In solid tumors, leukemias, and lymphomas, increased frequencies of functional CD4+CD25high regulatory T cells (Treg cells) have been previously demonstrated. In healthy individuals, Treg cells consist not only of memory but also of naive T cells, which can undergo peripheral expansion and are characterized by a relative enrichment for autoreactive T-cell receptors. Here, we demonstrate in patients with premalignant monoclonal gammopathy of undetermined significance and patients with multiple myeloma that functional FoxP3+ Treg cells of naive, central, and effector memory phenotype as determined by CCR7 and CD45RA expression are significantly expanded. Low frequencies of T-cell receptor excision circles in naive Treg cells in both healthy controls and multiple myeloma patients point to peripheral expansion as the prominent mechanism of increased frequencies of naive Treg cells in these cancer patients. These findings strongly suggest that the increase of functional Treg cells in cancer patients is a response to the process of malignant transformation.
Introduction
The lack of clinically sufficient antitumor immune responses has been attributed to soluble inhibitory factors such as TGFβ11,2 or PGE23-5 as well as the induction and expansion of regulatory cells.6,7 CD4+CD25high regulatory T cells (Treg cells) were shown to be expanded in murine tumor models.8 Moreover, their deletion reinstated an efficient antitumor immune response leading to complete tumor regression.9-11 We and others have demonstrated that CD4+CD25highFoxP3+ Treg cells are also expanded in patients with solid tumors,12-19 Hodgkin lymphoma,20,21 or B-cell chronic lymphocytic leukemia (CLL).22
Both in humans and in animal models, Treg cells have been described as anergic cells exerting strong suppression after T-cell receptor (TCR) stimulation.23-25 As demonstrated in murine models, natural Treg cells usually originate from the thymus,26,27 although cells with similar characteristics can also be generated in the periphery under appropriate conditions.28 More recently, the diversity and developmental stage of thymic emigrants with a Treg-cell phenotype as well as CD4+CD25+ Treg cells within peripheral blood were examined.29,30 In healthy individuals, the levels of T-cell receptor excision circles (TRECs) were comparable in both conventional CD4+CD25– and regulatory CD4+CD25+ thymic populations. However, the number of TRECs was significantly higher in thymic emigrants than in peripheral blood–derived T cells, which strongly suggests thymic development of human CD4+CD25high Treg cells.30 Nevertheless, conventional CD4+CD25– T cells from peripheral blood of healthy donors contained higher TREC numbers than their CD4+CD25+ counterparts, which is in line with the possibility of extrathymic expansion particularly within the Treg-cell subset.29 Until recently, CD4+CD25high Treg cells have been described to belong to the memory T-cell compartment.24,31-33 Valmori et al,34 however, identified a Treg-cell population with a naive phenotype (CCR7+CD45RA+), which they termed natural naive Treg cells (NnTregs). As expected, the frequency of these NnTregs was relatively low in healthy individuals. NnTregs were shown to vigorously proliferate in response to contact with autologous antigen-presenting cells, suggesting that particularly this subpopulation is enriched in T cells bearing self-reactive T-cell receptors.34 Most recently, Seddiki et al35 described the persistence of a population of naive CD45RA+ Treg cells in adult life.
Little is known about the differentiation, origin, and mechanisms of expansion of Treg cells in cancer patients. We and others have observed that increase of Treg-cell frequency correlates with disease state,13,22 which might be explained by an antigen-dependent mechanism of peripheral expansion in response to tumor progression. However, it is unknown if these Treg cells are also more differentiated toward a central or even effector memory phenotype.
Most recently, Prabhala et al36 published surprising results concerning reduced numbers of CD4+FoxP3+ T cells in patients with multiple myeloma (MM) as well as in premalignant monoclonal gammopathy of undetermined significance (MGUS). Whether these cells were coexpressing CD25 was not determined in this study. This is of particular interest since MM has been associated with immune dysfunctions,37-40 which alternatively might be explained by an increase of regulatory or immune inhibitory mechanisms.41 MM has been associated with decreased T-cell responses to mitogenic and TCR-mediated stimulations37-40 that might be—at least in part—due to induction of regulatory T cells.41,42
Based on these recent findings, we were interested to answer 2 main questions. First, we wanted to assess frequency and function of CD4+CD25highFoxP3+ Treg cells in MM patients using an experimental approach previously applied by us and others in patients with other types of cancer.12-22 Second, we were interested if naive Treg cells exist in patients with MGUS and MM. Therefore, we have assessed frequency, function, and phenotype of Treg cells in 9 MGUS and 67 MM patients. We demonstrate a significantly increased frequency of CD4+CD25highFoxP3+ Treg cells with strong inhibitory function in MGUS as well as untreated or treated MM patients. Unexpectedly, not only Treg cells of central or effector memory phenotype were expanded, but also naive CCR7+CD45RA+ Treg cells. We further demonstrate TREC expression in both MM patients and healthy individuals to be restricted to T cells of a naive phenotype, with the largest TREC numbers expressed by conventional CD4+CD25– T cells, followed by CD4+CD25low T cells, and the lowest numbers in CD4+CD25high Treg cells. These data strongly suggest that increased frequencies observed in patients with malignant disease are due to peripheral expansion of not only Treg cells of central and effector memory but also of Treg cells of a naive phenotype.
Patients, materials, and methods
Patients and clinical parameters
Following approval by our institutional review board (University Ethics Committee, Cologne, Germany), peripheral blood (PB) from 42 healthy individuals, 9 MGUS patients, and 67 MM patients as well as bone marrow (BM) from 6 MM patients was taken after informed consent was obtained in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were obtained using Ficoll/Hypaque (Amersham, Uppsala, Sweden) density centrifugation and used immediately or stored in liquid nitrogen until further use, leading to similar phenotypic and functional results. Patients included for phenotypical or functional analysis were either untreated or had not received cytoreductive treatment for a period of at least 1 month prior to investigation. Staging was performed according to the Durie and Salmon classification for MM. Characteristics of the patients studied are summarized in Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Antibodies and fluorescence-activated cell sorter (FACS) analysis
Phenotype of T cells was defined by flow cytometry using the following antibodies: CD4-FITC, OX40-FITC, cytotoxic T lymphocyte–associated protein 4 (CTLA4)–PE, CD45RA-PE-Cy-5, CD4-APC, CD4-APC-Cy-7 (all from Becton Dickinson PharMingen, Heidelberg, Germany), CD25-PE, CD62L-PE, CD25-PE-Cy7 (all from Becton Dickinson Biosciences, Heidelberg, Germany), CCR7-FITC (R&D Systems, Wiesbaden, Germany), as well as the corresponding isotype control antibodies (BD PharMingen). Intracellular staining was performed with the following antibodies: fork-head box P3 (FoxP3)–PE (eBioscience, San Diego, CA), glucocorticoid-induced TNFR-related protein (GITR)–FITC, IL-10–FITC (both from R&D Systems), TGFβ1-PE (IQ-Products, Groningen, the Netherlands), CTLA4-PE, or with the appropriate isotype controls (all from BD PharMingen).22 Cells were stained according to the manufacturer's recommendations.
Samples were acquired on a FACSCalibur or FACSCanto and analyzed with FACSDiva software (BD Biosciences) or WinMDI 2.8 (http://facs.scripps.edu/software.html). CD25high T cells were gated as previously described.22 Frequencies of CD4+CD25high T cells in PB are shown as percent values of CD4+ T cells. To determine cells positive for Treg-cell markers, we used stringent gating criteria, setting gates at the 1% level of the respective isotype control.
CD4+ T-cell isolation and culture
CD4+ T cells were purified from PBMCs using CD4 MACS Beads (Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously.22 To assess polyclonal CD4+ T-cell activation, 1 × 105 CD4+ T cells/well were activated in AIM-V (Gibco Invitrogen, Karlsruhe, Germany)/EX-Cell 610 (JRH Biosciences, Lenexa, KS) with pokeweed mitogen (PWM, 10 μg/mL), phytohemagglutinin (PHA, 10 μg/mL; both from Sigma-Aldrich, Taufkirchen, Germany), or anti-CD3 (0.2 μg/mL, OKT-3) and anti-CD28 mAb (0.2 μg/mL; kind gift of Dr L. M. Nadler) in 96-well plates. Proliferation of T cells was monitored by measuring BrdU (5-bromo-2′-deoxyuridine) incorporation (Roche Diagnostics, Mannheim, Germany).
Isolation of CD4+CD25high and CD4+CD25– T cells
For functional analysis, CD4+CD25high T cells were purified from PBMCs. Briefly, CD4 MACS Multisort Beads (Miltenyi Biotec) were used for isolation of CD4+ T cells.22 After detaching, cells were washed and CD4+CD25high T cells were positively selected using CD25 microbeads (2 μL beads/107 CD4+ cells). The described technique is optimized for the isolation of human CD4+CD25high T cells with high purity.43 Use of higher concentrations of microbeads for isolation results in better recovery but decreases the purity of the Treg-cell population. The negative fraction of CD4+CD25– T cells from healthy controls was used as effectors to assess Treg-cell function independently of potential defects of conventional CD4+ T cells. Cells were reanalyzed after sorting and routinely showed more than 95% purity.
RNA extraction and real-time RT-PCR for FOXP3
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was used to assess the Treg-cell–associated transcription factor FOXP3. The same primers and conditions were used as previously described.22 Relative FOXP3 expression in CD4+CD25– and CD4+CD25high T cells was normalized to GAPDH expression.
Assessment of inhibitory function of CD4+CD25high T cells
To assess the suppressive activity of CD4+CD25high T cells, a modified allogeneic mixed lymphocyte reaction (MLR) was performed as previously described.22 Briefly, after magnetic separation both highly purified CD4+CD25– and CD4+CD25high T cells were incubated for 20 hours with 10 U/mL IL-2 (Proleukin; Chiron, Munich, Germany) and 0.5 μg/mL anti-CD3 mAb in X-VIVO 15 (BioWhittakker, Verviers, Belgium).22,44-46 Subsequently, CD4+CD25– T cells (5 × 104/well) were cocultured with irradiated allogeneic PBMCs (2 × 105/well) in X-VIVO 15 supplemented with 10% fetal calf serum, 100 U/mL penicillin/streptomycin, and 2 mM glutamine (all from Gibco Invitrogen). Purified allogeneic CD4+CD25high T cells were added at different concentrations as indicated.34,47,48 On day 4, the cells were pulsed with BrdU, and BrdU incorporation was analyzed 20 hours later. There was no influence on the inhibitory effect of CD4+ CD25high T cells when CD4+CD25– T cells were not preactivated prior to the inhibition assay. Alternatively, 5,6-carboxyfluorescein-diacetate-succinimidyl-ester (CFSE; Sigma-Aldrich)–stained CD4+CD25– T cells (5 × 104/well) were stimulated with magnetic beads (Dynal Biotech, Oslo, Norway) coated with 5% anti-CD3 (OKT3), 14% anti-CD28 (9.3, kind gift of Drs C. June and J. Riley), and 81% anti–MHC class I (W6/32) at a ratio of 3:1 (cells-beads). PKH-26 (Sigma-Aldrich)–labeled allogeneic CD4+CD25high T cells were added at a 1:1 ratio to the culture, and proliferation of CD4+CD25– T cells was determined by assessing CFSE dilution after 4 days of culture. Use of autologous CD4+CD25– T cells yielded comparable results; however, for better comparability of Treg cells from healthy controls, MGUS patients, and MM patients, allogeneic CD4+CD25– T cells were used for most experiments.
Cytometric bead array (CBA) for chemokines
The concentration of IFN-γ in cell culture supernatants was measured using the human Th1/Th2 cytokine kit II (BD PharMingen). In brief, capture beads were mixed with culture supernatants and PE detection reagent and incubated for 3 hours at room temperature. The beads were then washed with wash buffer and analyzed.
Isolation of CD4+CD25–, CD4+CD25low, and CD4+CD25high T cells for DNA isolation and assessment of T-cell receptor excision circles
Briefly, CD4 MACS Beads (Miltenyi Biotec) were used for isolation of CD4+ T cells.22 After staining with CCR7-FITC (R&D Systems), CD25-PE, CD45RA-PE-Cy-5, and CD4-APC (all from BD PharMingen) according to the manufacturer's recommendations, CD4+C25–, CD4+CD25low, and CD4+CD25high T cells and the respective T-cell subsets, CCR7+CD45RA+ Tnaive, CCR7+CD45RA– TCM, and CCR7– CD45RA– TEM cells, were purified using a FACSDiVa Cell Sorter (BD Biosciences).
DNA was isolated from purified Tnaive,TCM, and TEM cell subsets of the CD4+CD25–, CD4+CD25low, and CD4+CD25high T-cell subpopulations using a DNA Isolation Kit (Roche Diagnostics) following the manufacturer's instructions.
Relative TREC levels were determined using real-time PCR with a LightCycler (Roche Diagnostics) based on specific primers and general fluorescence detection with SYBR Green. All PCRs were performed using LightCycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics). All samples were studied in duplicate reactions using the human TREC primer kit (Search-LC, Heidelberg, Germany). The number of TREC molecules in the sample was calculated as number of copies per 104 cells (detection limit ≥ 10 molecules).
Statistical analysis
Comparison between paired or unpaired groups was performed using the appropriate Student t test. A P value less than .05 was defined as statistically significant. Due to the explorative nature of this study, no multiplicity adjustment procedures were performed. All statistical analyses were performed using the SPSS statistical software package (SPSS 12.0 for Windows; SPSS, Chicago, IL).
Results
Increased frequencies of CD4+CD25highFoxP3+ Treg cells in peripheral blood and bone marrow of patients with MGUS and MM
Flow cytometry was used to assess frequencies of CD4+CD25high Treg cells in PB of MGUS patients (n = 9), MM patients (n = 67), and healthy controls (n = 26, Figure 1A). The frequency of Treg cells in controls (4.5% ± 1.1%) was similar to previously published results (Figure 1B).22,47 In contrast, individuals with MGUS (n = 9, 6.4% ± 2.2%, P < .05) as well as untreated stage I MM patients (n = 8, 8.0% ± 1.7%, P < .001), treated stage II MM patients (n = 11, 9.6% ± 3.8%, P < .005), and treated stage III MM patients (n = 43, 10.6% ± 3.9%, P < .001) showed significantly increased frequencies of Treg cells compared with healthy individuals (Figure 1B). There was a trend toward higher frequencies of Treg cells in untreated MM patients compared with MGUS patients; however, this did not reach statistical significance. In contrast, there was no statistically significant difference of Treg-cell frequencies between treated and untreated MM patients, suggesting that the treatment of MM patients had no selective effect on T-cell subpopulations. However, the absolute number of T cells was generally decreased after therapy of MM patients (data not shown). For 6 patients, we obtained PB and BM at the same time point. Frequencies of Treg cells in BM were comparable with PB (8.8% ± 1.9% PB vs 9.4% ± 2.7% BM, Figure 1C), indicating that no preferential accumulation in either compartment occurs.
Next, we investigated changes in frequency of Treg cells over time (Figure 1D). In 13 patients, we obtained blood samples at 2 or more different time points more than 6 months apart (range, 9 to 30 months). In 3 MM patients treated with conventional chemotherapy (melphalan or bendamustine) no clear pattern emerged (Figure 1D, Tx), with 1 patient showing similar frequencies at both time points, and 1 patient with lower and 1 patient with higher frequencies after therapy. In 5 MM patients, we obtained blood prior to and after autologous stem cell transplantation (Figure 1D, SCT). Overall, the frequencies of Treg cells at these 2 time points did not differ significantly. For 5 MM patients who had undergone autologous SCT prior to first Treg-cell analysis, lower frequencies were observed at the second time point of analysis (Figure 1D, after SCT). Of interest, these patients were at least in partial remission for the observed time period (range, 9 to 19 months).
Among the 67 MM patients, 34 patients had progressive disease at time of analysis, while 33 had stable disease or were in partial or complete remission. When comparing these 2 patient cohorts irrespective of prior treatment, no significant difference in the proportion of Treg cells was observed, while both patient cohorts showed significantly higher frequencies of Treg cells compared with healthy controls (data not shown).
Next, we assessed FOXP3 expression on both mRNA and protein level. For the assessment of FOXP3 mRNA, we purified CD4+CD25– as well as CD4+CD25high T cells prior to real-time RT-PCR to avoid potential contamination of other cells expressing FOXP3 and to compare FOXP3 mRNA on a cell-to-cell basis. Increased expression of FOXP3 mRNA was observed in CD4+CD25high T cells from MM patients (n = 6) and healthy individuals (n = 7) compared with CD4+CD25– T cells (Figure 1E). Although FOXP3 mRNA concentrations in CD4+CD25high T cells from MM patients were higher than in healthy individuals, this did not reach statistical significance, suggesting that the amount of FOXP3 mRNA per cell is not increased. To assess the frequency of FoxP3+CD4+CD25high Treg cells in MGUS and MM patients, we assessed FoxP3 protein expression on single cells by intracellular flow cytometry (Figure 1F). In MGUS patients (MGUS, n = 8, 3.3% ± 1.4%, P < .01), untreated MM patients (MM no Tx, n = 7, 3.6% ± 2.1%, P < .005), as well as treated MM patients (MM Tx, n = 13, 4.0% ± 2.9%, P < .05), frequencies of FoxP3+CD4+CD25high Treg cells were statistically significantly augmented compared with healthy individuals (control, n = 14, 1.5% ± 0.8%).
Our data indicate that the expansion of CD4+CD25highFoxP3+ Treg cells previously described for patients with other types of cancer13,14,18,22,49 is also apparent in MM as well as the premalignant precursor MGUS. Increased frequencies of Treg cells might therefore be a general phenomenon in cancer patients.
Frequency of CD4+CD25highFoxP3+ T cells. (A) Flow cytometric analysis of CD4 and CD25 on peripheral blood–derived T cells from a healthy individual (top panel) and a multiple myeloma (MM) patient (bottom panel). Samples were stained with the appropriate isotype controls (data not shown) or CD4 and CD25 mAbs. CD4+CD25+ T cells were divided into CD25low and CD25high cells according to previously published data. 22,47 Numbers represent percentage of events within the respective rectangle. Settings shown here were used for the analysis of all samples under study. (B) Frequency of CD4+CD25high T cells in 26 healthy donors and 9 MGUS patients, 8 untreated and 2 treated stage I MM patients, 3 untreated and 11 treated stage II MM patients, and 43 treated stage III MM patients. Shown here are median, 75th percentile (box), standard deviation (whiskers), and outliers (dots) (*P < .05, Student t test). (C) Frequency of CD4+CD25high T cells in peripheral blood (PB) and bone marrow (BM) of 6 MM patients. (D) Serial analysis of CD4+CD25high T cells in MM patients at 2 different time points separated by at least 6 months (Tx indicates conventional chemotherapy [n = 3]; SCT, autologous stem cell transplantation [n = 5]; and after SCT, after autologous SCT [n = 5]). (E) Expression of FOXP3 by human CD4+CD25high T cells. CD4+CD25highT cells (dark gray bars) and CD4+CD25– T cells (light gray bars) were sorted by magnetic activated cell sorting (MACS) from peripheral blood in healthy controls (n = 7) and MM patients (n = 6). Real-time RT-PCR for FOXP3 was performed, and relative expression of FOXP3 in CD4+CD25highT cells as well as CD4+CD25– T cells was normalized to GAPDH as described. 22 Error bars represent standard deviation (SD). (F) Flow cytometric analysis of FoxP3 in CD4+CD25highTreg cells in MGUS (n = 8) as well as untreated (n = 7) and treated (n = 13) MM patients compared with healthy donors (n = 14). Shown are the median, 75th percentile (box), standard deviation (whiskers), and outliers (dots). *P < .05, Student t test.
Frequency of CD4+CD25highFoxP3+ T cells. (A) Flow cytometric analysis of CD4 and CD25 on peripheral blood–derived T cells from a healthy individual (top panel) and a multiple myeloma (MM) patient (bottom panel). Samples were stained with the appropriate isotype controls (data not shown) or CD4 and CD25 mAbs. CD4+CD25+ T cells were divided into CD25low and CD25high cells according to previously published data. 22,47 Numbers represent percentage of events within the respective rectangle. Settings shown here were used for the analysis of all samples under study. (B) Frequency of CD4+CD25high T cells in 26 healthy donors and 9 MGUS patients, 8 untreated and 2 treated stage I MM patients, 3 untreated and 11 treated stage II MM patients, and 43 treated stage III MM patients. Shown here are median, 75th percentile (box), standard deviation (whiskers), and outliers (dots) (*P < .05, Student t test). (C) Frequency of CD4+CD25high T cells in peripheral blood (PB) and bone marrow (BM) of 6 MM patients. (D) Serial analysis of CD4+CD25high T cells in MM patients at 2 different time points separated by at least 6 months (Tx indicates conventional chemotherapy [n = 3]; SCT, autologous stem cell transplantation [n = 5]; and after SCT, after autologous SCT [n = 5]). (E) Expression of FOXP3 by human CD4+CD25high T cells. CD4+CD25highT cells (dark gray bars) and CD4+CD25– T cells (light gray bars) were sorted by magnetic activated cell sorting (MACS) from peripheral blood in healthy controls (n = 7) and MM patients (n = 6). Real-time RT-PCR for FOXP3 was performed, and relative expression of FOXP3 in CD4+CD25highT cells as well as CD4+CD25– T cells was normalized to GAPDH as described. 22 Error bars represent standard deviation (SD). (F) Flow cytometric analysis of FoxP3 in CD4+CD25highTreg cells in MGUS (n = 8) as well as untreated (n = 7) and treated (n = 13) MM patients compared with healthy donors (n = 14). Shown are the median, 75th percentile (box), standard deviation (whiskers), and outliers (dots). *P < .05, Student t test.
CD4+CD25high T cells from MM patients coexpress CTLA4, GITR, OX40, TGFβ1, and IL-10
We also assessed previously described surface receptors associated with Treg cells,25,50-57 including CTLA4, GITR, CD62L, and OX40, on CD4+CD25high T cells in MGUS and MM patients as well as healthy individuals. As summarized in Table 1, the frequencies of Treg cells from PB expressing these surface molecules were always significantly increased in MM patients. This was also true for Treg cells derived from PB of MGUS patients or BM of MM patients, except for OX40.
Expression of proteins associated with Treg cells
. | . | PB MGUS . | . | PB MM . | . | BM MM . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein . | Control, mean (SD) . | Mean (SD) . | P . | Mean (SD) . | P . | Mean (SD) . | P . | |||
| FoxP3 | 1.5 (0.8) | 3.3 (1.4) | < .01 | 3.8 (2.6) | < .005 | ND | NA | |||
| CTLA4 (intra) | 1.7 (0.7) | 3.4 (1.5) | < .005 | 3.9 (1.4) | < .001 | 4.7 (1.3) | < .001 | |||
| CTLA4 (extra) | 0.4 (0.2) | ND | NA | 1.1 (0.5) | < .005 | ND | NA | |||
| GITR | 1.0 (0.3) | 1.8 (0.7) | < .005 | 2.9 (1.4) | < .001 | 1.7 (0.7) | < .01 | |||
| CD62L | 2.7 (1.0) | 5.3 (1.4) | < .001 | 6.5 (2.2) | < .001 | 6.7 (2.5) | < .01 | |||
| OX40 | 1.0 (0.3) | 0.8 (0.4) | NS | 1.8 (0.9) | < .05 | 1.1 (0.5) | NS | |||
| IL-10 | 0.3 (0.2) | ND | NA | 1.4 (0.8) | < .05 | ND | NA | |||
| TGFβ1 | 0.4 (0.2) | ND | NA | 1.7 (0.7) | < .001 | ND | NA | |||
. | . | PB MGUS . | . | PB MM . | . | BM MM . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein . | Control, mean (SD) . | Mean (SD) . | P . | Mean (SD) . | P . | Mean (SD) . | P . | |||
| FoxP3 | 1.5 (0.8) | 3.3 (1.4) | < .01 | 3.8 (2.6) | < .005 | ND | NA | |||
| CTLA4 (intra) | 1.7 (0.7) | 3.4 (1.5) | < .005 | 3.9 (1.4) | < .001 | 4.7 (1.3) | < .001 | |||
| CTLA4 (extra) | 0.4 (0.2) | ND | NA | 1.1 (0.5) | < .005 | ND | NA | |||
| GITR | 1.0 (0.3) | 1.8 (0.7) | < .005 | 2.9 (1.4) | < .001 | 1.7 (0.7) | < .01 | |||
| CD62L | 2.7 (1.0) | 5.3 (1.4) | < .001 | 6.5 (2.2) | < .001 | 6.7 (2.5) | < .01 | |||
| OX40 | 1.0 (0.3) | 0.8 (0.4) | NS | 1.8 (0.9) | < .05 | 1.1 (0.5) | NS | |||
| IL-10 | 0.3 (0.2) | ND | NA | 1.4 (0.8) | < .05 | ND | NA | |||
| TGFβ1 | 0.4 (0.2) | ND | NA | 1.7 (0.7) | < .001 | ND | NA | |||
FoxP3, CTLA4, GITR, CD62L, TGFβ1, and IL-10 expression was assessed on CD4+ T cells coexpressing CD25 by either cell surface or intracellular multicolor flow cytometry. Results are expressed as percent of CD4+ T cells expressing CD4, CD25, and the respective marker.
PB indicates peripheral blood; MGUS indicates monoclonal gammopathy of undetermined significance; MM, multiple myeloma; BM, bone marrow; P, P value, Student t test; ND, not done; NA, not applicable; and NS, not significant.
Intracellular expression of TGFβ1 and IL-10 in PB was assessed since these 2 cytokines have been associated with Treg-cell function.58,59 Similar to the Treg-cell–associated receptors, TGFβ1 and IL-10 were significantly increased in CD4+CD25high T cells from MM patients (Table 1). Overall, CD4+ T cells expressing high levels of CD25, FoxP3, CTLA4, CD62L, GITR, OX40, TGFβ1, and IL-10 are significantly increased in MM patients.
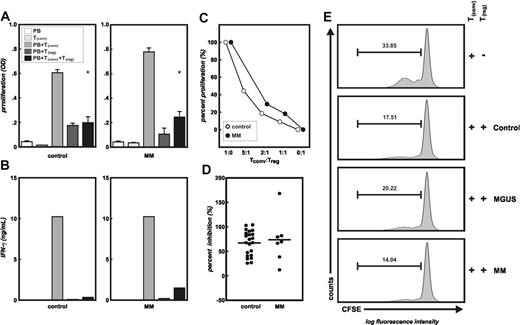
Strong inhibitory function of CD4+CD25high T cells from MM patients
In 7 treated MM patients, sufficient numbers of highly purified CD4+CD25high T cells were isolated to analyze their inhibitory function in comparison with Treg cells from healthy controls (n = 22). Regulatory function of CD4+CD25high T cells was assessed using an allogeneic MLR of CD4+CD25– T cells and allogeneic irradiated PBMCs as stimulators.22,25,34,46-48 As we and others have previously shown, the CD4+CD25high T-cell population in healthy controls exhibits strong inhibitory function on allogeneic T-cell proliferation.22,25,46 As exemplified in Figure 2A, proliferation of allogeneic conventional CD4+CD25– T cells was significantly inhibited when highly purified CD4+CD25high T cells from a healthy donor were added at a 1:1 ratio (P < .001). This was corroborated by a complete loss of IFN-γ production by allogeneic conventional T cells in the presence of Treg cells (Figure 2B). We next assessed highly purified CD4+CD25high Treg cells from treated MM patients at the same ratios for their inhibitory function on allogeneic T-cell proliferation and cytokine production (Figure 2A-B). On a cell-to-cell basis, these Treg cells showed an equally strong inhibitory function on conventional CD4+CD25– T-cell proliferation (Figure 2A) and IFN-γ release (Figure 2B). Titration experiments as exemplified in Figure 2C demonstrated that the inhibitory function of Treg cells from healthy individuals and MM patients was comparable also at lower Treg/Tconv ratios. When comparing all 7 treated MM patients with the healthy controls, no statistically significant difference of inhibitory capacity of Treg cells was observed (Figure 2D). Proliferation of allogeneic or autologous conventional CD4+CD25– T cells stimulated by beads coated with anti-CD3 and anti-CD28 mAbs was used as the readout to assess inhibitory function of CD4+CD25high T cells isolated from MGUS patients (n = 3) or untreated MM patients (n = 3) in comparison with Treg cells isolated from healthy individuals (n = 8, Figure 2E). For better comparison, Figure 2E shows a representative experiment of Treg cells from a healthy individual, an MGUS patient, and an MM patient using the same allogeneic conventional CD4+CD25– T cells as the readout for T-cell suppression by Treg cells. Proliferation of CD4+CD25– T cells was inhibited by addition of CD4+CD25high T cells from healthy donors, MGUS patients, or untreated MM patients at similar levels, confirming full inhibitory function of CD4+CD25high T cells from patients with either MGUS or MM. Similar results were obtained in an MGUS patient when using autologous conventional CD4+CD25– T cells as the readout (data not shown).
Functional analysis of CD4+CD25high T cells. Highly purified CD4+CD25– T cells were stimulated by allogeneic irradiated PBMCs in either the presence or absence of highly purified CD4+CD25highT cells derived from MM patients or healthy donors (both allogeneic). Treg cells from 22 controls and 7 MM patients were assessed in these MLRs. As a function of T-cell inhibition, proliferation (A) and IFN-γ production (B) were measured. Alternatively, Treg cell–induced reduction of proliferation by CD4+CD25– T cells stimulated with beads coated with anti-CD3 and anti-CD28 mAbs was assessed by flow cytometry (E). Panel A shows representative experiments froma healthy control and an MM patient. White bars (PB) indicate background proliferation of irradiated allogeneic PBMCs; light gray bars (Tconv), background proliferation of CD4+CD25– conventional T cells; gray bars (PB+Tconv), alloantigen-induced proliferation of CD4+CD25– conventional T cells; dark gray bars (PB+Treg), background proliferation of CD4+CD25high Treg cells; and black bars (PB+Tconv+Treg), proliferation of CD4+CD25– conventional T cells in the presence of CD4+CD25highTreg cells, at a 1:1 ratio, (*P < .001, Student t test). Error bars represent SD. (B) Measurement of IFN-γ by cytokine bead array in the supernatants from cultures described in panel A. (C) Inhibition of proliferation of CD4+CD25– conventional T cells by CD4+CD25highTreg cells at different ratios (responders to suppressors) from a healthy donor (○) and an MM patient (▪). (D) Percentages of inhibition of proliferation of CD4+CD25– conventional T cells by CD4+CD25highTreg cells at a 1:1 ratio in all healthy donors (n = 22) and MM patients (n = 7). (E) Proliferation of allogeneic CD4+CD25– T cells alone or in presence of CD4+CD25highT cells derived from a healthy donor, an MGUS patient, and an MM patient. One representative experiment of at least 3 for each group is shown here. Percentage of proliferating cells is indicated within the figure.
Functional analysis of CD4+CD25high T cells. Highly purified CD4+CD25– T cells were stimulated by allogeneic irradiated PBMCs in either the presence or absence of highly purified CD4+CD25highT cells derived from MM patients or healthy donors (both allogeneic). Treg cells from 22 controls and 7 MM patients were assessed in these MLRs. As a function of T-cell inhibition, proliferation (A) and IFN-γ production (B) were measured. Alternatively, Treg cell–induced reduction of proliferation by CD4+CD25– T cells stimulated with beads coated with anti-CD3 and anti-CD28 mAbs was assessed by flow cytometry (E). Panel A shows representative experiments froma healthy control and an MM patient. White bars (PB) indicate background proliferation of irradiated allogeneic PBMCs; light gray bars (Tconv), background proliferation of CD4+CD25– conventional T cells; gray bars (PB+Tconv), alloantigen-induced proliferation of CD4+CD25– conventional T cells; dark gray bars (PB+Treg), background proliferation of CD4+CD25high Treg cells; and black bars (PB+Tconv+Treg), proliferation of CD4+CD25– conventional T cells in the presence of CD4+CD25highTreg cells, at a 1:1 ratio, (*P < .001, Student t test). Error bars represent SD. (B) Measurement of IFN-γ by cytokine bead array in the supernatants from cultures described in panel A. (C) Inhibition of proliferation of CD4+CD25– conventional T cells by CD4+CD25highTreg cells at different ratios (responders to suppressors) from a healthy donor (○) and an MM patient (▪). (D) Percentages of inhibition of proliferation of CD4+CD25– conventional T cells by CD4+CD25highTreg cells at a 1:1 ratio in all healthy donors (n = 22) and MM patients (n = 7). (E) Proliferation of allogeneic CD4+CD25– T cells alone or in presence of CD4+CD25highT cells derived from a healthy donor, an MGUS patient, and an MM patient. One representative experiment of at least 3 for each group is shown here. Percentage of proliferating cells is indicated within the figure.
It needs to be emphasized that these assays are normalized for the same numbers of Treg cells from healthy controls and MGUS as well as MM patients. Therefore, since MGUS and MM patients have an increased frequency of these cells, the overall inhibitory effect of the whole increased Treg-cell population in MGUS and MM patients will be even more pronounced in vivo. This might also be reflected by an overall reduction of proliferation of CD4+ T cells in response to mitogenic (PWM, PHA) or even supraphysiologic stimulation with anti-CD3 and anti-CD28 mAbs in MM patients compared with healthy controls (Figure S1).
Increase of Treg cells in MGUS and MM patients includes cells with a naive phenotype
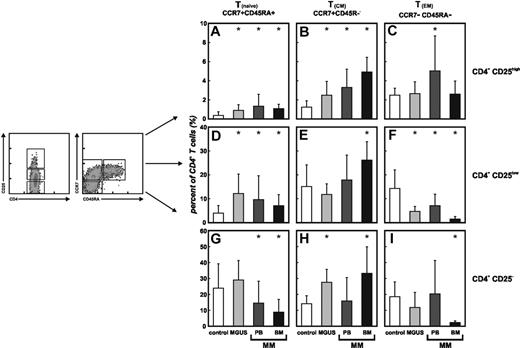
An important question concerning the increase of CD4+CD25highFoxP3+ Treg cells in cancer patients including MGUS and MM is the contribution of different T-cell subsets such as naive, central, and effector memory cells. These cells are defined by differential expression of the cell surface receptors CD45RA and CCR7.60,61 By multicolor flow cytometry, we determined the frequency of naive, central, and effector memory cells within the CD4+CD25high Treg-cell compartment from healthy individuals as well as MGUS and MM patients as previously described by Valmori et al34 and compared those with the distribution within conventional CD4+CD25– T cells and CD4+CD25low T cells (Figure 3). In healthy individuals (white bars), naive CCR7+CD45RA+CD4+CD25high Treg cells were hardly detectable (Figure 3A). Treg cells were almost exclusively of memory phenotype (Figure 3B-C) with a slightly higher frequency of CD4+CD25high TEM cells (Figure 3C). In contrast, in PB of MGUS (light gray bars) and MM patients (dark gray bars) a significant expansion of CD4+CD25high Treg cells with a CCR7+CD45RA+ naive phenotype was observed (Figure 3A). This was further accompanied by an increase of Treg cells with a central memory phenotype in MGUS as well as MM patients (Figure 3B), while effector memory Treg cells were augmented in PB of MM patients (Figure 3C). CD4+CD25low T cells, assumed to be mostly activated T cells, showed an increase of Tnaive cells (Figure 3D) with a concomitant decrease of TEM cells (Figure 3F) in PB of MGUS and MM patients, while frequencies of TCM cells remained unchanged (Figure 3E). Conversely, among conventional CD4+CD25– T cells, Tnaive cells (Figure 3G) were significantly lower in MM patients than in healthy controls, whereas no statistically significant differences were evident for the TCM (Figure 3H) and TEM (Figure 3J) subset. In MGUS patients, frequencies of TCM cells were augmented, while Tnaive and TEM cells were unaffected.
Increase of naive CD4+CD25high T cells in MGUS and MM patients. Frequencies of CCR7+CD45RA+ naive T cells (Tnaive), CCR7+CD45RA– central memory T cells (TCM), and CCR7–CD45RA– effector memory T cells (TEM) were assessed in peripheral blood (PB, n = 18) and bone marrow (BM, n = 6) of MM patients, MGUS patients (MGUS, n = 8), and healthy individuals (control, n = 30). CD4+ T cells were further subdivided into conventional CD4+CD25–, CD4+CD25low, and regulatory CD4+CD25highT cells according to their CD25 expression. Significant differences (P < .05, Student t test) between healthy donors, MGUS patients, and MM patients are marked by an asterisk. Error bars represent SD. (A) Increase of naive CD4+CD25highT cells in PB of patients with MGUS or MM as well as in BM of MM patients. (B) Enrichment of CD4+CD25highTCM cells in PB and BM of patients with MM as well as in MGUS patients. (C) PB of MM patients shows higher frequencies of CD4+CD25high TEM cells. (D) In PB and BM of MM as well as in MGUS patients, we observed an increase of Tnaive cells (F) with a concomitant decrease of TEM cells in the CD4+CD25low T-cell subsets, (E) whereas TCM cells were increased in BM of MM patients. (H) Higher frequencies of TCM cells were also observed in CD4+CD25– T cells in BM of MM patients as well as in PB of MGUS patients, (G) while reduced naive conventional CD4+CD25– T cells were detected in PB and BM of MM patients. (I) CD4+CD25– TEM cells were diminished only in BM of MM patients.
Increase of naive CD4+CD25high T cells in MGUS and MM patients. Frequencies of CCR7+CD45RA+ naive T cells (Tnaive), CCR7+CD45RA– central memory T cells (TCM), and CCR7–CD45RA– effector memory T cells (TEM) were assessed in peripheral blood (PB, n = 18) and bone marrow (BM, n = 6) of MM patients, MGUS patients (MGUS, n = 8), and healthy individuals (control, n = 30). CD4+ T cells were further subdivided into conventional CD4+CD25–, CD4+CD25low, and regulatory CD4+CD25highT cells according to their CD25 expression. Significant differences (P < .05, Student t test) between healthy donors, MGUS patients, and MM patients are marked by an asterisk. Error bars represent SD. (A) Increase of naive CD4+CD25highT cells in PB of patients with MGUS or MM as well as in BM of MM patients. (B) Enrichment of CD4+CD25highTCM cells in PB and BM of patients with MM as well as in MGUS patients. (C) PB of MM patients shows higher frequencies of CD4+CD25high TEM cells. (D) In PB and BM of MM as well as in MGUS patients, we observed an increase of Tnaive cells (F) with a concomitant decrease of TEM cells in the CD4+CD25low T-cell subsets, (E) whereas TCM cells were increased in BM of MM patients. (H) Higher frequencies of TCM cells were also observed in CD4+CD25– T cells in BM of MM patients as well as in PB of MGUS patients, (G) while reduced naive conventional CD4+CD25– T cells were detected in PB and BM of MM patients. (I) CD4+CD25– TEM cells were diminished only in BM of MM patients.
For BM, a preferential TCM phenotype has been described,62 which we could also observe in BM of patients with MM irrespective of CD25 expression (Figure 3B,E,H black bars) with a concomitant decrease of TEM cells (Figure 3C,F,J). However, comparable with PB of MGUS and MM patients, we observed a significant increase of naive CD4+CD25high Treg cells in BM of MM patients (Figure 3A). This increase of Tnaive cells, similar to PB, could also be observed in the CD4+CD25low T cells (Figure 3D), while the decrease of naive conventional CD4+CD25– T cells was also apparent in BM of patients with MM (Figure 3G).
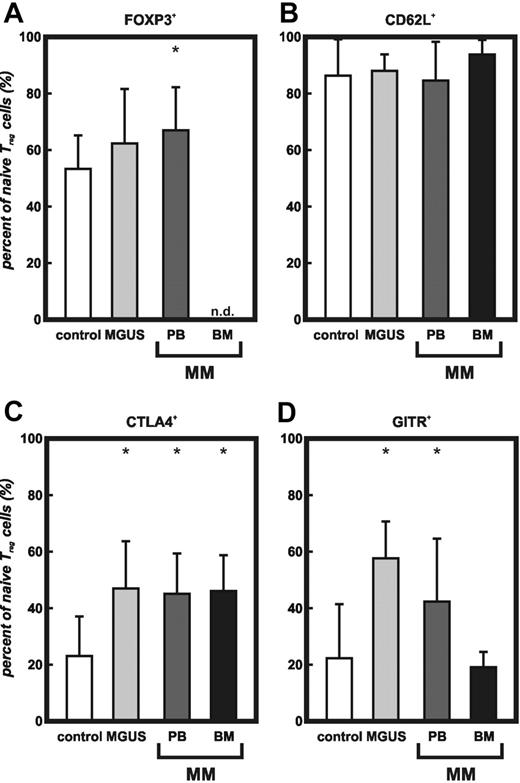
To further characterize the increased subset of naive CD4+CD25high Treg cells, we analyzed the expression of molecules previously associated with Treg cells, particularly FoxP3. In addition to the expression of CD45RA and CCR7, we found significantly increased levels of FoxP3 on naive CD4+CD25high Treg cells in PB of MGUS and MM patients (Figure 4A). CD62L was expressed in comparable amounts on naive CD4+CD25high Treg cells (Figure 4B). Similar to previously published data, expression of intracellular CTLA4 was detectable in naive Treg cells from healthy individuals.34 Analysis of the increased subset of naive Treg cells in PB and BM from MM patients as well as MGUS patients revealed a significantly higher percentage of CTLA4+ naive Treg cells (Figure 4C). Equivalent expression of GITR was observed in naive Treg cells of healthy individuals and in BM of patients with MM, whereas naive Treg cells from PB of MM and MGUS patients showed significantly augmented GITR expression (Figure 4D).
Increase of FoxP3, CTLA4, and GITR expression on naive CD4+CD25high Treg cells from MGUS and MM patients.FoxP3, CD62L, CTLA4, and GITR expression of naive CD4+CD25highT cells in PB of healthy donors, MGUS patients, as well as PB or BM of MM patients coexpressing CD45RA and CCR7 by multicolor flow cytometry. Results are expressed as percent of CCR7+CD45RA+ CD4+CD25high T cells. Error bars represent SD. (A) Intracellular FoxP3 (*MGUS and PB, P < .05, Student t test), (B) extracellular CD62L, (C) intracellular CTLA4 (*MGUS, PB, and BM, P < .01, Student t test), (D) intracellular GITR (*MGUS and PB, P < .05, Student t test). Control indicates peripheral blood from healthy individuals (n = 13); MGUS, peripheral blood from MGUS patients (n = 8); PB, peripheral blood from MM patients (n = 7); BM, bone marrow from MM patients (n = 6); and n.d., not done.
Increase of FoxP3, CTLA4, and GITR expression on naive CD4+CD25high Treg cells from MGUS and MM patients.FoxP3, CD62L, CTLA4, and GITR expression of naive CD4+CD25highT cells in PB of healthy donors, MGUS patients, as well as PB or BM of MM patients coexpressing CD45RA and CCR7 by multicolor flow cytometry. Results are expressed as percent of CCR7+CD45RA+ CD4+CD25high T cells. Error bars represent SD. (A) Intracellular FoxP3 (*MGUS and PB, P < .05, Student t test), (B) extracellular CD62L, (C) intracellular CTLA4 (*MGUS, PB, and BM, P < .01, Student t test), (D) intracellular GITR (*MGUS and PB, P < .05, Student t test). Control indicates peripheral blood from healthy individuals (n = 13); MGUS, peripheral blood from MGUS patients (n = 8); PB, peripheral blood from MM patients (n = 7); BM, bone marrow from MM patients (n = 6); and n.d., not done.
Peripheral expansion of naive CD4+CD25high Treg cells in patients with MM
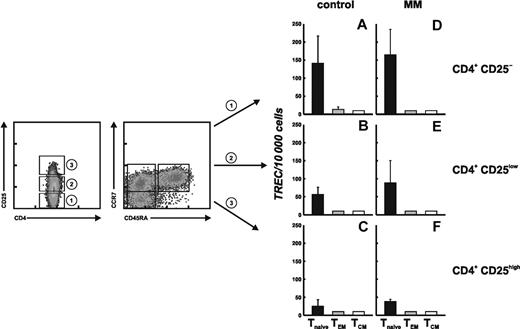
As Treg cells with a naive phenotype are increased in patients with MM, we were interested if the increase of Treg cells results from peripheral expansion or possibly thymic generation of CD4+CD25high T cells. The level of TRECs has been used as a marker to estimate the developmental vicinity of T cells to the thymus and their division history.30 We therefore applied TREC analysis to further characterize Treg cells with a naive phenotype in MM patients. PB-derived CD4+ T cells from 2 MM patients and 2 age-matched healthy individuals were first sorted into conventional CD4+CD25–, CD4+CD25low, and regulatory CD4+CD25high T cells according to their CD25 expression. Subsequently, the cells were separated into the appropriate T-cell subsets, namely Tnaive, TCM, and TEM, defined by their expression of CCR7 and CD45RA.
TREC values for all 9 highly purified T-cell subsets were assayed by real-time PCR. As expected, the TREC content of conventional CD4+CD25– Tnaive cells from healthy individuals was significantly higher than that of CD4+CD25– TCM or TEM cells (Figure 5A). CD4+CD25low T cells (Figure 5B), primarily consisting of recently activated T cells, showed lower TREC levels in Tnaive cells than in conventional CD4+CD25– T cells, and TREC levels were below the detection limit in TCM and TEM cells in CD25– T cells (Figure 5A) and CD25low T cells (Figure 5B) from healthy individuals. These data are in accordance with the concept of T-cell activation and memory T-cell generation, as CD4+CD25low T cells should consist predominantly of activated antigen-experienced memory T cells. CD4+CD25high Treg cells showed even lower TREC contents in the naive T-cell population, and TREC levels were below the detection threshold in the TCM and TEM subsets (Figure 5C). These observations are in line with the concept of antigen-driven peripheral expansion of Treg cells in healthy individuals rather than recent thymic emigration.
Despite significant differences in the frequencies of the distinct T-cell subsets in patients with MM, the TREC content was comparable between MM patients and healthy controls on the single-cell level (Figure 5D-F), suggesting that the underlying mechanism of Treg-cell generation and differentiation is the same in healthy individuals and MM patients. This implies that the increased frequency of naive as well as central and effector memory Treg cells in MM patients is due to peripheral expansion of Treg cells rather than newly generated Treg cells within the thymus.
Discussion
Here, we have addressed 2 main questions concerning CD4+CD25highFoxP3+ Treg cells in MGUS and MM patients. First, are Treg cells similarly expanded and functional in MGUS and MM patients, as has been shown for other cancer patients including solid tumors as well as CLL? Second, are Treg cells with a naive phenotype present in cancer patients? Using previously published experimental approaches, we demonstrate an increase of fully functional Treg cells in MGUS and MM patients. For the first time, we demonstrate that not only Treg cells with a memory phenotype but also naive CCR7+CD45RA+ CD4+CD25highFoxP3+ T cells are expanded in cancer patients. This cell compartment is further characterized by an increase of cells coexpressing CTLA4 and GITR, well-known markers associated with Treg cells. In addition, by TREC analysis we demonstrate that increased numbers of naive Treg cells in MM patients are most likely explained by an increased peripheral expansion of these cells. The assessment of FoxP3 protein expression by CD4+CD25high T cells has clearly revealed that there is an increase in frequency of these cells rather than an increase of expression within individual cells. Costaining experiments of intracellular FoxP3 with other previously suggested Treg-cell markers once adequate antibody combinations are available will further elucidate the functional relationship of these proteins on Treg cells, not only in MM patients, but also healthy individuals.
Replicative history of CD4+ T-cell populations defined by the expression of CD45RA, CCR7, and CD25. CD4+ T cells were separated into conventional CD4+CD25– (1), CD4+CD25low (2), and regulatory CD4+CD25highT cells (3) defined by their expression of CD25. These subsets were further sorted according to their CD45RA and CCR7 expression in 3 subsets each, namely Tnaive (CD45RA+CCR7+), TCM (CD45RA–CCR7+), and TEM cells (CD45RA–CCR7–). These CD4+ T-cell subsets were then assessed for TREC content. Genomic DNA of sorted subsets was isolated, and the number of TRECs was determined by quantitative real-time PCR. Data are shown as the mean values obtained for 2 independent donors and 2 MM patients. Error bars represent SD. (A) Conventional CD4+CD25–, (B) CD4+CD25low, and (C) CD4+CD25highT cells from 2 healthy individuals. (D) Conventional CD4+CD25–, (E) CD4+CD25low, and (F) CD4+CD25highT cells from 2 MM patients.
Replicative history of CD4+ T-cell populations defined by the expression of CD45RA, CCR7, and CD25. CD4+ T cells were separated into conventional CD4+CD25– (1), CD4+CD25low (2), and regulatory CD4+CD25highT cells (3) defined by their expression of CD25. These subsets were further sorted according to their CD45RA and CCR7 expression in 3 subsets each, namely Tnaive (CD45RA+CCR7+), TCM (CD45RA–CCR7+), and TEM cells (CD45RA–CCR7–). These CD4+ T-cell subsets were then assessed for TREC content. Genomic DNA of sorted subsets was isolated, and the number of TRECs was determined by quantitative real-time PCR. Data are shown as the mean values obtained for 2 independent donors and 2 MM patients. Error bars represent SD. (A) Conventional CD4+CD25–, (B) CD4+CD25low, and (C) CD4+CD25highT cells from 2 healthy individuals. (D) Conventional CD4+CD25–, (E) CD4+CD25low, and (F) CD4+CD25highT cells from 2 MM patients.
Most recently, Prabhala et al36 reported lower frequencies of CD4+FoxP3+ T cells in MM patients. So far, only increased frequencies of CD4+CD25+ Treg cells with strong inhibitory function were observed in cancer patients,12-19 Hodgkin lymphoma,20,21 and CLL.22 When assessing FoxP3 expression in the context of CD25 expression, we found increased frequencies of CD4+CD25highFoxP3+ Treg cells with normal inhibitory function in MGUS and MM patients. The different results obtained by the previous study are most likely explained by the different experimental approaches chosen. For example, Treg-cell function in the prior study was assessed using bulk PBMCs depleted of CD25+ cells, while we and others used purified cells excluding effects that might be due to other cells within PBMCs. In fact, the significantly lower proliferation of CD25– PBMCs in MGUS and MM patients reflects differences that already exist between healthy controls and patients that cannot be contributed to Treg-cell function. It has been previously stated by Baecher-Allan et al63 that the assessment of human Treg cells is still difficult and the use of different assays sometimes makes it difficult to compare different studies. We and others have previously observed that the analysis of Treg cells in cancer patients can be hampered by concomitant defects in the conventional T-cell compartment.19,22 This is particularly true in situations where tumor patients already show a reduced proliferative response of total CD4+ T cells (Figure S1), where further inhibitory effects induced by Treg cells are not easily assessed. To circumvent such limitations, we have deployed 2 allogeneic systems where conventional T cells from a healthy donor are used as a readout system to ensure comparability of Treg-cell function of healthy donors and MGUS or MM patients, thereby reducing variables that might have an impact on the outcome of the assessment of Treg-cell function.
An important issue of Treg-cell biology in cancer is to better understand why there is an expansion and how the expansion might be explained. Recent studies have clearly indicated that antigen-specific expansion of Treg cells must occur in cancer, since Treg-cell clones were established and shown to recognize tumor antigen in an MHC class II–restricted fashion.42 Furthermore, in healthy individuals it was demonstrated that Treg cells exist at all differentiation states, namely naive, central, and effector memory.30,34,35 We therefore addressed whether the increase of Treg cells would be solely driven by an expansion of memory Treg cells. Surprisingly, we did find a significant expansion not only of Treg cells with a central or effector memory phenotype, but also of naive CCR7+CD45RA+ Treg cells. This was not restricted to MM patients but was also observed in MGUS patients, suggesting that the pool of naive Treg cells is also expanded at an early disease state and during tumor progression. Similar to observations in healthy individuals,34,35 this opened the question whether naive Treg cells might be recent thymic emigrants or whether peripheral expansion might account for the increased number of these cells. At least for conventional naive T cells, peripheral expansion is well established.64 The analysis of TREC is prone to answer this critical question. By isolation of 9 T-cell subsets dependent on expression of CD25 and differentiation state, we clearly demonstrate that only T cells of a naive phenotype expressing CCR7 and CD45RA—irrespective of CD25 expression—contain detectable amounts of TRECs. In contrast, TRECs were not detected in central and memory effector T cells of healthy individuals and MM patients. Of interest, the number of TRECs differed between CD25–, CD25low, and CD25high naive T-cell subpopulations. In both MM patients and healthy individuals, we found the highest numbers of TRECs in conventional CD25– T cells followed by the CD25low population and lowest numbers in the CD25high Treg cells. We conclude from these findings that the increased frequency of naive Treg cells is most likely due to peripheral expansion. Differences in frequencies of Treg cells in different compartments as observed in bone marrow or peripheral blood from MM patients most likely reflect different migration patterns of Treg cells with a naive, central memory, or effector memory phenotype. In murine model systems, it has recently been demonstrated that appropriate localization is crucial for in vivo activity of Treg cells.65
Our findings open new aspects of Treg-cell biology in cancer patients. It will be an important issue to address whether antigen-specific Treg-cell clones can be established only from the central or effector memory phenotype subpopulations, or whether the Treg-cell compartment with a naive phenotype already contains such cells.42 Furthermore, it will also be interesting to see whether Treg-cell expansion in solid tumors is also accompanied by the expansion of naive Treg cells and whether there are differences in different compartments (eg, blood, secondary lymphoid organs, and bone marrow) or at the tumor site.
Taken together, CD4+CD25highFoxP3+ Treg cells are increased and fully functional in MGUS as well as early untreated or late-stage–treated MM patients, which might play a crucial role for some of the immune dysfunctions observed in MM patients. By analysis of CCR7 and CD45RA, we demonstrate the expansion of naive Treg cells. According to our TREC analysis, this increase is due to peripheral expansion of naive Treg cells. These findings open new avenues to study Treg-cell biology in the context of tumor development and progression.
Prepublished online as Blood First Edition Paper, January 12, 2006; DOI 10.1182/blood-2005-09-3671.
Supported by the Sofja Kovalevskaja Award of the Alexander von Humboldt Foundation (J.L.S.), the Wilhelm-Sander Stiftung (J.L.S. and M.K.), and the Nationales Genomforschungsnetz (J.L.S.). P.A.K. and E.E. are supported in part by grant HBFG-109-517.
M.B. designed research, performed research, wrote the paper, and analyzed data; M.K. designed research and analyzed data; T.G. contributed vital analytic tools and performed research; E.E. contributed vital analytic tools and performed research; M.R.W. performed research; P.A.K. contributed vital analytic tools; S.C. performed research; and J.L.S. designed research, wrote the paper, and analyzed data.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to our patients for their commitment to this study. We thank I. Büchmann and J. Claasen for excellent technical assistance, K.-H. Grips and D. Gerecke for referral of patients, C. June and J. Riley for providing us with the 9.3 antibody, and B. Gathof and the Division of Transfusion Medicine for providing us with blood samples from healthy individuals.

![Figure 1. Frequency of CD4+CD25high FoxP3+ T cells. (A) Flow cytometric analysis of CD4 and CD25 on peripheral blood–derived T cells from a healthy individual (top panel) and a multiple myeloma (MM) patient (bottom panel). Samples were stained with the appropriate isotype controls (data not shown) or CD4 and CD25 mAbs. CD4+CD25+ T cells were divided into CD25low and CD25high cells according to previously published data. 22,47 Numbers represent percentage of events within the respective rectangle. Settings shown here were used for the analysis of all samples under study. (B) Frequency of CD4+CD25high T cells in 26 healthy donors and 9 MGUS patients, 8 untreated and 2 treated stage I MM patients, 3 untreated and 11 treated stage II MM patients, and 43 treated stage III MM patients. Shown here are median, 75th percentile (box), standard deviation (whiskers), and outliers (dots) (*P < .05, Student t test). (C) Frequency of CD4+CD25high T cells in peripheral blood (PB) and bone marrow (BM) of 6 MM patients. (D) Serial analysis of CD4+CD25high T cells in MM patients at 2 different time points separated by at least 6 months (Tx indicates conventional chemotherapy [n = 3]; SCT, autologous stem cell transplantation [n = 5]; and after SCT, after autologous SCT [n = 5]). (E) Expression of FOXP3 by human CD4+CD25high T cells. CD4+CD25highT cells (dark gray bars) and CD4+CD25– T cells (light gray bars) were sorted by magnetic activated cell sorting (MACS) from peripheral blood in healthy controls (n = 7) and MM patients (n = 6). Real-time RT-PCR for FOXP3 was performed, and relative expression of FOXP3 in CD4+CD25highT cells as well as CD4+CD25– T cells was normalized to GAPDH as described. 22 Error bars represent standard deviation (SD). (F) Flow cytometric analysis of FoxP3 in CD4+CD25highTreg cells in MGUS (n = 8) as well as untreated (n = 7) and treated (n = 13) MM patients compared with healthy donors (n = 14). Shown are the median, 75th percentile (box), standard deviation (whiskers), and outliers (dots). *P < .05, Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/10/10.1182_blood-2005-09-3671/2/m_zh80100695740001.jpeg?Expires=1767730597&Signature=Y0hQFgi1cIhKS7l5uElYF4nrsRWnfY5gjeCMO0hBZAtRMHscNUhjVFyfyzeB4KFd7lG8soEnvWA~SiIX-s4cVsyutH6hbAfuo9XwPQbe9b8NuBMGewgM5WAFMKD32GGZiewtd9yPH-yAG8MHvCqba-JeCeZd9Y4qY0BN-BFEUzp3Qcgzt3j~qwT1BzNToGCttdcr~ZUMsFCxJfgeTQuxPgXs9y8jzMNsUzk47BJWE2pzJ1JLxSdA91eMdHHz6V~1VHxWXXFP0wMsEeQVn9AnEGYETLpr185ny~2a6w4ZVtLXYKzAb414WuqG3Iu818mkuTzSVoXmQqLQmk4-pNEpWQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal