Anemia of chronic disease (ACD) is frequently found in patients with chronic immune activation. Since most studies on ACD pathophysiology were performed with cell culture or animal models but not in humans, we examined 37 ACD patients suffering from autoimmune diseases or infections, 10 subjects with iron-deficiency anemia (IDA), 10 anemic patients with hereditary spherocytosis (HS), and 27 age-matched controls. Although hemoglobin concentrations were comparable between ACD and IDA patients, the latter presented with significantly higher serum erythropoietin concentrations than ACD patients. The significant negative correlation between erythropoietin and hemoglobin levels observed in IDA patients was also found in a group of anemic but not hypoferremic hereditary spherocytosis subjects, but not in ACD patients. Increased serum concentrations of the hepcidin precursor prohepcidin were paralleled by a decreased expression of the iron exporter ferroportin in circulating monocytes of ACD patients. In the latter cells, increased amounts of the iron storage protein ferritin and a reduced activity of iron-regulatory protein indicated monocyte iron accumulation. Our data indicate that hypoferremia in ACD may result from downregulation of ferroportin expression by hepcidin and cytokines with subsequent iron retention in monocytes. Together with a diminished erythropoietin formation, the impaired iron recirculation from monocytes may be central in the pathophysiology of ACD in humans.
Introduction
Anemia of chronic disease (ACD), also termed as anemia of inflammation, is likewise the most frequent anemia in hospitalized patients. This mild to moderate normocytic to microcytic anemia is found with a frequency between 8% and 95% in patients suffering from diseases that are associated with chronic immune activation, such as autoimmune disorders including rheumatoid arthritis and malignancies and chronic infections including HIV.1-3
At least 3 major immunity-driven mechanisms contribute to the development of ACD. These include the development of hypoferremia with subsequent limitation of iron availability for erythroid progenitor cells, antiproliferative effects of cytokines toward the proliferation and differentiation of erythroid progenitor cells, and, last but not least, an impaired production and reduced biologic activity of erythropoietin (Epo).
The development of hypoferremia results from a complex interaction of acute-phase proteins and cytokines together with an anticipated reduction in the lifespan of erythrocytes. Thereby, the absorption of iron from the gut is reduced while iron acquisition and retention by cells of the reticulo-endothelial system (RES) is greatly stimulated, leading to the development of iron-restricted erythropoiesis and anemia. The liver-derived acute-phase protein hepcidin plays a central role in this setting, since it reduces both duodenal iron absorption and iron export from monocytes/macrophages.4-6 These effects can be referred to as interaction of hepcidin with the transmembrane protein ferroportin,7 which is responsible for the transfer of iron from enterocytes and monocytes/macrophages to the circulation.8,9 The expression of hepcidin by hepatocytes is induced by lipopolysaccharide (LPS) and interleukin-6 (IL-6), resulting in the development of hypoferremia in mice within a few hours after injection.6,10
Moreover, proinflammatory and anti-inflammatory cytokines play an important role in the pathophysiology of ACD by increasing iron accumulation and storage by monocytes/macrophages by hepcidin-independent pathways. The injection of tumor necrosis factor alpha (TNF-α) and interleukin 1 (IL-1) into mice resulted in the development of hypoferremia,11 which was referred to as cytokine-inducible synthesis of the major iron storage protein ferritin and subsequent iron storage in cells of the RES.12,13 In addition, interferon-gamma (IFN-γ) and LPS affect iron homeostasis in monocytes by inducing the formation of short-lived radicals such as nitric oxide (NO), hydrogen peroxide, and superoxide anion, all of which affect the posttranscriptional regulation of iron homeostasis via iron regulatory proteins (IRPs),14-17 thereby modulating ferritin translation and the mRNA stability of transferrin receptor (TfR), which is responsible for the uptake of transferrin-bound iron into cells.18-20 These inflammatory stimuli also increase the uptake of ferrous iron into monocytes via induction of divalent metal transporter 1 (DMT-1) expression,21 DMT-1 being a divalent metal/proton symporter that is responsible for ferrous iron incorporation into cells and iron delivery to the cytoplasm.22 Iron accumulation by activated macrophages is further enhanced by erythrophagocytosis, which can be aggravated by toxic radical-mediated damage of erythrocyte membranes as a consequence of TNF-α exposure.23,24 On the other hand, IFN-γ/LPS downregulate ferroportin mRNA expression, thus aggravating iron retention in monocytes/macrophages.21,25 Interestingly, antiinflammatory cytokines, such as IL-4, IL-10, and IL-13, play distinct roles in iron regulation during ACD. These cytokines induce TfR-mediated iron uptake into cells, enhance the acquisition of hemoglobin/haptoglobin complexes via CD163, and increase iron storage by monocytes/macrophages via IRP deactivation and induction of ferritin translation.26,27
Thus, hypoferremia in ACD appears to be the result of a complex network of cytokines, immune cells, and acute-phase proteins28 that has not been sufficiently studied in humans so far, since most of our knowledge originates from cell culture and animal models.
Patients, materials, and methods
Patients
A total of 84 patients were included in the study. Informed consent for obtaining additional blood samples for scientific purposes during routine blood drawing was obtained from each subject before the procedure. Patients were considered to suffer from ACD when (i) they had a chronic infection or autoimmune disease; (ii) they were anemic with a hemoglobin level of less than 130 g/L (13 g/dL) for men and less than 120 g/L (12 g/dL) for women; and (iii) they had low transferrin saturation (TfS < 16%) but normal or increased serum ferritin concentrations (> 100 μg/L [100 ng/mL]).1 Among the 37 patients with ACD, 8 suffered from recurrent pneumonia, 6 from rheumatoid arthritis, 4 from large-cell vasculitis, 3 from chronic osteomyelitis, 3 from pyelonephritis, 3 from adult Still syndrome, 2 from cholangitis, 2 from endocarditis, 2 from chronic soft-tissue infections, 2 from pulmonary tuberculosis, 1 from systemic lupus erythematosus, and 1 from a chronic peritonsilliar abscess. Although some patients received antibiotics at enrollment, none of the patients with newly diagnosed autoimmune disorders had been treated with immunosuppressive drugs before study enrollment.
Patients were considered to have iron-deficiency anemia (IDA) when hemoglobin levels and both TfS and ferritin concentrations (< 30 μg/L [30 ng/mL])29 were decreased. The diseases underlying IDA were chronic bleeding episodes due to hypermenorrhea in 6 cases, erosive ulcerative gastritis in 2 cases, and bleeding from nonmalignant adenoma of the colon in 2 cases.
For studying the response of erythropoietin to anemia other than IDA and ACD, we retrospectively analyzed blood samples from a group of anemic patients suffering from hereditary spherocytosis (HS). Among these patients, 5 harbored mutations within spectrin, 2 harbored mutations within ankyrin, and 3 patients had band 3–deficient HS.30 All HS patients investigated herein were not splenectomized. None of these HS patients had any clinical signs of inflammation.
We also studied a group of age-matched controls with no signs of anemia, normal serum iron status, and no signs of inflammation (normal serum concentrations of C-reactive protein < 0.7 mg/dL). We did not include patients with malignancies, since a radiotherapeutic and/or chemotherapeutic regimen as well as bone marrow infiltration by the tumor alter the pathophysiology of the anemia compared with subjects with ACD on the basis of an autoimmune or infectious disease.1 In addition, patients with renal insufficiency (serum creatinine concentrations above the upper limit normal, n = 2) and hemolysis (decreased haptoglobin levels and/or positive Coombs tests, n = 1) were excluded from the study. None of our patients were under treatment with iron or recombination human erythropoietin and/or received blood transfusion before study entry.
The baseline characteristics of the different patient groups are shown in Table 1.
Patient baseline characteristics
. | Control . | ACD . | IDA . | HS . |
|---|---|---|---|---|
| No. patients | 27 | 37 | 10 | 10 |
| Age, y | 52.0 ± 19.0 | 67.0 ± 20.0* | 43.1 ± 19.0† | |
| Sex, F/M | 9/18 | 16/19 | 8/2ठ| 5/5 |
| Hb level, g/L | 151 ± 11 | 107 ± 14‡ | 100 ± 17‡ | 102 ± 13‡ |
| MCH level, pg | 31.0 ± 1.5 | 29.3 ± 2.5* | 23.5 ± 5.3‡ | 28.7 ± 1.5‡ |
| MCV, fL | 92.1 ± 3.51 | 87.9 ± 7.2* | 78.4 ± 8.3‡ | 83.0 ± 7.7‡ |
| Fe level, μM | 19.4 ± 7.7 | 5.5 ± 2.4‡ | 7.1 ± 4.0‡ | 19.6 ± 8.0§ |
| Ferritin level, μg/L | 113.5 ± 86.9 | 589.0 ± 647.2‡ | 12.9 ± 10.0‡§ | 304.4 ± 199.0‡§ |
| Transferrin level, g/L | 2.78 ± 0.38 | 1.6 ± 0.5‡ | 3.94 ± 0.54‡† | 2.78 ± 0.39† |
| TfS, % | 27.9 ± 9.8 | 13.6 ± 5.7‡ | 9.3 ± 7.7‡ | 40.4 ± 17.9† |
| Folic acid level, μg/L | 9.2 ± 2.7 | 9.6 ± 13.7 | 9.9 ± 6.3 | ND |
| Cobalamine level, ng/L | 473.0 ± 214.0 | 531.0 ± 420.0 | 343.0 ± 136.0 | ND |
| Epo level, IU/L | 10.4 ± 19.4 | 16.6 ± 10.6 | 37.7 ± 36.3*§ | 66.1 ± 36.2‡† |
. | Control . | ACD . | IDA . | HS . |
|---|---|---|---|---|
| No. patients | 27 | 37 | 10 | 10 |
| Age, y | 52.0 ± 19.0 | 67.0 ± 20.0* | 43.1 ± 19.0† | |
| Sex, F/M | 9/18 | 16/19 | 8/2ठ| 5/5 |
| Hb level, g/L | 151 ± 11 | 107 ± 14‡ | 100 ± 17‡ | 102 ± 13‡ |
| MCH level, pg | 31.0 ± 1.5 | 29.3 ± 2.5* | 23.5 ± 5.3‡ | 28.7 ± 1.5‡ |
| MCV, fL | 92.1 ± 3.51 | 87.9 ± 7.2* | 78.4 ± 8.3‡ | 83.0 ± 7.7‡ |
| Fe level, μM | 19.4 ± 7.7 | 5.5 ± 2.4‡ | 7.1 ± 4.0‡ | 19.6 ± 8.0§ |
| Ferritin level, μg/L | 113.5 ± 86.9 | 589.0 ± 647.2‡ | 12.9 ± 10.0‡§ | 304.4 ± 199.0‡§ |
| Transferrin level, g/L | 2.78 ± 0.38 | 1.6 ± 0.5‡ | 3.94 ± 0.54‡† | 2.78 ± 0.39† |
| TfS, % | 27.9 ± 9.8 | 13.6 ± 5.7‡ | 9.3 ± 7.7‡ | 40.4 ± 17.9† |
| Folic acid level, μg/L | 9.2 ± 2.7 | 9.6 ± 13.7 | 9.9 ± 6.3 | ND |
| Cobalamine level, ng/L | 473.0 ± 214.0 | 531.0 ± 420.0 | 343.0 ± 136.0 | ND |
| Epo level, IU/L | 10.4 ± 19.4 | 16.6 ± 10.6 | 37.7 ± 36.3*§ | 66.1 ± 36.2‡† |
Data are shown as means ± SD in each group.
Hb indicates hemoglobin; MCH, mean corpuscular Hb; MCV, mean corpuscular volume; TfS, transferrin saturation; ND, not done; and Epo, erythropoietin.
P < .05 when comparing control subjects with ACD, IDA, HS.
P < .001 when comparing ACD with IDA or HS by means of Student t test.
P < .001 when comparing control subjects with ACD, IDA, or HS.
P < .05 when comparing ACD with IDA or HS by means of Student t test.
Blood samples were drawn on a routine basis, and laboratory parameters (eg, ALT level, AST level, hemoglobin level, blood cell count) and serum iron parameters were examined by routinely used automated laboratory tests: ferritin concentration by an immunoassay and transferrin concentration by a turbitometric method. Serum specimens were drawn during this routine examination and stored at –70°C until cytokine assays were performed. Determinations of serum concentrations of TNF-α, IL-4, IL-6, and IL-10 were all carried out by commercially available enzyme-linked immunosorbent assay (ELISA) kits obtained from R&D Systems (Quantikine HS ELISA Kit; Minneapolis, MN). The detection limits were 0.1 pg/mL for TNF-α, 0.1 pg/mL for IL-4, 0.04 pg/mL for IL-6, and 0.01 pg/mL for IL-10. Determinations of serum erythropoietin and prohepcidin concentrations in serum and urine were carried out by commercially available ELISA kits obtained from DRG (Heidelberg, Germany). The detection limits were 2.0 IU/L for Epo and 4 ng/mL for prohepcidin.
Isolation of monocytes
Peripheral-blood mononuclear cells (PBMCs) were freshly isolated from whole blood by Ficoll-Paque separation (Pharmacia, Uppsala, Sweden) as previously described.31 For monocyte isolation by plastic adherence, PBMCs were resuspended with RPMI 1640 (Biochrom AG, Berlin, Germany) supplemented with 2 mM L-glutamine, 10% (vol/vol) heat-inactivated fetal calf serum (PAA Laboratories Gmbh, Pasching, Austria), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Biochrom AG). Cells were seeded into a 100-mm dish (Becton Dickinson, Franklin Lakes, NJ) and allowed to adhere in a 5% CO2 incubator at 37°C for 2 hours. Nonadherent cells were removed and the adherent cells were washed carefully, at least twice, with prewarmed PBS (Biochrom AG) before being harvested. The purity of the resulting cell suspension was randomly tested by fluorescence-activated cell sorter (FACS) analysis and yielded greater than 96% of monocytes.
Quantitative determination of monocyte mRNA expression by real-time PCR
Total RNA was extracted from monocytes using a guanidiniumisothiocyanate-phenol-chloroform–based procedure as previously described.21 Reverse transcription was performed with 1 μg of total RNA, random hexamer primers (5 μM), dNTPs (62.5 μM; Roche, Mannheim, Germany), and 200 U MMLV reverse transcriptase (GIBCO, Gaithersburg, MD) in 1 × reverse transcription buffer for 1.5 hours at 37°C. TaqMan reverse transcriptase–polymerase chain reaction (RT-PCR) primers and probes were designed, and quantification of target genes by RT-PCR was carried out exactly as described.32 The following primers and TaqMan probes were used: human TfR (huTfR), 5′-TCCCAGCAGTTTCTTTCTGTTTT-3′,5′-CTCAATCAGTTCCTTATAGGTGTCCA-3′,5′-CGAGGACACAGATTATCCTTATTTGGGTACCACC-3′; huDMT-1 (IRE-form), 5′-TGCTGCTATCATTCCAACACTAAATT-3′, 5′-ATATAGCCTGGTTAAGAATCATGCA-3′, 5′-CAGCTCAGTTTATCCTTCGGAGAGACAAGGAT-3′; huferroportin, 5′-TGACCAGGGCGGGAGA-3′, 5′-GAGGTCAGGTAGTCGGCCAA-3′, 5′-CACAACCGCCAGAGAGGATGCTGTG-3′; huDcytb, 5′-GTCACCGGCTTCGTCTTCA-3′, 5′-CAGGTCCACGGCAGTCTGTA-3′, 5′-CCAGGGCATCGCCATCATCGT-3′; 18s rRNA, 5′-CCTGCCCTTTGTACACACCG-3′, 5′-CGATCCGAGGGCCTCAC-3′, 5′-CCGTCGCTACTACCGATTGGATGGTTT-3′.
Western blotting
Protein extracts were prepared from peripheral monocytes as described32 and run on a 15% sodium dodecyl sulfate (SDS)–polyacrylamide gel. Proteins were transferred onto a nylon membrane (Hybond-P; Amersham-Pharmacia, Vienna, Austria) and, after blocking, incubated with either a rabbit anti–human ferritin antibody (2 μg/mL; Dako, Vienna, Austria), a rabbit anti–human ferroportin antibody (1:250; kindly provided by Andrew McKie33 ), or a mouse anti–human β-actin antibody (2 μg/mL; Sigma, St Louis, MO), which was used as a loading control. The ferritin concentration in cellular monocyte extracts was determined by a commercially available ELISA (Alpha Diagnostic International, San Antonio, TX).
Gel retardation assay
Detergent cell extracts from monocytes were prepared as described34 and band shifts were carried out using a 32P-labeled IRE probe that was generated by an in vitro transcription procedure15 using the following DNA template: 5′-GGGATCCGTC CAAGCACTGT TGAAGCAGGA TCCCTATAGT GAGTCGTATT A-3′.
Analysis of RNA/protein complexes was carried out by nondenaturing gel electrophoresis and subsequent autoradiography.34
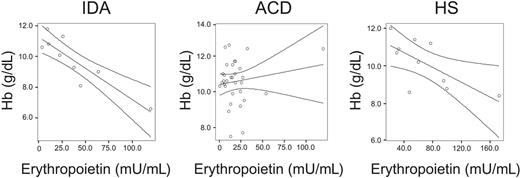
Correlations between hemoglobin (Hb) and serum erythropoietin levels in IDA, ACD, and HS patients. Correlations between Hb and serum erythropoietin levels in IDA patients (R = –0.892, P = .001), ACD patients (R = 0.163, P = .372), and HS patients (R = 0.674, P = .04) as determined by Spearman rank correlation technique are shown. The regression line and the 95% confidence interval are plotted.
Correlations between hemoglobin (Hb) and serum erythropoietin levels in IDA, ACD, and HS patients. Correlations between Hb and serum erythropoietin levels in IDA patients (R = –0.892, P = .001), ACD patients (R = 0.163, P = .372), and HS patients (R = 0.674, P = .04) as determined by Spearman rank correlation technique are shown. The regression line and the 95% confidence interval are plotted.
Data analysis
Statistical analysis was carried out using a SPSS 13.0 (Chicago, IL) statistical package. Calculations for statistical differences between the various groups were carried out by Student t test or by nonparametric Kruskal-Wallis test. Associations among the various parameters in the different groups were calculated using the Spearman rank correlation technique and Bonferroni correction for multiple tests.
Results
Body iron homeostasis and endogenous erythropoietin response in ACD, IDA, and HS
As shown in Table 1, patients with ACD were normochromic and normocytic, however, they were significantly different from controls and IDA patients, the latter presenting with microcytic and hypochromic anemia. Iron status showed the typical characteristics for ACD and IDA. Both groups had significantly decreased serum iron levels and TfS compared with controls, whereas ferritin and transferrin concentrations moved in opposite directions in ACD and IDA subjects.1 A ratio of ferritin versus transferrin levels made this difference even stronger (3.68 for ACD patients versus 0.033 for IDA; P < .001). Whether such a ratio may be helpful to identify patients with ACD and true iron deficiency in a comparable fashion to the determination of soluble TfR remains to be shown.35 Only one patient in the IDA and ACD group, respectively, presented with a moderate cobalamine deficiency, whereas folate levels were in the normal range in all subjects. When studying serum Epo levels, we found them to be significantly increased in IDA but not in ACD subjects compared with controls (Table 1). Although, the degree of anemia as determined by hemoglobin levels was not different between ACD and IDA patients (Table 1), Epo levels were significantly lower in ACD than in IDA patients.
For further exploration of the Epo response to anemia and to see whether this is affected by iron deficiency, we investigated a group of patients suffering from HS. These patients were not iron deficient but had mean hemoglobin levels that were comparable to those found in ACD and IDA subjects (Table 1). Endogenous Epo levels were significantly higher in HS compared with ACD subjects (Table 1).
Interestingly, we found that Epo levels were not correlated to hemoglobin concentrations in ACD, whereas the anticipated significant negative correlation between these 2 parameters was found in IDA and HS subjects, respectively (Figure 1).
Prohepcidin and cytokine concentrations in ACD and IDA
Since hepcidin has been shown to be centrally involved in the induction of hypoferremia during inflammation,6,10,36 we studied serum levels of the hepcidin precursor prohepcidin in our patients,37 since currently no assay is available to determine hepcidin concentrations in serum directly. Although as yet it is not clear whether prohepcidin levels are a surrogate for hepcidin concentrations in serum, we found serum prohepcidin levels to be significantly increased in ACD patients compared with controls whereas they were significantly lower in IDA patients compared with both control and ACD subjects (Table 2). This was also true for urinary prohepcidin levels that were significantly correlated to serum prohepcidin concentrations (r = 0.664; P = .02 by means of Spearman rank correlation technique; details not shown).
Serum cytokine, CRP, and prohepcidin concentrations in ACD, IDA, and control subjects
. | Control . | ACD . | IDA . |
|---|---|---|---|
| CRP concentration, mg/dL | 0.5 ± 0.8 | 9.3 ± 7.5* | 0.4 ± 0.4† |
| IL-4 concentration, pg/L | 20.0 ± 69.9 | 150.8 ± 258.0‡ | 0 ± 0 |
| IL-6 concentration, pg/mL | 2.2 ± 2.1 | 23.4 ± 28.0* | 1.4 ± 1.6† |
| IL-10 concentration, pg/mL | 1.1 ± 0.7 | 5.9 ± 11.2‡ | 1.1 ± 0.8† |
| TNF-α concentration, pg/mL | 1.6 ± 2.08 | 3.2 ± 2.3‡ | 1.3 ± 0.5† |
| Prohepcidin concentration, ng/mL | 110.7 ± 44.0 | 154.0 ± 62.1‡ | 74.6 ± 24.3† |
. | Control . | ACD . | IDA . |
|---|---|---|---|
| CRP concentration, mg/dL | 0.5 ± 0.8 | 9.3 ± 7.5* | 0.4 ± 0.4† |
| IL-4 concentration, pg/L | 20.0 ± 69.9 | 150.8 ± 258.0‡ | 0 ± 0 |
| IL-6 concentration, pg/mL | 2.2 ± 2.1 | 23.4 ± 28.0* | 1.4 ± 1.6† |
| IL-10 concentration, pg/mL | 1.1 ± 0.7 | 5.9 ± 11.2‡ | 1.1 ± 0.8† |
| TNF-α concentration, pg/mL | 1.6 ± 2.08 | 3.2 ± 2.3‡ | 1.3 ± 0.5† |
| Prohepcidin concentration, ng/mL | 110.7 ± 44.0 | 154.0 ± 62.1‡ | 74.6 ± 24.3† |
Data are shown as means ± SD in each group.
CRP indicates C-reactive protein.
P < .001 when comparing controls with ACD or IDA.
P < .001 when comparing ACD with IDA by means of nonparametric Kruskal-Wallis test.
P < .05 when comparing controls with ACD or IDA.
When investigating circulating cytokine levels, not unexpectedly, we found them to be increased in ACD patients compared with controls. However, no significant correlations between these cytokine parameters (IL-4, IL-6, IL-10, and TNF-α) and either prohepcidin or Epo levels were observed when calculating Spearman rank correlations either in IDA or ACD patients, respectively (details not shown).
Monocyte iron homeostasis in ACD and IDA patients
Since monocytes/macrophages are believed to be of pivotal importance in the pathogenesis of ACD by retaining iron and limiting its delivery for erythropoiesis,1,2,23,38 we studied monocyte iron homeostasis in ACD patients. This was of importance, since most of the knowledge on the mechanisms of iron acquisition by the RES during inflammatory processes has been obtained from studies in animals or in vitro cell culture models.39 To our knowledge no comparable studies have been performed so far in humans with ACD.
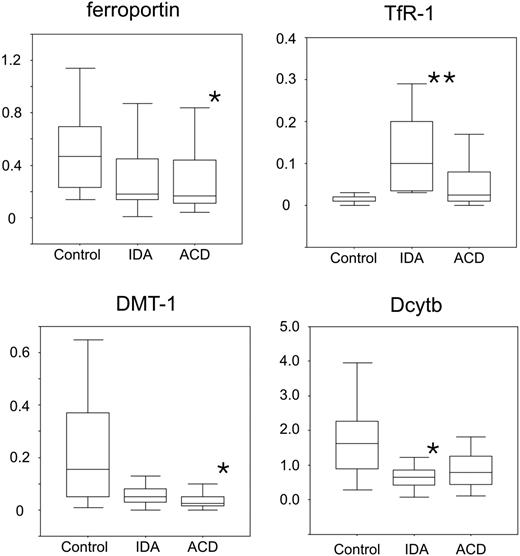
We found that TfR mRNA expression is significantly increased in patients with IDA but not obviously changed in patients with ACD compared with controls (Figure 2). On the other hand, the mRNA expression of the central molecule for the uptake of ferrous iron, DMT-1, was significantly decreased in ACD patients but also reduced in IDA subjects (P = .07) compared with controls. DcytB greatly paralleled the changes in DMT-1 expression and was decreased in IDA and ACD patients compared with controls (Figure 2).
When investigating the expression of the only known transmembrane iron exporter ferroportin, we found that ferroportin mRNA expression was significantly reduced in monocytes of ACD subjects compared with controls (Figure 2). In addition, no significant differences were observed for the expression of HFE and NRAMP-1 between the 3 groups (details not shown).
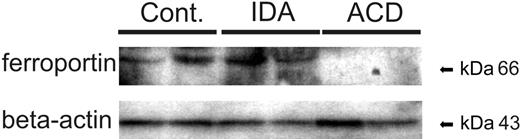
Since hepcidin regulates ferroportin expression after translation,7 we performed Western blots to determine ferroportin protein levels in circulating monocytes. In cellular monocyte extracts we observed decreased ferroportin protein expression in ACD patients compared with both control and IDA subjects (Figure 3). This demonstrated that the changes in ferroportin mRNA expression (Figure 2) were accompanied by corresponding alterations of its protein expression.
Expression profiles of iron transporter mRNAs in isolated human monocytes. Isolated blood monocytes were subjected to RNA preparation, followed by reverse transcription and quantitative TaqMan PCR. Specific values of target genes were normalized to those of 18sRNA. Data are shown as means ± SD for relative abundances. **P < .005 or *P < .05 when comparing control with iron-deficiency anemia (IDA) or anemia of chronic disease (ACD) patients, respectively, by means of Student t test. Data are shown as means (horizontal lines) ± SD (error bars) for relative abundance.
Expression profiles of iron transporter mRNAs in isolated human monocytes. Isolated blood monocytes were subjected to RNA preparation, followed by reverse transcription and quantitative TaqMan PCR. Specific values of target genes were normalized to those of 18sRNA. Data are shown as means ± SD for relative abundances. **P < .005 or *P < .05 when comparing control with iron-deficiency anemia (IDA) or anemia of chronic disease (ACD) patients, respectively, by means of Student t test. Data are shown as means (horizontal lines) ± SD (error bars) for relative abundance.
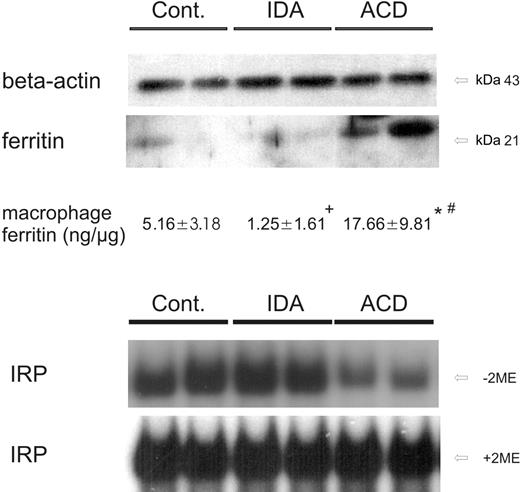
To study the consequences of these regulatory changes of iron transporter expression on monocyte iron homeostasis, we determined IRP activity and cellular ferritin levels as estimates of intracellular iron availability. Upon determination of monocyte ferritin levels by means of Western blotting and quantitative ELISA measurement, we found low amounts of ferritin in controls and even less in patients with IDA (Figure 4). In contrast, ferritin protein expression was significantly increased in monocytes of ACD patients compared with the 2 other groups, thus indicating cellular iron storage (Figure 4).
When studying IRP binding affinity as an indicator of intracellular iron availability, we found IRP to be activated in controls and in IDA patients, suggesting intracellular iron depletion. In contrast, IRP binding affinities were low in ACD patients, being suggestive for increased intracellular iron availability (Figure 4).
Ferroportin protein expression in circulating monocytes. Freshly isolated blood monocytes were subjected to cell-extract preparation and subsequent Western blotting for quantification of ferroportin protein expression. Determination of β-actin protein expression was used as an internal control (Cont). One of 4 representative experiments is shown.
Ferroportin protein expression in circulating monocytes. Freshly isolated blood monocytes were subjected to cell-extract preparation and subsequent Western blotting for quantification of ferroportin protein expression. Determination of β-actin protein expression was used as an internal control (Cont). One of 4 representative experiments is shown.
IRP binding activity and ferritin protein levels in circulating monocytes. (A) Freshly isolated blood monocytes were subjected to cell-extract preparation and subsequent Western blotting for quantification of ferritin protein expression. β-actin was used as an internal control. One of 4 representative experiments is shown. In addition, intracellular ferritin concentrations of monocytes were determined by ELISA technique. The means ± SD are shown for 15 control, 9 IDA, and 13 ACD patients. Results are expressed as ng ferritin per μg total monocyte protein. *P < .001 or +P < .005 when comparing controls with ACD or IDA patients by means of Student t test, respectively; #P < .001 for comparison of ACD with IDA subjects. (B) Cellular extracts from corresponding monocytes were analyzed for IRP binding affinity by means of band shift assays. IRP binding affinity in monocytes of controls, IDA, and ACD patients is shown in the top panel. The total IRP binding capacity was determined by preincubation of extracts with 2-mercaptoethanol (+ 2-ME, bottom panel). One of 6 representative experiments is shown.
IRP binding activity and ferritin protein levels in circulating monocytes. (A) Freshly isolated blood monocytes were subjected to cell-extract preparation and subsequent Western blotting for quantification of ferritin protein expression. β-actin was used as an internal control. One of 4 representative experiments is shown. In addition, intracellular ferritin concentrations of monocytes were determined by ELISA technique. The means ± SD are shown for 15 control, 9 IDA, and 13 ACD patients. Results are expressed as ng ferritin per μg total monocyte protein. *P < .001 or +P < .005 when comparing controls with ACD or IDA patients by means of Student t test, respectively; #P < .001 for comparison of ACD with IDA subjects. (B) Cellular extracts from corresponding monocytes were analyzed for IRP binding affinity by means of band shift assays. IRP binding affinity in monocytes of controls, IDA, and ACD patients is shown in the top panel. The total IRP binding capacity was determined by preincubation of extracts with 2-mercaptoethanol (+ 2-ME, bottom panel). One of 6 representative experiments is shown.
Discussion
In this study we investigated the alterations of monocyte iron homeostasis and endogenous Epo formation in patients with ACD and their potential pathophysiologic role in this frequent clinical condition. These results were compared with those obtained in controls and subjects suffering from IDA and from HS.
When studying endogenous Epo levels in the different patient groups, we found Epo levels to be significantly higher in IDA and HS patients compared with ACD subjects, although hemoglobin concentrations were not significantly different between the various patient groups (Table 1). This supports the notion of an inadequate Epo formation for the degree of anemia in patients with ACD40-42 ; however, some studies, especially in children with cancer or juvenile arthritis, did not confirm this prediction43,44 and this may be due to various factors.45 On the one hand, cytokine patterns differ according to the underlying disease, and the relative balance of stimulating and inhibitory cytokines may variably affect Epo production in the kidneys. Additionally, the antiproliferative effects of radiotherapeutic and chemotherapeutic interventions can further impair Epo formation in cancer patients.1 Moreover, therapeutic interventions in autoimmune or infectious diseases can alter endogenous Epo formation directly or indirectly, via modulation of cytokine activities and immune function. Accordingly, treatment of healthy subjects with corticosteroids resulted in a significant increase of serum Epo concentrations.46 Finally, since an impaired Epo formation in comparison to the degree of ACD was not always observed, especially in children,43,44 one might suggest that children may harbor a greater capacity than adults to compensate the inhibitory effects of cytokines toward Epo production.
Interestingly, we found that in patients with HS, Epo levels were even higher than in IDA subjects although they had comparable hemoglobin levels. In contrast to IDA subjects, HS patients had normal iron levels and no hypoferremia, which indicates that hypoxia as a consequence of anemia rather than iron deficiency is the primary driving force for Epo formation in the kidneys of anemic patients.
Importantly, while we found a strong negative correlation between hemoglobin concentrations and serum Epo levels in IDA and HS subjects, this was not the case for ACD patients. The lack of such a correlation in ACD subjects may be referred to as a defective oxygen sensing mechanism in the kidneys and/or based on negative regulatory effects of cytokines toward Epo formation, as found in cell culture and animal models.47-49 However, we did not observe a significant correlation between serum Epo and selected cytokine levels (Table 2). Notably, this does not imply that cytokines do not affect Epo expression in humans under chronic inflammatory conditions. There are several cytokines, chemokines, or acute-phase proteins expressed under inflammatory conditions, as observed during autoimmune disorders or systemic infections,50,51 a setting that is much different from the controlled application of a single cytokine or LPS during an experiment. Therefore, the in vivo situation, as investigated in our study, reflects the net effects of several agonistic and antagonistic cytokines toward a specific target (eg, Epo formation or monocyte iron homeostasis), and thus the potential effect of a single immune effector molecule found in experiments may be masked in this in vivo setting.
When investigating serum prohepcidin concentrations as a surrogate for endogenous hepcidin levels, we found them to be increased in ACD patients compared with controls whereas they were significantly lower in IDA patients compared with ACD and control subjects (Table 2). These data very nicely resemble the associations between hepcidin and iron status found in mouse models and in patients with hepcidin-producing hepatic adenomas, where an increased hepcidin expression was found to be associated with anemia of inflammation, whereas hepcidin concentrations were low in iron-deficiency anemia.6,52,53
Upon examination of monocyte iron transporters, the most striking difference between IDA and ACD patients was observed with ferroportin expression. Since macrophages have multiple pathways to acquire iron, among which erythrophagocytosis-, DMT-1–, and TfR-mediated iron uptake may be the most important ones,39 so far only one pathway has been identified for monocyte iron export, namely via ferroportin. Thus, modulation of ferroportin expression is a powerful tool to control monocyte/macrophage iron homeostasis irrespective of divergent iron uptake mechanisms. Accordingly, we observed a drastic decrease of ferroportin expression in monocytes of ACD patients that was paralleled by increased serum and urine levels of prohepcidin. In accordance with published experimental evidence whereupon hepcidin interacts with ferroportin thereby resulting in its internalization and degradation7,54 and given that prohepcidin is a surrogate for circulating hepcidin, our data would imply that this interaction also takes place in monocytes of humans with ACD. This would also fit the inverse observation of high ferroportin expression together with low prohepcidin levels in IDA subjects.
Nonetheless, at least in ACD patients, ferroportin appears to be regulated at multiple levels. While hepcidin controls ferroportin expression at the posttranslational level, we also observed reduced ferroportin mRNA concentrations in monocytes of ACD patients, which according to in vitro data should result from downregulation of ferroportin transcription by inflammatory stimuli such as IFN-γ or LPS.21,25
Importantly, the decreased expression of ferroportin was paralleled by an increased iron storage in monocytes of ACD patients as estimated by increased ferritin levels and decreased IRP binding affinity. As a functional consequence of decreased ferroportin expression and the subsequent reduction of cellular iron export, intracellular iron levels will increase. This results in a reduction of the binding affinities of IRPs for IREs and a derepression of IRP-mediated inhibition of ferritin translation, thus promoting iron storage.18
The activation of IRP binding affinity can also explain the changes in the expression of ferritin and TfR mRNA in IDA. Thereby, the increased activity of IRPs, as a consequence of iron depletion, stabilizes TfR mRNA but at the same time blocks ferritin translation by interacting with the IRE motif located within its 5′ untranslated region.18,19,55
Nevertheless, since TfR and DMT-1 mRNA expressions were rather reduced in monocytes of ACD patients compared with controls, it is suggestive that these 2 pathways are of minor importance for iron uptake into chronically activated monocytes compared with the predicted increase in iron acquisition via erythrophagocytosis.23 However, the quantification of erythrophagocytosis and its definitive role for the development of ACD still waits for experimental confirmation in humans. The described in vitro effects of cytokines such as TNF-α on DMT-1 and TfR mRNA expression were not observed in our monocytes.21,56,57 This again indicates that in vivo a number of (as yet not elucidated) regulators differently affect the expression of these iron uptake proteins, as IL-4, -10, and -13 have already been demonstrated to upregulate TfR expression in activated macrophages and to partly counteract DMT-1 induction by proinflammatory cytokines.21,27,58,59 Moreover, our study investigated monocytes in a chronic inflammatory state where we measured the sum of inducing and counterbalancing signals, which may also suggest that the regulatory effects of cytokines and acute-phase proteins on iron homeostasis may change over time and thus be different between the acute or chronic phase of an infection or an inflammatory process.
In summary, we have shown that patients with ACD present with an inadequate formation of endogenous erythropoietin for the degree of anemia. In addition, hypoferremia in association with ACD may result from increased iron retention in monocytes as a consequence of ferroportin downregulation by hepcidin and cytokines.
Supported by grants from the Austrian National Bank, P-10543 (G.W.) and the Austrian Research Fund, P-15943 (G.W.).
I.T. and G.W. designed the research; all authors performed the research and examination of patients; I.T., M.M., and G.W. controlled and analyzed data; G.W. wrote the paper; and all authors checked the final version.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 24, 2006; DOI 10.1182/blood-2005-08-3364.
The authors would like to thank Dr A. McKie (Kings College, London) for his kind gift of ferroportin antibody.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal