanoma.4,5 CXCR3 and its ligands, the CXC chemokines CXCL11 (I-TAC), CXCL10 (IP-10), and CXCL9 (Mig), play a role in Th1-dependent inflammatory responses, among them rheumatoid arthritis,6 multiple sclerosis,7,8 graft rejection,9,10 and hepatitis C.11,12
Binding of chemokines to CXCR3 induces cellular responses that are involved in leukocyte trafficking, most notably integrin activation, cytoskeletal changes, and chemotactic migration.13-15 CXCR3 activates multiple signaling pathways, including the Ras/ERK, Src, and PI3K pathways.3 The exposure of CKRs to high concentrations of chemokines often results in the rapid attenuation of receptor responsiveness and reduced biological response. This process, called “desensitization,” is the consequence of a combination of different mechanisms. These mechanisms include receptor phosphorylation followed by uncoupling of the receptor from heterotrimeric G proteins and internalization of cell-surface receptors to intracellular endocytic vesicles.16 For most G protein–coupled receptors (GPCRs), including CKRs, agonist-induced phosphorylation occurs at the serine and threonine residues of the carboxyl terminus domain and/or the third intracellular (3i) loop. Internalization of both CCR5 and CXCR4 appears to require the serine/threonine-rich C tail. A highly conserved carboxyl-terminal, the Leu-Leu motif, has also been demonstrated to involve the endocytosis of various membrane proteins, including G protein–coupled b2-AR.17 Dileucine or Ile-Leu, present as the LLKIL or LKIL motif in CXCR2 and CXCR4, is also involved in the internalization of these receptors.18,19 In contrast to CXCR1, 2, and 4, it was recently suggested that the C terminal domain of human CXCR3 is not essential for CXCL11-dependent internalization of human CXCR3 overexpressed in murine 300-19 pre-B cells, and that the third extracellular domain of CXCR3 in these cells is predominantly involved in this process. Moreover, it was also shown that migration of murine 300-19 pre-B cells used in this study was dependent on the C terminal domain of CXCR3.20
Our findings indicate that CXCL11-dependent internalization of CXCR3 is regulated by the membrane proximal carboxyl terminus and the LLL motif. The migration of cells in response to CXCL11 was also dependent on the membrane proximal carboxyl terminus domain, whereas adhesion was regulated by the 3i loop S245, most likely through regulation of actin polymerization.
Materials and methods
Materials
Human recombinant CXCL9 and CXCL10 were purchased from R&D Systems (Minneapolis, MN) and CXCL11 from PeproTech (Rocky Hills, NJ). PMA was obtained from Sigma (St Louis, MO). Protein kinase C (PKC) inhibitors staurosporin and GF 109203X (GF) were obtained from Sigma, and rottlerin was obtained from Biomol Research Laboratories (Plymouth Meeting, PA). Pertussis toxin (PTx) was purchased from List Biological Laboratories (Campbell, CA). The recombinant protein human soluble vascular cell adhesion molecule-1 (sVCAM-1) was a generous gift from Dr R. Lobb (Biogen, Cambridge, MA). Human serum albumin (HSA; fraction V) was from Merck Bioscience (Schwalbech, Germany).
Human cell lines
Human embryonal kidney 293 (HEK 293) cells were maintained in Dulbecco modified Eagle medium (DMEM), whereas YTS, Jurkat, and RBL cells were maintained in RPMI (Gibco Laboratories, Grand Island, NY), both supplemented with 10% fetal bovine serum (FBS), 1 mM l-glutamine, 100 U/mL penicillin, and 0.01 mg/mL streptomycin (all from Biological Industries, Kibbutz Beth Haemek, Israel) (full medium) at 37°C in 5% CO2 atmosphere. HEK 293 stable transfectant cells were maintained in 450 to 700 mg/mL G418 (Calbiochem, San Diego, CA).
Plasmid construction and stable transfectants
Human CXCR3 cDNA in pcDNA3-CXCR3 was kindly donated by B. Moser (University of Bern, Switzerland). The cDNAs of the COOH terminally truncated CXCR3 were amplified by the standard polymerase chain reaction (PCR) method. CXCR3 cDNA, EcoRI-XbaI gel-purified fragment of pcDNA3-CXCR3 served as a template. A common 5′ primer containing the BamHI site and 3′ mutagenic primers containing the EcoRI site were used to generate the truncations 349stopΔ20, 332stopΔ37, and 327stopΔ42 by altering the codons 349, 332, or 327, respectively, into a stop codon. The 5′ primer was 5′-GTTAGGATCCAGCCAGAGCACCAGCCC-3′ and the 3′ mutagenic primers were: 349stopΔ20 5′-CATGAATTCTCATGGCTGCCTCTGGAGCC-3′, 332stopΔ37 5′-CATGAATTCTCACATCCACATCCGCTCCCG-3′, and 327stopΔ42 5′-CATGAATTCCTACCGGAACTTGACCCCTACAAA-3′ (stop codons are underlined, restriction sites are in italics). The PCR products were 5′ and 3′ terminally digested with BamHI and EcoRI, and ligated with pcDNA3 cut with the same enzymes. Integrity of CXCR3 was confirmed by sequencing. CXCR3 332-334L→A and S245A site-directed mutagenesis were generated by PCR using the QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) on pcDNA3-CXCR3 as a template. The mutagenic complimentary primers spanning the LLL motif at CXCR3 c-tail were: sense primer 5′-AAGTTCCGGGAGCGGATGTGGATGGCAGCTGCACGCCTGGGCTGCCCCAACCAGAGA-3′ and antisense primer 5′-TCTCTGGTTGGGGCAGCCCAGGCGTGCAGCTGCCATCCACATCCGCTCCCGGAACTT-3′. The mutagenic complimentary primers spanning 3i loop S245 were: sense primer 5′-CGTGCTGCTGGTTGCAAGGGGCCAGCGGC-3′ and antisense primer 5′-GCCGCTGGCCCCTTGCAACCAGCAGCACG-3′ (Nucleotides encoding for alanine are in bold). The PCR mix was preheated at 95°C for 10 minutes, and then PFU polymerase was added followed by 18 cycles at 95°C for 30 seconds, 55°C for 1 minute, and 68°C for 14.5 minutes. The PCR product, which was a reconstitution of the whole plasmid containing mutated CXCR3, was then transformed into bacteria. Positive colonies were screened by sequencing of CXCR3. All transfections of HEK 293 cells were carried out by FueGene 6 (Roche, Indianapolis, IN). Stable colonies derived by selection with G418 were screened for CXCR3 cell-surface expression by immunofluorescence flow cytometry.
Flow cytometric analysis
Cells (2-4 × 105) were resuspended in 0.2 mL fluorescence-activated cell-sorting (FACS) buffer (phosphate-buffered saline [PBS], 0.1% bovine serum albumin [BSA; Biological Industries], and 0.01% NaN3) and incubated with 1% BSA for 15 minutes on ice. Then, blocked cells were mixed with either PE-conjugated human-specific CXCR3 49801.111 monoclonal antibody (mAb) (R&D Systems; 1:40) or isotype-matched controls (IQ Products, Groningen, The Netherlands; 1:40) for 20 minutes on ice, and were washed with FACS buffer. Immunostained cells were analyzed by FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA), and data were analyzed using CellQuest software (BD Biosciences, San Jose, CA). Events (10 000) were acquired in list mode. Analyzed cells were also stained with 1 mg/mL propidium iodide (Sigma), and dead cells were gated out.
Binding assay of biotinaleted CXCL11 to 293 cells expressing CXCR3
293-CXCR3 wild-type (WT) and mutant cells were trypsinized and counted. Cells (4 × 105) were suspended in 400 mL DMEM and 1% FCS and incubated for 10 minutes on ice. Biotinylated interferon-inducible T-cell alpha chemoattractant (I-TAC; Exalpha Biologicals, Watertown, MA) was then added and the cells were incubated for 30 minutes on ice and then washed in FACS buffer. Biotinylated I-TAC was added to the cells in the following concentrations: 5 nM, 20 nM, and 50 nM. Following incubation with Biotinylated I-TAC, cells were incubated for 30 minutes on ice with Strepavidin FITC (Jackson Immunoresearch Laboratories, West Grove, PA), diluted 1:700, and then washed with FACS buffer and suspended in 450 mL FACS buffer.
Analysis of receptor internalization
Cells (4 × 105) expressing the WT or mutated CXCR3 were incubated in 0.2 mL DMEM containing 1% FBS with chemokine or PMA at 37°C for the indicated time periods (HEK 293 were trypsinized prior to assay and incubated in full DMEM containing 10% FBS for 2 hours at 37°C to allow recovery). The cells were then rapidly cooled by dilution with 10-fold volumes of ice-cold FACS buffer and placed on ice. Cells were then stained with PE-conjugated specific anti-CXCR3 monoclonal antibodies at 4°C for 30 minutes and analyzed by flow cytometry. Percentage internalization of the receptor was calculated from the mean fluorescence intensity value for each sample compared with cells incubated with medium alone. When PKC inhibitors were used in assays, percentage of receptor internalization was calculated from the mean fluorescence intensity value for each sample compared with control cells treated with inhibitor alone, but without a stimulator. P values were calculated using the Student t test.
Migration assay
The migration of HEK 293 cells expressing WT and mutant CXCR3 was assessed by a 48-well microchemotaxis Boyden chamber technique as previously described.21 The migration of the human T-cell–derived leukemic Jurkat cells,22 Rat mast cell–derived leukemic RBL cells,23,24 and the human-derived NK leukemic cell line YTS25 were tested using a transwell migration assay as previously described.25 The effect of PTx on the migration of 293 cells was determined by preincubating the cells with the compound for 2 hours at 37°C, followed by washings. The statistical significance of the number of cells migrating in response to CXCL11 versus to BSA medium was evaluated using the Student t test.
Trunsduction of hematopoietic cell lines using lentiviral vectors
pRLL-hPGK-CXCR3-WPRE derived from self-inactivating (SIN) human immunodeficiency virus type 1 (HIV-1) carrying the WT or mutant human CXCR3 cDNAs was constructed by replacing the enhanced green fluorescent protein (EGFP) of the lentiviral vector pRLL.hPGK.EGFP.WPRE26 with the human WT or mutant CXCR3 cDNAs.
Gene transfer derived from lentiviruses was done as previously described.27 Briefly, 2 × 106 HEK 293T packaging cells were seeded 24 hours prior to transfection in a 10-cm plate in full medium. The HIV-based virions were generated by transfection of the 293T cells by a 3-plasmid system: 10 mg of the transfer vector-pRLL-hPGK-CXCR3-WPRE; 6.5 mg of the packaging construct-CMVDR8.91; and 3.5 mg of the envelope-coding plasmid VSV-G in the presence of 80 mL FuGene 6 and the serum-free medium OptiMEM (Gibco Laboratories) in a final volume of 900 mL. After transfection (24 hours), the transfection supernatant was replaced with the target cell-line culture medium. Later (24 hours), viral supernatant was collected and used for infection of target cells (first infection): adherent HEK 293 cells, seeded 1 day before infection in a 12-well plate at 5 × 105 cells/well, were centrifuged with the viral supernatant and 5 mg/mL polybrene at 420g for 2 hours at room temperature (RT). Then, cells were incubated for 24 hours at 37°C in 5% CO2 atmosphere. Suspension cell lines were seeded at 3 × 105 cells/150 mL/well on the day of infection. Virions supernatant was × 10 concentrated by ultracentrifugation at 50 000g at 4°C for 2 hours. The virion pellet was gently resuspended in culture medium and mixed with the target cells in the presence of polybrene. Infection was repeated 24 hours later (second infection). Clonally expanded transduced cells were screened and assayed for stably integrated CXCR3 cDNA and its level of expression was evaluated by FACS.
Calcium influx assay
Cells (1 × 106/mL) in DMEM containing 1% FBS were loaded with 10 μm of the Ca2+ indicator fluo-3 (Molecular Probes, Eugene, OR), which is nonfluorescent unless bound to Ca2+, for 30 minutes at 37°C. Cells were washed twice with modified Gay buffer (MGB; 5 mM KCl, 147 mM NaCl, 0.2 mM KH2PO4, 1.1 mM Na2HPO4, 5.5 mM glucose, 0.3 mM MgSO4.7H20, 1 mM MgCl2, 0.1% BSA, 10 mM HEPES [pH 7.4], and 1.3 mM Ca2+), resuspended at 1 × 106 cells/mL in the same buffer, and analyzed by FACS at FL1 (linear scale) versus time. Cells were acquired for 2 minutes before stimulation with chemokine, and 1 g/mL CXCL11 was added in 2-minute intervals to monitor calcium influx.
Actin filament polymerization
Cells (1 × 106) resuspended in DMEM containing 1% FBS were stimulated with 1 g/mL CXCL11 for the indicated time points at 37°C. Incubation was stopped by adding 3 volumes of 4% paraformaldehyde (PFA) at RT for 10 minutes, followed by washing with PBS and permeabilization on ice for 2 minutes with 0.2% Triton X-100 in PBS. Thereafter, cells were washed, stained with 2 mg/mL FITC-phalloidin (Sigma) at RT for 30 minutes, and washed again. F-actin was analyzed by FACS at FL1 in linear scale.
Receptor-binding assay
Binding assay was performed according to Colvin et al20 with modifications. WT or mutant CXCR3/HEK 293 or Jurkat cells (5 × 106) were incubated in a total volume of 150 μL binding buffer (0.5% BSA, 5 mM MgCl2, 1 mM CaCl2, 0.01% sodium azide, and 50 mM HEPES [pH 7.4]), 0.06 nM 125I-labeled CXCL11 (Amersham Biosciences, Piscataway, NJ), and increasing concentrations of unlabeled CXCL11 (R&D Systems). After 90 minutes at room temperature with shaking, the cells were washed 3 times with 1 mL binding buffer supplemented with 0.5 M NaCl. Radioactivity was measured in the dried pellet in a g counter.
Adhesion assays
The adhesion assays were performed in a flow chamber as previously described.25,28 Soluble, affinity-purified human sVCAM-1 and fibronectin (FN) were mixed in coating media (PBS buffered with 20 mM sodium bicarbonate [pH 8.5]) and absorbed as 10-mL drops on a polystyrene plate (60 × 15-mm Petri dish; Becton Dickinson, Lincoln Park, NJ) overnight at 4°C. The plate was then washed and blocked with human serum albumin (0.2% HSA) for 2 hours at 4°C. To coimmobilize CXCL11 with the adhesive substrates, the ligands (sVCAM-1 and FN) were coated in the presence of active (2 mg/mL) or heat-denatured CXCL11 and HSA (2 mg/mL), washed, and quenched as described previously. A polystyrene plate with coated adhesive substrates was assembled as the lower wall in a parallel plate flow chamber (260-mm gap), mounted on the stage of an inverted phase-contrast microscope (Diaphot 300; Nikon, Melville, NY) and extensively washed with binding medium. All flow experiments were conducted at 37°C. Jurkat cells were suspended in binding buffer perfused into the chamber and allowed to settle on the substrate-coated chamber wall for 1 minute. Flow was initiated and increased in 2- to 2.5-fold increments every 5 seconds, thereby generating controlled shear stresses on the wall generated by an automated syringe pump (Harvard Apparatus, Natick, MA). All flow experiments were recorded on videotape by a long integration camera (LIS-700 CCD; Applitech, Holon, Israel) and a SVHS time-lapse video recorder (AG-6730; Panasonic, Osaka, Japan), and analyzed as previously described.25,28
Statistical analysis
Mean values were compared using the ANOVA (analysis of variance) test in Figures 3, 4, 6B-C, and 7B Whenever ANOVA was statistically significant, multiple comparisons were done. Level of significance was set at a P value below .05. Values for all measurements were expressed as the mean plus or minus SE.
Results
Construction and expression of the CXCR3 mutants
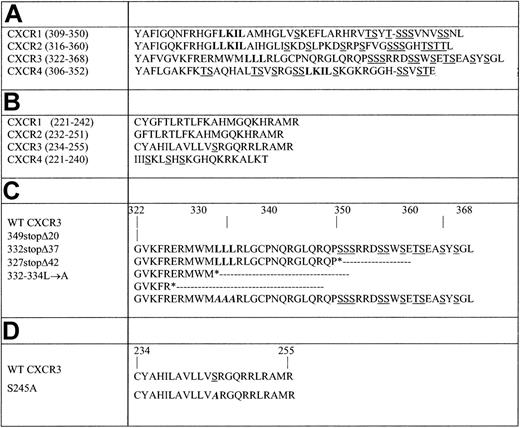
To identify the CXCR3 regions that regulate the receptor-mediated migration, adhesion, and internalization, a series of CXCR3 variants with progressive truncations and site-specific mutations was generated either in the carboxyl terminus domain and/or the 3i loop, based on previous observations on involvement of Ser-Thr and dileucine domains in these processes in CXCR1, CXCR2, and CXCR4 (Figure 1, Table 1).18,19,24,29-31 The shortest truncation of the carboxyl terminus, 349stopΔ20, removes all potential phosphorylation sites of serine-threonine (20 last amino acids). The longer truncations are 332stopΔ37, in which the 3 leucines (37 last amino acids) were removed, and 327stopΔ42, which extend to remove also the 5 amino acids upstream to the LLL motif (42 last amino acids). Additionally, a site-specific CXCR3 mutant, 332-334L→A, was generated by replacing the trileucine of the C-tail at positions 332, 333, and 334 with alanines (Table 1), and at the 3i loop, serine 245 was replaced with alanine, generating S245A (Table 1). 37/S245A, a double-mutant of 332stopΔ37 and S245A, was also produced. The WT and mutant CXCR3 receptors were transfected into HEK 293 cells, which do not express CXCR3 on their surface, and their expression was detected on the cell surface at levels comparable with WT CXCR3 (Figure 2A). Furthermore, the mutant CXCR3 receptor binds similarly to fluorescence-labeled CXCL11 (Figure 2B). Furthermore, WT CXCR3, 349stopΔ20, 332stopΔ37, 332-334L→A, and S245A mutants on 293 cells bound iodinated CXCL11 at similar levels and affinity as determined by inhibitory concentration at 50% (IC50) values (Figures 1, 2; Table 1).
Truncations and site-directed mutagenesis in the carboxyl terminus and 3i loop of CXCR3
CXCR3 receptor . | Surface binding of iodinated CXCL11 to CXCR3, % WT . | IC50, nM . |
|---|---|---|
| 293-WT | 100 | 8 |
| 293-349stopΔ20 | 70.4 | 8 |
| 293-332stopΔ37 | 71.2 | 6 |
| 293-332-334L→A | 70.0 | 4 |
| 293-S245A | 85.4 | 1 |
| Jurkat-WT | 100 | 0.4 |
| Jurkat-S245A | 115 | 0.2 |
CXCR3 receptor . | Surface binding of iodinated CXCL11 to CXCR3, % WT . | IC50, nM . |
|---|---|---|
| 293-WT | 100 | 8 |
| 293-349stopΔ20 | 70.4 | 8 |
| 293-332stopΔ37 | 71.2 | 6 |
| 293-332-334L→A | 70.0 | 4 |
| 293-S245A | 85.4 | 1 |
| Jurkat-WT | 100 | 0.4 |
| Jurkat-S245A | 115 | 0.2 |
Relative surface binding, to WT CXCR3, of iodinated CXCL11 to the different CXCR3 mutants is shown. IC50 values (nM) for the WT and mutants CXCR3 are also shown.
Truncations and site-directed mutagenesis in the carboxyl terminus and 3i loop of CXCR3. (A) Carboxyl terminus alignment of CXCR1, CXCR2, CXCR3, and CXCR4. (B) 3i loop subdomain of CXCR1, CXCR2, CXCR3, and CXCR4. (C) CXCR3 carboxyl-terminus. The carboxyl-terminal truncations of CXCR3 were generated by introducing stop codons (*) at Ser349, Leu332, or Glu327. 332-334L→A site-directed mutagenesis was generated as described in “Materials and methods.” (D) CXCR3 3i loop subdomain. Site-directed mutagenesis at position 245, replacing serine with alanine, was generated as described in “Materials and methods.” Serine and threonine residues that serve as potential phosphorylation sites are underlined, and the leucine motifs are in bold. The site-directed mutagenesis is in bold italics. Positions are indicated according to Feature Aligner of ExPASy, Swiss-Prot (Swiss Institute of Bioinformatics, Basel, Switzerland).
Truncations and site-directed mutagenesis in the carboxyl terminus and 3i loop of CXCR3. (A) Carboxyl terminus alignment of CXCR1, CXCR2, CXCR3, and CXCR4. (B) 3i loop subdomain of CXCR1, CXCR2, CXCR3, and CXCR4. (C) CXCR3 carboxyl-terminus. The carboxyl-terminal truncations of CXCR3 were generated by introducing stop codons (*) at Ser349, Leu332, or Glu327. 332-334L→A site-directed mutagenesis was generated as described in “Materials and methods.” (D) CXCR3 3i loop subdomain. Site-directed mutagenesis at position 245, replacing serine with alanine, was generated as described in “Materials and methods.” Serine and threonine residues that serve as potential phosphorylation sites are underlined, and the leucine motifs are in bold. The site-directed mutagenesis is in bold italics. Positions are indicated according to Feature Aligner of ExPASy, Swiss-Prot (Swiss Institute of Bioinformatics, Basel, Switzerland).
Cell-surface expression of WT and mutated CXCR3 receptors on HEK 293 cells. WT and mutant CXCR3 cell-surface expression on stable transfected HEK 293 cell clones was tested by immunostaining with anti-CXCR3 specific antibodies and analyzed by FACS (black line). Immunostaining with IgG1 isotype control antibodies is in gray line. (A) WT CXCR3. (B) 349stopΔ20 C-tail truncated. (C) 332stopΔ37 C-tail truncated. (D) 332-334L→A C-tail mutations. (E) S245 to A 3i loop mutation. (F) Double-mutant of 332stopΔ37 C-tail truncated and S245 to A 3i loop mutation. (G) HEK 293 cells. Counts indicate relative cell number. A representative histogram of at least 3 experiments performed is presented. (H) Binding of biotinylated I-TAC (mean florescence intensity [MFI]) at different concentrations (nM) to 293 cells expressing the different mutated or truncated CXCR3.
Cell-surface expression of WT and mutated CXCR3 receptors on HEK 293 cells. WT and mutant CXCR3 cell-surface expression on stable transfected HEK 293 cell clones was tested by immunostaining with anti-CXCR3 specific antibodies and analyzed by FACS (black line). Immunostaining with IgG1 isotype control antibodies is in gray line. (A) WT CXCR3. (B) 349stopΔ20 C-tail truncated. (C) 332stopΔ37 C-tail truncated. (D) 332-334L→A C-tail mutations. (E) S245 to A 3i loop mutation. (F) Double-mutant of 332stopΔ37 C-tail truncated and S245 to A 3i loop mutation. (G) HEK 293 cells. Counts indicate relative cell number. A representative histogram of at least 3 experiments performed is presented. (H) Binding of biotinylated I-TAC (mean florescence intensity [MFI]) at different concentrations (nM) to 293 cells expressing the different mutated or truncated CXCR3.
Migration mediated by CXCR3 toward CXCL11 depends mainly on its carboxyl terminus
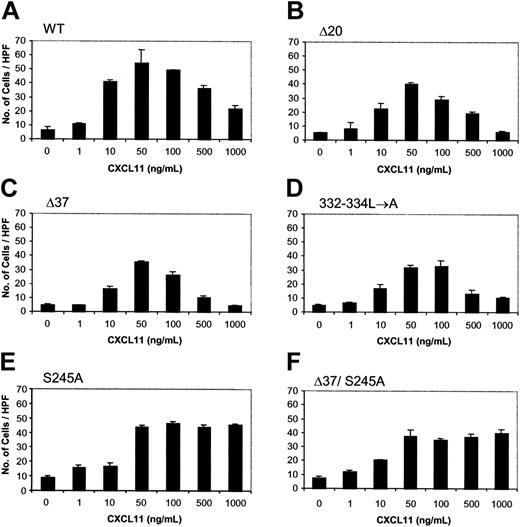
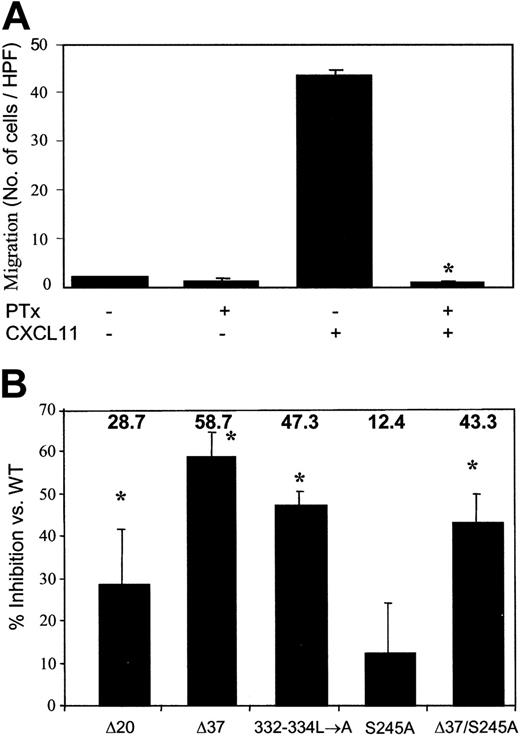
To investigate whether the CXCR3 carboxyl-terminus and/or 3i loop are involved in the regulation of CXCR3-mediated biological response, the migration of WT and mutant CXCR3-expressing HEK 293 cells in response to CXCL11 was assayed. The migration of HEK 293 cells expressing WT CXCR3 and the mutated or truncated form of the C terminal of the receptor was dose dependent and PTx sensitive (Figures 3,4A). The migration peaked at a concentration of 50 to 100 ng/mL CXCL11 and was reduced at the concentration of 500 to 1000 ng/mL of the ligand. The migration of cells in response to 50 ng/mL CXCL11 was slightly reduced (28.7% ± 13.0%) when HEK 293 cells expressing 349stopΔ20, including the portion of all potential phosphorylation sites of serine-threonine, were used (Figures 3B,4B). The migration of cells that express either the truncated CXCR3 C terminus, 332stopΔ37 (58.7% ± 5.6%), or CXCR3 specifically mutated in the trileucine motif, 332-334L→A (47.3% ± 3.2%), was significantly inhibited compared with control cells expressing WT CXCR3 (Figures 3C-D,4B). We therefore concluded that the migration of HEK 293 cells in response to CXCL11 was essentially regulated through the C terminus of CXCR3. However, the C terminus itself is not sufficient to control this biological effect, suggesting that another part of CXCR3 is involved in regulating migration.
Dose-response of migration of HEK 293 cells expressing WT or mutated CXCR3 receptors toward CXCL11. Migration was performed as described in “Materials and methods” in response to various concentrations of CXCL11 (ng/mL). (A) WT CXCR3. (B) 349stopΔ20 C-tail truncated. (C) 332stopΔ37 C-tail truncated. (D) 332-334L→A C-tail mutations. (E) S245 to A 3i loop mutation. (F) Double-mutant of 332stopΔ37 C-tail truncated and S245 to A 3i loop mutation. A representative experiment of at least 3 performed is presented. Each value represents the mean ± SD of triplicates of the representative experiment. ANOVA was used to determine the levels of difference between CXCL11 concentrations of 50, 100, 500, and 1000 ng/mL within each CXCR3 type. The differences in number of cells per high-power field (HPF) were statistically significant for all CXCR3 types except for D37/S245A. Therefore, multiple comparisons of number of cells per HPF between these concentrations in each receptor type were done by the Newman-Keuls test. For WT CXCR3, number of cells per HPF at 50 and 100 ng/mL were not statistically different. For the mutant 332-334L→A, number of cells/HPF at 50 versus 100 ng/mL and at 500 versus 1000 ng/mL were not statistically different, but these pairs of concentrations were statistically different from each other. For the mutant S245A, number of cells per HPF at 50 versus 100 ng/mL and 500 versus 100 ng/mL were statistically different. ANOVA also showed that the differences in number of cells per HPF were statistically significant for all CXCR3 types at 50 ng/mL CXCL11. Multiple comparisons of each mutant with WT CXCR3 at this concentration were performed by the Dunnett test. Except for S245A, all other mutants were different and statistically significant from the WT.
Dose-response of migration of HEK 293 cells expressing WT or mutated CXCR3 receptors toward CXCL11. Migration was performed as described in “Materials and methods” in response to various concentrations of CXCL11 (ng/mL). (A) WT CXCR3. (B) 349stopΔ20 C-tail truncated. (C) 332stopΔ37 C-tail truncated. (D) 332-334L→A C-tail mutations. (E) S245 to A 3i loop mutation. (F) Double-mutant of 332stopΔ37 C-tail truncated and S245 to A 3i loop mutation. A representative experiment of at least 3 performed is presented. Each value represents the mean ± SD of triplicates of the representative experiment. ANOVA was used to determine the levels of difference between CXCL11 concentrations of 50, 100, 500, and 1000 ng/mL within each CXCR3 type. The differences in number of cells per high-power field (HPF) were statistically significant for all CXCR3 types except for D37/S245A. Therefore, multiple comparisons of number of cells per HPF between these concentrations in each receptor type were done by the Newman-Keuls test. For WT CXCR3, number of cells per HPF at 50 and 100 ng/mL were not statistically different. For the mutant 332-334L→A, number of cells/HPF at 50 versus 100 ng/mL and at 500 versus 1000 ng/mL were not statistically different, but these pairs of concentrations were statistically different from each other. For the mutant S245A, number of cells per HPF at 50 versus 100 ng/mL and 500 versus 100 ng/mL were statistically different. ANOVA also showed that the differences in number of cells per HPF were statistically significant for all CXCR3 types at 50 ng/mL CXCL11. Multiple comparisons of each mutant with WT CXCR3 at this concentration were performed by the Dunnett test. Except for S245A, all other mutants were different and statistically significant from the WT.
Migration of HEK 293 cells in responses to CXCL11. (A) Migration of HEK 293 cells expressing WT CXCR3 receptors toward CXCL11 is PTx dependent. HEK 293-WT CXCR3 cells were preincubated with 100 ng/mL PTx for 2 hours at 37°C and washed prior to assay. Migration was performed as described in “Materials and methods” in response to 50 ng/mL CXCL11. A representative experiment of 3 performed is presented. Each value represents the mean SD of triplicates of the representative experiment. Using ANOVA and the Newman-Keuls test, the difference was significant for PTx/CXCL11 treatment (*P < .05). (B) Migration of HEK 293-CXCR3 was dependent mainly upon the receptor C-tail. Migration of HEK 293 cells expressing the various CXCR3 mutants was performed as described in “Materials and methods” in response to 50 ng/mL CXCL11. Percentage inhibition of migration by each mutant of CXCR3 was calculated as number of migrating cells expressing mutated CXCR3 per HPF versus the number of migrating cells expressing WT CXCR3 per HPF. Each value represents the mean ± SD of inhibition calculated of 3 independent experiments. Using ANOVA, the difference of percentage inhibition between the CXCR3 mutants was significant (*P < .05). Multiple comparisons between the mutants by the Newman-Keuls test showed that only S245A and D37 were different from each other.
Migration of HEK 293 cells in responses to CXCL11. (A) Migration of HEK 293 cells expressing WT CXCR3 receptors toward CXCL11 is PTx dependent. HEK 293-WT CXCR3 cells were preincubated with 100 ng/mL PTx for 2 hours at 37°C and washed prior to assay. Migration was performed as described in “Materials and methods” in response to 50 ng/mL CXCL11. A representative experiment of 3 performed is presented. Each value represents the mean SD of triplicates of the representative experiment. Using ANOVA and the Newman-Keuls test, the difference was significant for PTx/CXCL11 treatment (*P < .05). (B) Migration of HEK 293-CXCR3 was dependent mainly upon the receptor C-tail. Migration of HEK 293 cells expressing the various CXCR3 mutants was performed as described in “Materials and methods” in response to 50 ng/mL CXCL11. Percentage inhibition of migration by each mutant of CXCR3 was calculated as number of migrating cells expressing mutated CXCR3 per HPF versus the number of migrating cells expressing WT CXCR3 per HPF. Each value represents the mean ± SD of inhibition calculated of 3 independent experiments. Using ANOVA, the difference of percentage inhibition between the CXCR3 mutants was significant (*P < .05). Multiple comparisons between the mutants by the Newman-Keuls test showed that only S245A and D37 were different from each other.
Previous studies of CXCR4 reported that its 3i loop was involved in the chemotactic regulation of HEK 293 cells expressing this receptor.31 We next questioned whether the potential 3i loop of CXCR3 played a role in mediating the migratory response. The CXCR3 3i loop contains a single serine, compared with the CXCR4 3i loop that includes several serine residues, which may suggest the involvement of this amino acid in chemotactic regulation (Table 1). Therefore, the 3i loop S245A and the double-mutant 37/S245A were tested. HEK 293 cells that expressed the mutant S245A, like WT CXCR3-expressing cells, were capable of sensing low chemotactic levels of CXCL11 (up to 100 ng/mL). This mutation did not significantly affect the level of the response (12.4% ± 11.9%) compared with WT CXCR3-expressing cells. The 37/S245A that also lacks all the Ser-Thr and the trileucine motifs in the C terminus inhibited the migration at a low CXCL11 concentration (50 ng/mL) in a level similar to 332-334L→A versus the WT receptor (43.3% ± 6.7%) (Figures 3E-F,4B). These results suggest that CXCR3 domain(s) other than S245A play a role in this signaling event along with the C terminus, and remain to be discovered.
Inhibition of CXCR3-mediated migration at high concentrations of CXCL11 requires S245 in the 3i loop
Under an excess of chemoattractant, desensitization requires the discontinuation of leukocyte migration to reduce the inflammatory response.
Our observations demonstrate that the migration of HEK 293 cells expressing the WT and impaired C terminus of CXCR3 was reduced at high levels (500 and 1000 ng/mL) of CXCL11 (Figure 3A-D). However, at these high ligand levels, the cells that expressed the 3i loop mutant S245A lost their ability to desensitize the response toward CXCL11 (Figure 3E). 37/S245A, similarly to S245A, was unable to desensitize the migratory response at high CXCL11 concentrations (Figure 3F). Taken together, these findings suggest that the C terminus of CXCR3 is unnecessary to attenuate the biological response toward high levels of CXCL11, and serine 245 in the 3i loop regulates this response.
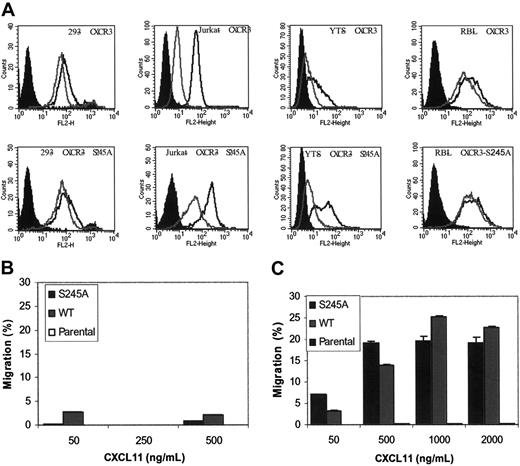
To further study the role that S245A plays in regulating the migration of cells in response to CXCL11, we expressed the WT and the S245A CXCR3 chemokine receptor mutant on a cell surface of human T-cell–derived leukemic Jurkat cells, human NK cell–derived YTS leukemic cell line, and the rat mast cell–derived leukemic RBL cells, and tested their ability to migrate in response to CXCL11 (Figure 5). Jurkat cells as well as the YTS human cells expressing CXCR4 were previously shown to migrate well in response to CXCL12.22,25 RBL rat cells were extensively used to study chemokine-induced migration and internalization of various chemokine receptors, including the CXCR1 and CXCR2 chemokine receptors.23,24 Surprisingly, we found that both RBL as well as YTS cells overexpressing the human CXCR3 chemokine receptor poorly migrate in response to CXCL11 (Figure 5B). It is important to note that overexpression of CXCR4 in YTS cells and overexpression of CXCR1 in RBL cells resulted in a significant migration of the cells toward the relevant chemokines CXCL12 and CXCL8.22,25 In contrast to RBL and YTS cells, the migration of Jurkat cells expressing WT CXCR3 was significant and dose dependent. WT CXCR3 and the S245A mutant bound iodinated CXCL11 at similarly high levels of affinity (0.2-0.4 nM) as determined by IC50 values (Figure 1; Table 1). Interestingly, Jurkat cells bound CXCL11 at an affinity 10-fold higher than that of HEK 293 cells. This may be the result of pretreatment of HEK 293 cells with trypsin prior to the performance of the binding assay. Jurkat cells that expressed the mutant S245A and the WT CXCR3 were capable of sensing low chemotactic levels of CXCL11 (50 ng/mL). Furthermore, this mutation did not significantly affect the level of migration of cells in response to CXCL11 (20% versus 25%). Similar to HEK 293 cells, the migration of Jurkat cells that expressed the 3i loop mutant S245A was not desensitized at high CXCL11 concentrations (Figure 5C). However, in contrast to HEK 293 cells, high levels of CXCL11 induced only a minor reduction in the migration of Jurkat cells that expressed the WT CXCR3. Taken together, these findings suggest that the response of cells toward a high concentration of CXCL11 is also dependent on the type of cell tested.
Expression and function of WT CXCR3 and S245A-mutated CXCR3 in Jurkat, YTS, and RBL hematopoietic cells. (A) Cell-surface expression of WT or the S245A mutated CXCR3 in HEK 293, Jurkat, YTS, and RBL cells (black line). Immunostaining with immunoglobulin G1 (IgG1) isotype control antibodies is shown in filled histograms. Cell-surface expression of CXCR3 or the S245A-mutated CXCR3 in cells incubated with 1 mg/mL CXCL11 for 1 hour is also shown (gray line). (B-C) The migration of parental-, CXCR3-, and CXCR3-S245A-expressing YTS (B) and Jurkat cells (C) in response to different concentrations of CXCL11 is shown. Each value represents the mean plus or minus SD of 3 independent experiments.
Expression and function of WT CXCR3 and S245A-mutated CXCR3 in Jurkat, YTS, and RBL hematopoietic cells. (A) Cell-surface expression of WT or the S245A mutated CXCR3 in HEK 293, Jurkat, YTS, and RBL cells (black line). Immunostaining with immunoglobulin G1 (IgG1) isotype control antibodies is shown in filled histograms. Cell-surface expression of CXCR3 or the S245A-mutated CXCR3 in cells incubated with 1 mg/mL CXCL11 for 1 hour is also shown (gray line). (B-C) The migration of parental-, CXCR3-, and CXCR3-S245A-expressing YTS (B) and Jurkat cells (C) in response to different concentrations of CXCL11 is shown. Each value represents the mean plus or minus SD of 3 independent experiments.
Internalization of CXCR3 induced by ligand and PMA is differentially regulated through its membrane-proximal carboxyl terminus
Desensitization of a biological response can be regulated through the chemokine receptor internalization. We therefore tested the role of the carboxyl terminal domains and/or the S245 3i loop of CXCR3 in the receptor internalization.
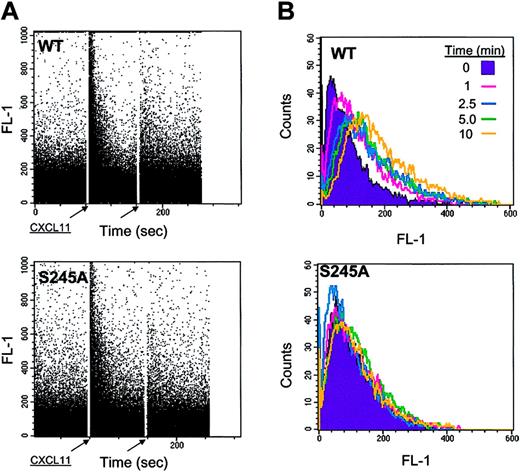
To determine the kinetics of CXCR3 internalization induced by the ligand CXCL11 and PMA, an internalization assay was performed in a time-course manner in WT CXCR3-expressing HEK 293 cells. These cells were stimulated with either 1 mg/mL CXCL11 or 100 ng/mL PMA. CXCL11 induced a marked internalization of CXCR3 within 2 hours (61.9 ± 2.8%), whereas the internalization mediated by PMA was slower, reaching a similar extent after 5 hours (68.8 ± 1.4%) (Figure 6A). Several members of the CXC CKR family, such as CXCR4, are subject to modulation of internalization by the PKC pathway. We found that the phorbol ester PMA could also induce internalization of CXCR3 in interleukin-2 (IL-2)–activated NK and CXCR3-HEK 293 cells (data not shown). In comparing the pattern of CXCL11 effect on CXCR3 internalization between the hematopoietic and CXCR3-HEK 293 cells, we found that RBL-expressing CXCR3 and CXCR3-HEK 293 cells similarly reduced their CXCR3 expression by approximately 40% after 1 hour of incubation with 1 mg/mL CXCL11, whereas both Jurkat and YTS cells rapidly reduced their CXCR3 expression by approximately 80% (Figure 7A). However, slow or rapid internalization of CXCR3 did not correlate with the migration potential of the cells since in the slow internalizer (RBL cells) and the fast internalizer (YTS cells), migration in response to CXCL11 was poor. These studies using 4 different cell types confirm not only that HEK 293 cells are suitable for studying CXCR3, but suggest that the function and biochemical behavior of CXCR3 are dependent on the type of cells used.
Reduction of CXCR3 cell-surface expression following CXCL11 and PMA stimulation. (A) Time course of the reduction of CXCR3 expression in response to CXCL11 and PMA in 293-CXCR3 cells. CXCR3-expressing HEK 293 cells were trypsinized and incubated with either 1 mg/mL CXCL11 or 100 ng/mL PMA for the indicated time points at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. Each value represents the mean plus or minus SE of 3 to 4 independent experiments. (B) Effect of PKC inhibitors on ligand- or PMA-induced CXCR3 down-modulation on the surface of 293 cells. 293-CXCR3 cells were trypsinized and incubated in medium alone or with a PKC inhibitor (staurosporin [0.5 μM], GF [5 μM], or rottlerin [5 μM]) for 30 minutes at 37°C, and then challenged with 1μg/mL CXCL11 or 100 ng/mL PMA for 1 hour at 37°C. The cells were then washed, and surface expression of CXCR3 receptors was analyzed. Each value represents the mean ± SE of 4 independent experiments. *P < .05 by ANOVA and Dunnett test for internalization induced by PMA with staurosporin versus without staurosporin, and with GF versus without GF. (C) The CXCR3-expressing HEK 293 cells, WT CXCR3, 349stopΔ20 C-tail truncated, 332stopΔ37 C-tail truncated, and 332-334L→A C-tail mutations were trypsinized and incubated with CXCL11 (1 μg/mL) or PMA (100 ng/mL) for 1 hour at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. Each value represents the mean ± SE of at least 3 independent experiments. *P < 05 by ANOVA and Dunnett test for D37 CXCL11- and PMA-induced internalization versus WT.
Reduction of CXCR3 cell-surface expression following CXCL11 and PMA stimulation. (A) Time course of the reduction of CXCR3 expression in response to CXCL11 and PMA in 293-CXCR3 cells. CXCR3-expressing HEK 293 cells were trypsinized and incubated with either 1 mg/mL CXCL11 or 100 ng/mL PMA for the indicated time points at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. Each value represents the mean plus or minus SE of 3 to 4 independent experiments. (B) Effect of PKC inhibitors on ligand- or PMA-induced CXCR3 down-modulation on the surface of 293 cells. 293-CXCR3 cells were trypsinized and incubated in medium alone or with a PKC inhibitor (staurosporin [0.5 μM], GF [5 μM], or rottlerin [5 μM]) for 30 minutes at 37°C, and then challenged with 1μg/mL CXCL11 or 100 ng/mL PMA for 1 hour at 37°C. The cells were then washed, and surface expression of CXCR3 receptors was analyzed. Each value represents the mean ± SE of 4 independent experiments. *P < .05 by ANOVA and Dunnett test for internalization induced by PMA with staurosporin versus without staurosporin, and with GF versus without GF. (C) The CXCR3-expressing HEK 293 cells, WT CXCR3, 349stopΔ20 C-tail truncated, 332stopΔ37 C-tail truncated, and 332-334L→A C-tail mutations were trypsinized and incubated with CXCL11 (1 μg/mL) or PMA (100 ng/mL) for 1 hour at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. Each value represents the mean ± SE of at least 3 independent experiments. *P < 05 by ANOVA and Dunnett test for D37 CXCL11- and PMA-induced internalization versus WT.
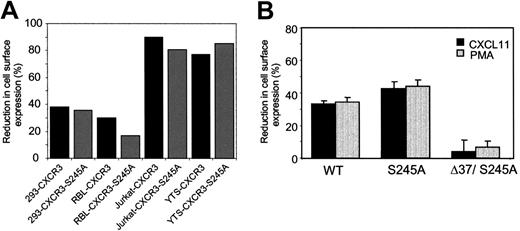
Reduction of S245A-mutant CXCR3 receptor expression on the cell surface of HEK 293 cells in response to CXCL11 and PMA. (A) The WT CXCR3, S245 to A 3i loop mutation CXCR3-expressing HEK 293, RBL, Jurkat, and YTS cells, were incubated with CXCL11 (1 mg/mL) for 1 hour at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. The reduction of cell-surface expression of CXCR3 is shown. (B) The WT CXCR3, S245 to A 3i loop mutation CXCR3, and the double mutant of 332stopΔ37 C-tail truncated and S245 to A 3i loop mutation expressing HEK 293 were incubated with CXCL11 (1 mg/mL) for 1 hour at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. The reduction of HEK 293 cell-surface expression of CXCR3 is shown. *P < .05 by ANOVA and Dunnett test for CXCL11- and PMA-induced reduction in cell-surface expression of D37/S245A versus WT. Data are expressed as the means plus or minus SD of 3 independent experiments.
Reduction of S245A-mutant CXCR3 receptor expression on the cell surface of HEK 293 cells in response to CXCL11 and PMA. (A) The WT CXCR3, S245 to A 3i loop mutation CXCR3-expressing HEK 293, RBL, Jurkat, and YTS cells, were incubated with CXCL11 (1 mg/mL) for 1 hour at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. The reduction of cell-surface expression of CXCR3 is shown. (B) The WT CXCR3, S245 to A 3i loop mutation CXCR3, and the double mutant of 332stopΔ37 C-tail truncated and S245 to A 3i loop mutation expressing HEK 293 were incubated with CXCL11 (1 mg/mL) for 1 hour at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. The reduction of HEK 293 cell-surface expression of CXCR3 is shown. *P < .05 by ANOVA and Dunnett test for CXCL11- and PMA-induced reduction in cell-surface expression of D37/S245A versus WT. Data are expressed as the means plus or minus SD of 3 independent experiments.
To determine whether similar mechanisms were involved in ligand- and PMA-mediated internalization of CXCR3, the effect of PKC inhibitors on the receptor internalization was investigated. CXCR3-expressing HEK 293 cells were pretreated with staurosporin, a general inhibitor of PKC, and then challenged with a ligand or PMA. Subsequently, cell-surface CXCR3 levels were determined by FACS (Figure 6B). Staurosporin completely inhibited the PMA-induced internalization of CXCR3. In contrast, the ligand-induced internalization of the receptor was not blocked. To identify which PKC isotype was involved in the PMA-induced internalization process, similar experiments were performed with GF 109203X or rottlerin, preferential inhibitors of PKCα or PKCδ, respectively. The results suggest that PKCα is involved in CXCR3 PMA-induced internalization in HEK 293 cells, as GF 109203X significantly blocked its internalization. In contrast, the ligand-induced internalization was not affected by either one of the PKC inhibitors. These results indicate that ligand- and PKC-induced internalization of CXCR3 are regulated differentially.
The mechanisms of ligand- and PMA-induced internalization of CXCR3 are not known. We therefore examined the role of CXCR3 carboxyl-terminus and third intracellular loop in receptor internalization.
To reduce the effect of receptor recycling on the levels of CXCR3 cell surface to a minimum, the internalization of the mutant receptors was assayed in response to CXCL11 and PMA after 1 hour. The truncated 349stopΔ20 CXCR3 internalized to a similar extent as that of the WT receptor in response to both CXCL11 and PMA. In contrast, 332stopΔ37, the membrane proximal truncation, significantly affected the internalization induced by both stimulators. The internalization stimulated by CXCL11 was significantly reduced (60% inhibition), while that mediated by PMA was totally abolished (Figure 5C). The truncation 327stopΔ42 had no additional effect on CXCR3 internalization and resulted in a comparable level of internalization of the 332stopΔ37 truncation within 1 hour (data not shown). Taken together, this mutant analysis indicates that the serine-threonine domain in the C terminus of CXCR3 is not essential for the receptor internalization induced by both CXCL11 and PMA.
Dileucine or Ile-Leu motif present in CXCR2 and CXCR4, was shown to regulate the internalization of these receptors.18,19 To assess its role in CXCR3 internalization, a site-directed mutagenesis of the candidate LLL in the membrane proximal portion of CXCR3 C-tail was generated (332-334L→A). The 332-334L→A mutant affected the internalization induced by CXCL11 similar to that seen in the CXCR3 332stopΔ37 truncated receptor (50% and 60% inhibition, respectively). However, the internalization mediated by PMA was not affected by CXCR3 332-334L→A mutant receptor (Figure 6C). This mutant analysis indicates that the membrane proximal region of CXCR3 carboxyl terminus that includes the LLL motif regulates receptor internalization stimulated by PKC or CXCL11. However, whereas the LLL motif plays a role in the regulation of ligand-induced internalization, the motif that regulates PKC-induced internalization has yet to be discovered. It is known that the 3i loop domain of GPCR, including the chemokine receptor CXCR4, is partially involved in internalization.32,33 Therefore, the partial ligand-induced internalization of CXCR3, missing its C-tail, suggests that the 3i loop domain may contribute to the process of internalization.
The 3i loop, S245, is involved in the inhibition of the migratory response to CXCL11 (Figures 3,5C). We found that the S245A CXCR3 mutant was equally well internalized compared with the WT CXCR3 receptor in all cell lines tested (Figure 7A-B). Interestingly, when the S245A mutation was introduced in the CXCR3 truncated receptor 332stop→37, it reduced the internalization percentage from 17% (60% inhibition) to 4% (86% inhibition). These phenomena may indicate that the 3i serine 245 acquired the ability to regulate the internalization of the truncated receptor in the absence of the C terminus. To conclude, thus far our findings suggest that in the CXCR3 receptor, the C terminus, and mainly its membrane proximal domain, regulates receptor internalization induced by CXCL11 and PMA and migration, whereas inhibition of the migration is regulated specifically by the 3i loop serine 245 and not by internalization.
S245 3i loop of CXCR3 regulates actin polymerization and adhesion, but not calcium mobilization
To further study the mechanism by which the 3i loop S245 regulates migration, we tested the intracellular calcium mobilization induced by CXCL11 in HEK 293 clones expressing WT CXCR3 and the 3i loop mutant S245A. Both cells responded equivalently to a primary dose of CXCL11 by inducing a transient rise of intracellular free Ca2+, which was desensitized by a subsequent challenge with a similar dose of the ligand (Figure 8A).
Among the requirements for chemokine-dependent integrin-mediated adhesion and migration are an increased rate of actin polymerization and an extensive reorganization of the F-actin–based cytoskeleton. We therefore analyzed actin-filament polymerization in HEK 293 cells expressing WT or mutated S245A CXCR3. Cells were pretreated with CXCL11 at various time points, and promotion of actin polymerization was measured by binding of FITC-phalloidin, that stabilizes the filaments against depolymerization. Chemotactic concentration of 100 ng/mL CXCL11 within 1 minute did not induce a remarkable change of actin polymerization in either WT or S245 CXCR3-expressing cells (data not shown). However, 1 mg/mL CXCL11 promoted a time-dependent burst of actin polymerization in WT CXCR3-expressing cells, while such response was abrogated by the S245A mutant (Figure 8B).
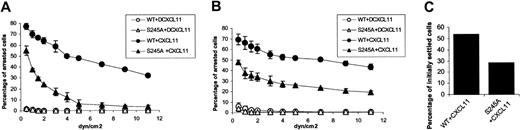
An increased rate of actin polymerization was associated with an increased integrin activation resulting in firm adhesion, as well as in reduced migration. To study the involvement of CXCR3 serine 245 in integrin-mediated adhesion, we used Jurkat cells expressing endogenous very late antigen 4 (VLA-4) and either the WT CXCR3 or the S245A mutant CXCR3. The influence of immobilized CXCL11 on VLA-4–dependent Jurkat cell adhesion and spreading was tested on plates coated with sVCAM and CXCL11 (2 mg/mL), or FN (5 mg/mL) and CXCL11 (2 mg/mL). As a negative control, the plates were coated with either sVCAM or FN and denatured CXCL11. The cells were allowed to settle on the substrate-coated chamber wall for 1 minute. Flow was initiated and increased in 2- to 2.5-fold increments every 5 seconds. In the presence of shear stress, without functional CXCL11 cells failed to arrest on VCAM-1 or FN (Figure 9A-B). When VCAM-1 or FN were coimmobilized with CXCL11, the Jurkat cells expressing the WT CXCR3 firmly arrested on VCAM-1 or FN (Figure 9A-B). In contrast, Jurkat cells expressing the S245A mutant CXCR3 exhibited attenuated adhesion to VCAM-1 or FN (Figure 9A-B and Movies S1-S2, which are available on the Blood website; see the Supplemental Movies link at the top of the online article). Furthermore, when subjected to an immediate pulse of high shear stress (5 dyn/cm2), mutant expressing cells immediately detached from the VCAM-1/CXCL11 substrate, whereas cells expressing WT CXCR3 remained firmly bound (Figure 9C). In order to test the ability of adherent cells to spread on the integin ligand in response to CXCL11, cells were allowed to settle on the substrate for 1 minute and were subjected for a constant shear stress of 5 dyn/cm2 for 15 minutes. The interaction of cells with the coated substrate under shear flow were recorded on a videotape with a long-integration LIS-700 CCD video camera and SVHS video recorder with time-lapse. We found that whereas the Jurkat cells expressing the WT CXCR3 readily spread on the coated substrate, the mutant-expressing cells failed to spread on the VCAM-1/CXCL11-coated substrate (Movies S3-S4). Thus, the 3i loop S245 is essential for both rapid VLA-4 activation triggered by CXCL11 as well as for subsequent VLA-4–mediated cell spreading induced by the CXCR3 ligand.
Ca2+ mobilization and F-actin polymerization in response to CXCL11 in HEK 293 cells expressing WT and the S245A CXCR3. (A) Intracellular Ca2+ mobilization mediated by CXCR3 in HEK 293-CXCR3 cells. Fluorescence changes in HEK 293 cells expressing WT and S245A CXCR3 loaded with 10 mM Fura-3 were measured upon stimulation with 1 mg/mL CXCL11 as indicated by arrows and described in “Materials and methods.” (B) F-actin polymerization in HEK 293 cells expressing WT and S245A CXCR3 in response to 1 mg/mL CXCL11 at various time points. F-actin polymerization was monitored by FACS as described in “Materials and methods.”
Ca2+ mobilization and F-actin polymerization in response to CXCL11 in HEK 293 cells expressing WT and the S245A CXCR3. (A) Intracellular Ca2+ mobilization mediated by CXCR3 in HEK 293-CXCR3 cells. Fluorescence changes in HEK 293 cells expressing WT and S245A CXCR3 loaded with 10 mM Fura-3 were measured upon stimulation with 1 mg/mL CXCL11 as indicated by arrows and described in “Materials and methods.” (B) F-actin polymerization in HEK 293 cells expressing WT and S245A CXCR3 in response to 1 mg/mL CXCL11 at various time points. F-actin polymerization was monitored by FACS as described in “Materials and methods.”
Adhesion of Jurkat cells expressing WT and the S245A CXCR3 to VCAM-1 and FN in response to CXCL11. (A) Denatured and intact CXCL11 induced adhesion of Jurkat cells, expressing WT or the S245A CXCR3, to immobilized recombinant human (rh) VCAM-1 are shown. Adhesion of cells is monitored over a period of 1 minute while detachment forces (dyn/cm2) continuously increased. (B) Denatured and intact CXCL11-induced adhesion of Jurkat cells, expressing WT or the S245A CXCR3, to immobilized fibronectin are shown. Adhesion of cells is monitored over a period of 1 minute while detachment forces (dyn/cm2) continuously increased. (C) CXCL11-induced adhesion of Jurkat cells, expressing WT or the S245A CXCR3, to immobilized rhVCAM-1 5 seconds after the detachment force was increased to 5 dyn/cm2.
Adhesion of Jurkat cells expressing WT and the S245A CXCR3 to VCAM-1 and FN in response to CXCL11. (A) Denatured and intact CXCL11 induced adhesion of Jurkat cells, expressing WT or the S245A CXCR3, to immobilized recombinant human (rh) VCAM-1 are shown. Adhesion of cells is monitored over a period of 1 minute while detachment forces (dyn/cm2) continuously increased. (B) Denatured and intact CXCL11-induced adhesion of Jurkat cells, expressing WT or the S245A CXCR3, to immobilized fibronectin are shown. Adhesion of cells is monitored over a period of 1 minute while detachment forces (dyn/cm2) continuously increased. (C) CXCL11-induced adhesion of Jurkat cells, expressing WT or the S245A CXCR3, to immobilized rhVCAM-1 5 seconds after the detachment force was increased to 5 dyn/cm2.
These results suggest a role for S245 at 3i loop in regulating both integrin adhesion strengthening as well as cell spreading on integrin ligands in response to CXCL11 signals under shear flow.
Discussion
Studies of CXCR3 involvement in the trafficking of immune cells to sites of inflammation and its biological effects are widespread. However, little is known about the regulation of migration, adhesion, and internalization of this receptor.
The carboxyl terminus of CXCR3 consists of two main defined domains, the potential phosphorylation Ser-Thr and the trileucine domains, which are separated by an undefined domain of 14 amino acids (amino acids 335-348). This organization differs from that of the carboxyl terminus of the other CXC CKRs, CXCR1, CXCR2, and CXCR4. In CXCR1 and CXCR2, the Ser-Thr domain is separated from the L-LKIL by only 6 amino acids, while in CXCR4 the Ser and Thr residues are dispersed also upstream to the LKIL motif. CXCR3 3i loop also diverges in the organization and frequency of the potential Ser-Thr and leucines. In contrast to CXCR1 and CXCR2 and like CXCR4, the 3i loop of CXCR3 contains an additional serine, which is a potential phosphorylation site (Table 1).
In this research, we have constructed a progressive truncated (349stopΔ20, 332stopΔ37, and 327stopΔ42) and mutated (332-334L→A) C-tail, a mutated 3i loop (S245A), and double-mutant (332stopΔ37/S245A) CXCR3 receptors. The WT and the mutated CXCR3 receptors were transfected into HEK 293 cells or Jurkat cells and expressed comparably on the cell surface. It is important to emphasize this fact, since in other studies, the cell-surface expression of certain truncated or mutated CXC CKRs receptors was weaker compared with that of the WT receptor.31 This impaired expression should be taken under consideration when conclusions are made from mutant analysis.
To study the CXCR3 parts that regulate the receptor function, we first established the relevance of HEK 293 cells in CXCR3-mediated migration toward its ligand CXCL11. Heterotrimeric G proteins of the Gαi subclass have been shown to mediate CXCR3-induced migration in T cells.34 We showed that this mechanism is also valid in our system, as pretreatment of HEK 293-CXCR3 with PTx completely abolishes CXCR3-mediated migration toward CXCL11. Thus, Gαi primarily couples to CXCR3 in HEK 293-CXCR3 and regulates the downstream signaling event of receptor activation consistent with the other CXC CKRs, CXCR1, CXCR2, and CXCR4 studied in T and HEK 293 cells.21,31,35-37
CXCR3-mediated cell migration is of importance to determine the type of inflammatory responses. The migration of HEK 293 cells expressing WT CXCR3 and the mutated or truncated form of the C terminal of the receptor was dose dependent and desensitized at high doses of CXCL11. This pattern of migratory response is typical for other CXC CKRs. The study of the mechanism of CXCR3 migratory response revealed that the migration under low and chemotactic dose of CXCL11 is regulated mainly through the C terminus of the receptor, while most of the regulation is attributed to the trileucine motif, as the 332-334L→A induced approximately 50% reduced migration when compared with WT CXCR3. The importance of the human carboxyl-terminus in regulating the migration of the murine 300-19 pre-B cells was also recently described by Colvin et al.20 The involvement of the carboxyl-terminus in the migratory function resembles that of other CXC CKRs. For CXCR2, it was reported that the membrane proximal portion of the carboxyl terminus of the receptor, which contains the LLKIL motif, has a major role in regulating the migration response in HEK 293 cells, while the distal region of the C-terminus of this receptor, containing Ser and Thr residues, does not affect agonist-mediated migration.30,38 Another domain that is a candidate regulator of biological function of GPCRs is the 3i loop. We have found that S245 of the 3i loop of CXCR3 is not essential for mediating migration since inhibition of this response by S245A was insignificant compared with that observed by the C terminus. Therefore, CXCR3 is different from CXCR4 wherein the second and the third intracellular loops as well as the C terminus were reported to be essential for mediating migration.31
Cell migration is likely to be important in an inflammatory response, and is required to be stopped by adhesion where excess of ligand may persist for a period of time. Our studies demonstrate that the carboxyl terminus of CXCR3 lacks any role in the desensitizing process of the CXCR3/CXCL11-mediated migration response and reveals S245 in the 3i loop as an exclusive role in the adhesion event. We have observed that the absence of this serine alone causes persistence of the migratory responsiveness at high doses of the agonist both in human HEK 293 and Jurkat T cells. Serine residues in the 3i loop of chemokine receptors have not been previously shown to be a regulator of adhesion or internalization. However, CXCR2 studies on HEK 293 cells proposed that the Ser-Thr residues that are present in the membrane distal portion of the carboxyl terminus of the receptor were regulatory elements of desensitization through internalization.30
Desensitization is a consequence of a combination of several mechanisms, which include uncoupling of the receptor from heterotrimeric G proteins in response to receptor phosphorylation, and internalization of cell-surface receptors to intracellular compartments. Internalization of a chemokine receptor is important since it may serve as a mechanism to reduce the chemotactic activity of leukocytes under conditions of high exposure to inflammatory stimuli, thereby preventing their continued migration and departure from the site of inflammation. Our observation shows that in cells expressing the CXCR3 S245A mutant, internalization and Ca2+ influx are not affected. This observation suggests that a different mechanism is involved in reducing the migration of cells expressing the CXCR3 S245A mutant at high levels of CXCL11. We show here that cells expressing the CXCR3 S245A mutant have a defect in adhesion and spreading to integrin ligands such as FN and VCAM-1.
Rearrangement of the actin cytoskeleton is an early cellular event in the migration response to chemokines. Since CXCR3 plays a role in the trafficking of immune cells and the actin cytoskeleton is involved in cell morphologic changes and motility, it seemed reasonable that the inhibition of migration by enhancing the adhesion of cells to the extracellular membrane (ECM) and integrin ligands would involve reorganization of the actin-based cytoskeleton. The loss of S245 in the 3i loop of CXCR3 significantly reduced actin polymerization in response to CXCL11. Sequential actin assembly and disassembly are necessary to regulate the balance between adhesion and chemotaxis. An increased rate of actin polymerization is associated with an increased integrin function and firm adhesion. Since the difference of accumulation in actin polymerization between the WT and S245A CXCR3 was observed only at high levels and not at low and chemotactic doses of the ligand, it is therefore possible that high concentrations of CXCL11 stimulate the adhesion of HEK 293 cells to collagen and prevent their migration. Indeed, we show that Jurkat cells that express the mutated S245 CXCR3 and migrate well in response to CXCL11 failed to adhere to VCAM-1 or FN in response to CXCL11. We therefore suggest that loss of S245 in the 3i loop of CXCR3 reduces actin polymerization and adhesion and allows the cells to continue migrating at high concentrations of the ligand.
Recently, it has been shown that the phosphorylated 3i loop of CXCR4 binds β-arrestin, and that this interaction strongly influences receptor internalization and extracellular signal–regulated kinase activation.32 It is therefore possible that binding of β-arrestin to the phosphorylated form of S245 in CXCR3 may be followed by uncoupling of the receptor from Gαi, resulting in a shutoff of the migratory response and an increased signaling through molecular pathways that enhance actin polymerization and adhesion. Consequently, enhanced adhesion of cells to the extracellular matrix will reduce their ability to migrate in response to the chemokine.
Receptor internalization provides an additional pathway for regulating receptor expression and/or its adhesion. Our results indicate that ligand- and PKC-induced internalization of CXCR3 occurs through independent intracellular pathways. This phenomenon involving different mechanisms of receptor internalization may imply that the intracellular signaling pathways that activate PKC could lead to the internalization of CXCR3. The fact that PKC is not involved in agonist-induced CXCR3 internalization is supported by previous studies done on CXCR3 and CXCR4 in T cells.2,39 In this study, we have shown that of the PKC isotypes, PKCα regulates the PKC-induced internalization in HEK 293 cells. It is important to note that the PKC isotype may depend on the type of the immune response and the type of the immune cells involved in that response.
The mechanism regulating CXCL11-induced internalization of CXCR3 is similar to that of CXCR2 studied in HEK 293 cells, since in both receptors, the carboxyl serine-threonine domain is not involved in the receptor internalization induced by its ligand.29 This mechanism is different from that of CXCR1 since its C terminus phosphorylation sites are required for the ligand-mediated internalization of the human receptor in HEK 293 and RBL-2H3 cells,24,29 and the rabbit receptor in CHO cells.40 Several studies of CXCR4 internalization mediated by its ligand CXCL12 (SDF-1) demonstrate, both in COS-1 and HEK 293 cells, that the dileucine motif and serines in its C terminal are required to trigger this process.19 The CXCR4 3i loop has been shown to be dispensable for ligand-induced internalization, while another study claims that both the 3i loop and the C terminus of CXCR4 regulate its internalization via a direct interaction with β-arrestin.31,32
The dileucine and Ile-Leu motifs are critical for internalization of GPCRs,17 including the CKRs CXCR218 and CXCR4.19 Trileucine in tandem are present in the carboxyl terminus of CXCR3, suggesting a potential role of this motif in the internalization of the receptor. The dileucine motif has been shown to mediate association of GPCRs, specifically of CXCR2, with the AP-2-clathrin-adaptor complex.18
In contrast to the results presented in this study, it was recently suggested that the C terminal domain of human CXCR3 is not essential for CXCL11-dependent internalization of human CXCR3 overexpressed in murine 300-19 pre-B cells and that the third extracellular domain of CXCR3 in these cells is predominantly involved in this process.20 Our studies using 4 different cell lines overexpressing the S245A CXCR3, suggest that the serine localized in the 3i loop is not essential for the internalization of the CXCR3 receptor. In the studies performed by Colvin et al,20 the entire CXCR3 3i loop was replaced with the CXCR1 3i loop. This may completely change the folding and performance of the CXCR3 receptor and may in fact distract the internalization process. We found that WT and S245A CXCR3 are expressed and internalized similarly in response to CXCL11 by rat RBL cells and human HEK 293 cells. However, RBL cells did not migrate in response to CXCL11. Similar to RBL cells, WT and S245A CXCR3 are expressed and internalized in YTS cells; however, these cells also poorly migrate in response to CXCL11. In contrast to RBL and YTS cells, Jurkat cells both internalized and migrated well in response to CXCL11. These results suggest that CXCR3-dependent internalization and migration of cells in response to CXCL11 is strongly influenced by the type of cells tested. The use of nonhuman murine 300-19 pre-B cells in Colvin et al's studies may explain the differences between the 2 observations.20
This study provides a novel insight into elements in the carboxyl-terminus and third intracellular loop of CXCR3 that are involved in the regulation of CXCR3-induced internalization, migration, and adhesion. Furthermore, this study suggests that at least for the chemokine receptor CXCR3, the type of cells used to study its function may significantly influence the results. This may not be surprising since CXCR3 is used mainly by activated T and NK cells to direct their trafficking to the Th1 response sites. It is known that activation of these cells differentially affects the expression of CXCR3 compared with the CXC chemokine receptors CXCR1, 2, and 4.41 Moreover, during activation of T and NK cells, the gene-expression pattern of these cells is significantly changed. This may lead to a unique cytoplasmic microenvironment in which the CXCR3 receptor is functioning. It is therefore not clear whether using nonhuman, nonactivated cells, such as mast cells (RBLs) or murine 300-19 pre-B cells, would be appropriate to study CXCR3 expression and function. More studies are needed in order to find out whether Jurkat T cells and HEK293 cells are indeed of relevance for the study of CXCR3 receptor expression and function.
Prepublished online as Blood First Edition Paper, December 20, 2005; DOI 10.1182/blood-2004-01-0214.
Supported by grants from the Israel Ministry of Health (Grant No. 5052), the Israel Cancer Research Fund (grant No. 20020085), and the Israel Cancer Association through the donation from Mr David Brown, Miami, FL, USA, in memory of Melvin M. Brown.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Yoram Altschuler, Department of Pharmacology, the Hebrew University of Jerusalem, Israel; Chai Ezerzer, for helping with the PCR of the site-directed mutagenesis; and P. Loetscher and B. Moser, University of Bern, Switzerland, for supplying us with the WT CXCR3 construct.

![Figure 2. Cell-surface expression of WT and mutated CXCR3 receptors on HEK 293 cells. WT and mutant CXCR3 cell-surface expression on stable transfected HEK 293 cell clones was tested by immunostaining with anti-CXCR3 specific antibodies and analyzed by FACS (black line). Immunostaining with IgG1 isotype control antibodies is in gray line. (A) WT CXCR3. (B) 349stopΔ20 C-tail truncated. (C) 332stopΔ37 C-tail truncated. (D) 332-334L→A C-tail mutations. (E) S245 to A 3i loop mutation. (F) Double-mutant of 332stopΔ37 C-tail truncated and S245 to A 3i loop mutation. (G) HEK 293 cells. Counts indicate relative cell number. A representative histogram of at least 3 experiments performed is presented. (H) Binding of biotinylated I-TAC (mean florescence intensity [MFI]) at different concentrations (nM) to 293 cells expressing the different mutated or truncated CXCR3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/10/10.1182_blood-2004-01-0214/2/m_zh80100695830002.jpeg?Expires=1767957082&Signature=r3IyB1gpuaZ8yQVd6s8rM8RhTjH35SkQ1BxTHXfYqlYBMPkWS5f~9taWSNiYEFcd3fkgu-Si72JUu1c-dVe5VPonpCB7gTL6mJsN-WrNiVIPSye7A~2xkwH1oRew1FH1XZs1Nl44VyX6~dXCErQ7gPYhaCrVDvPWA2TFZLM1FJK5uv0-QkOsQkMUIDyJk9U7Z28cFbgwkJMewDOUHygWLAXXob0bXSJ-pcMoQElZqXJjs4Nm93HIbbcxsiLzdZBzkLGDFP31odhz0Unrm0UVc8rKIZSWB6UeZ56CkDeA2Nyyz2f030nfQp~7YVX286iYoH0mcfsVDeF28RjDLKmSpQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Reduction of CXCR3 cell-surface expression following CXCL11 and PMA stimulation. (A) Time course of the reduction of CXCR3 expression in response to CXCL11 and PMA in 293-CXCR3 cells. CXCR3-expressing HEK 293 cells were trypsinized and incubated with either 1 mg/mL CXCL11 or 100 ng/mL PMA for the indicated time points at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. Each value represents the mean plus or minus SE of 3 to 4 independent experiments. (B) Effect of PKC inhibitors on ligand- or PMA-induced CXCR3 down-modulation on the surface of 293 cells. 293-CXCR3 cells were trypsinized and incubated in medium alone or with a PKC inhibitor (staurosporin [0.5 μM], GF [5 μM], or rottlerin [5 μM]) for 30 minutes at 37°C, and then challenged with 1μg/mL CXCL11 or 100 ng/mL PMA for 1 hour at 37°C. The cells were then washed, and surface expression of CXCR3 receptors was analyzed. Each value represents the mean ± SE of 4 independent experiments. *P < .05 by ANOVA and Dunnett test for internalization induced by PMA with staurosporin versus without staurosporin, and with GF versus without GF. (C) The CXCR3-expressing HEK 293 cells, WT CXCR3, 349stopΔ20 C-tail truncated, 332stopΔ37 C-tail truncated, and 332-334L→A C-tail mutations were trypsinized and incubated with CXCL11 (1 μg/mL) or PMA (100 ng/mL) for 1 hour at 37°C. The cells were then washed, immunostained with anti-CXCR3–specific antibodies, and FACS analyzed. Each value represents the mean ± SE of at least 3 independent experiments. *P < 05 by ANOVA and Dunnett test for D37 CXCL11- and PMA-induced internalization versus WT.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/10/10.1182_blood-2004-01-0214/2/m_zh80100695830006.jpeg?Expires=1767957082&Signature=v6NLr-~sAP6itwHokyFJqQa99n99fNIKM5FeFkm-EViBmtqklL3I40PET9lcUbvuMHL0kN574FEifaSndqnZPJN1fVBllqBdohyiKVGW2fO5hdEoCWAgjNsRbkBwpgr015kkGenQKnhDvnD12FA9pVKn0swdgdK7QzvuSsx~jdVKYLGWoEjrf~CisNe2-Inbo6Nb~VVGTnVcyHQ5bcZwA2cVtGL8KKAadAFHI5Z~dn4re5gg6NfWf2SNDWhi~7TafkY6rwtq8OCdyHlDeCQuMEk0HKfedVCKRGg60TS6dk6WYx6T29yRzPFYoHuTKon7~jHOILB0z7VSLT5vbOeFJw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal