Abstract
We have recently shown that the leukotriene B4 (LTB4)–BLT1 pathway is important in early effector T-cell recruitment in mouse models of inflammation. Here we characterize the phenotype and function of human peripheral blood BLT1+ T cells in health and illustrate their involvement in asthma and acute infection. In healthy individuals, BLT1+ T cells are a rare peripheral blood T-cell population enriched for the activation markers CD38 and HLA-DR. Compared with BLT1– T cells, a larger proportion of peripheral blood BLT1+ T cells express the effector cytokines IFNγ and IL-4 and inflammatory chemokine receptors, CCR1, CCR2, CCR6, and CXCR1. Consequently, in healthy individuals peripheral blood BLT1+ T cells are a rare antigen-primed T-cell subset with unique phenotypic, migratory, and functional properties. BLT1 expression on T cells is tightly regulated by inflammation and only transiently expressed after naive T-cell activation by dendritic cells. Although rare in the peripheral blood of healthy individuals, BLT1+ T cells are markedly increased in frequency in the peripheral blood in response to acute Epstein-Barr virus (EBV) infection and moderately increased in the airways of asymptomatic allergic asthmatics. Our studies provide novel insights into the LTB4-BLT1 lipid chemoattractant pathway in human T-cell responses, and how it may link innate and adaptive immunity.
Introduction
Recently, novel roles for bioactive lipids, such as the leukotrienes and sphingosines, in immune cell trafficking and regulation have been elucidated.1 A key feature of bioactive lipid chemoattractants is the rapidity with which they are produced as they are synthesized in minutes as opposed to the hours required to synthesize peptide chemoattractants.2,3 Leukotriene B4 (LTB4) is one such molecule derived by 5-lipoxygenation of arachidonic acid in activated innate leukocytes at inflammatory sites.4 LTB4 mediates its activity via 2 G protein–coupled 7 transmembrane spanning receptors named BLT1 and BLT2.5,6 BLT1 is the high-affinity receptor and is preferentially expressed on peripheral leukocytes, while BLT2 is a lower affinity receptor expressed more ubiquitously.
Since discovery in 1982, LTB4 has been a known potent chemoattractant for myeloid cells.4,5,7 Nevertheless, it has recently been appreciated that LTB4 is also a potent chemoattractant for effector T cells.8-10 We have recently described that BLT1 mRNA is highly expressed in mouse effector T cells, and LTB4 potently induces their firm adhesion and chemotaxis.8 Although mouse BLT1 protein levels have not been characterized, we have shown that the LTB4-BLT1 pathway performs an important function recruiting early effector T cells to sites of antigen rechallenge using BLT1-deficient mice in an active immunization model of asthma.
Nevertheless, little is known as to how these findings in mice can be extended to human T-cell trafficking or disease pathogenesis. Prior to the identification of BLT1, it was shown that LTB4 bound a small proportion of human peripheral blood T cells11,12 and could induce their chemotaxis both in vitro and in vivo after topical application of LTB4.11,13 Studies have noted very low-level expression of BLT1 in human peripheral blood T cells as measured by RNA levels or cell surface protein expression.14,15 The phenotype and function of human BLT1+ T cells and the functional significance of the LTB4-BLT1 lipid chemoattractant pathway in human adaptive immune cell trafficking, therefore, still remain to be defined.
A cardinal feature of adaptive immunity is the ability to mount swift recall responses to cognate antigen challenge.16,17 To implement effector functions, naive and memory T cells are first activated by cognate antigen-presenting cells, usually in lymphoid organs, and then home to tissue sites of inflammation.18,19 Based on CCR7-mediated lymphoid homing, memory cells have recently been broadly defined as CCR7+ central memory and CCR7– effector memory populations.20 However, in part because of the stochastic nature of in vivo priming events and antigen re-exposure at different tissue sites, memory T cells are a very heterogeneous population.21-24 Thus, memory T cells exist across a broad spectrum with regard to phenotype, function, and differentiation.
Signals regulating T-cell activation or differentiation are also tightly linked to the expression of specific chemokine receptors involved in T-cell trafficking.25,26 In fact, distinct patterns of responsiveness to migratory cues have helped define the function of different memory/effector T-cell subsets both in terms of differentiation stage, as well as tissue localization and cellular interactions. Subsets of memory/effector T cells, such as Th1- or Th2-polarized T cells, follicular T helper cells, and tissue-specific T cells, all have unique chemokine receptor and adhesion molecule profiles.27
Here we define the phenotype and function of human BLT1+ T cells and extend our prior observation that LTB4 mediates effector T-cell homing prior to that induced by peptide chemoattractants. We present novel findings that BLT1 expression defines a rare but unique subset of peripheral blood antigen-primed T cells during health, capable of rapidly secreting polarizing cytokines and trafficking into tissues early in inflammatory responses. Further, during active acute inflammatory responses, these cells transiently expand into a much larger effector population, supporting a role for this receptor in peripheral effector trafficking.
Patients, materials, and methods
Study subjects
Atopic asthmatics were identified by skin testing to cat allergen. Bronchoalveolar lavage (BAL) cells and peripheral blood mononuclear cell (PBMC) samples were obtained from cat allergen–sensitive atopic asthmatics as described.28 Normal BAL cells were obtained from healthy lung transplant donors. Donors were studied in the acute and chronic/latent phases of Epstein-Barr virus (EBV) infection. PBMCs were obtained from healthy volunteers or fresh buffy coats from the Massachusetts General Hospital blood bank. All human subject protocols were approved by the institutional review board. Informed consent was obtained from all study participants, in accordance with the Declaration of Helsinki.
Cell preparation and culture
PBMCs were prepared by density gradient centrifuge on Histopaque-1077 (Sigma-Aldrich, St Louis, MO). Untouched CD4+ T cells and CD8+ T cells, and CD45RA+/CD45RO+ CD4+ or CD8+ T cells, were isolated using magnetic bead depletion (Miltenyi Biotec, Auburn, CA). Dendritic cells (DCs) were prepared from fresh buffy coats as previously described.29 CD4+ or CD8+ T cells were cultured with DCs as described.30 γδ and natural killer T (NKT) cell cultures were obtained by labeling fresh PBMCs with fluorochrome-conjugated γδ and Vα24 antibodies and by performing magnetic bead positive selection (Miltenyi Biotec); cells were activated with 2 μg/mL PHA. PB CD1d-restricted NKT cells clones were generated from healthy donors as previously described.31,32 BAL cells were isolated by passing BAL through gauze to remove mucus, then washed and resuspended in 1% FCS PBS.
Flow cytometry reagents and staining
Unconjugated monoclonal antibodies to BLT1 (clones 202/7B1 and 203/14F11) were kindly donated by C. Owman15 (Lund University, Sweden), and FITC-conjugated BLT1 mAb (202/7B1) was obtained from Serotec (Oxford, United Kingdom); monoclonal antibodies were analyzed with appropriate isotype control antibody. Abs to CCR1 (53504.111), CXCR1 (42705.111), CCR2 (48607.121), CXCR2 (48311.211), CCR3 (61828.111), CXCR5 (51505.111), CCR6 (53103.111), and CCR7 (150503) were obtained from R&D Systems (Minneapolis, MN). Abs to CXCR3 (1C6), CCR4 (1G1), CXCR4 (12G5), CCR5 (3A9), CCR6 (11A9), CD3 (UCHT1), CD4 (SK3), CD8 (RPA-T8), CD19 (SJ25C1), CD69 (FN50), CD25 (M-251), CD38 (HIT2), HLA-DR (G46-6), CD27 (M-T271), CD28 (CD28.2), CD45RO (UCHL1), CD45RA (HI100), γδ (B1), perforin (27-35), and 6B11 were purchased from BD PharMingen (San Diego, CA). Abs to CX3CR1 (2A9-1) were purchased from MBL (Nakaku Nagoya, Japan); CD57 (NC1) and Vα24 (C15) were purchased from Beckman Coulter (Fullerton, CA). The 6B11 antibody is directed against an invariant Vα24JαQ epitope possessed by NKT cells. Dual staining with Vα24 and 6B11 enables specific identification of a canonical TCR rearrangement on circulating NKT cells, comparable in specificity to tetramer staining.32 The following human leukocyte antigen (HLA) tetramers were used for analysis of EBV responses: HLA-B8–restricted FLRGRAYGL (EBNA-3A) and QAKWRLQTL (EBNA-3A), HLA-B8–restricted RAKFKQLL (BZFL1), and HLA-A2–restricted GLCTLVAML (BMLF1). Cells were blocked with 10% human serum before staining. Intracellular cytokine and perforin staining was performed as described.33
Fluorescence-activated cell sorter (FACS) analysis and sorting
Surface expression of various markers was assessed using CellQuest software on a FACSCalibur (BD Biosciences, Mountain View, CA) flow cytometer. Events (1-2 million) per PBMC sample were obtained to acquire several hundred BLT1+ CD4+ and CD8+ T events. Samples were stained with CD14 (M5E2; BD PharMingen) or 7AAD (BD PharMingen) to exclude monocytes or dying cells to define forward scatter (FSC)hi lymphocyte scatter gates. Cells were sorted on a Cytomation MoFlo high-speed cell sorter (Cytomation Systems, Fort Collins, CO). Purity of sorted cells was more than 85%.
Quantitative PCR
Quantitative polymerase chain reaction (PCR) was performed on cDNA obtained from total RNA extracted from sorted cells as described.29 The primers used to amplify BLT1 mRNA were 5′GCCCTGGAAAACGAACATGA and 3′TTAGATGGAAGGCCCGGTG.
Chemotaxis and calcium flux
Sorted cells were rested in 0.5% FCS RPMI overnight, resuspended in 1% BSA RPMI, and loaded in a disposable chemotaxis apparatus (ChemoTx; Neuroprobe, Gaithersburg, MD). Chemokines were placed in the lower wells. After incubation for 2 hours (in vitro–activated cell lines) or 3 hours (ex vivo–sorted cells) at 37°C, cell counts in lower wells were determined with an inverted microscope. In some experiments, CP-105696 (obtained from Pfizer, Groton, CT), a small molecule that blocks binding of LTB4 to its receptor,34 was added at different concentrations to upper and lower wells of the chamber. Calcium flux was performed on NKT clones as described.32
Statistical analyses
Unpaired Student t test was used (2-tailed). P values less than .05 were considered significant.
Results
A minor subset of peripheral blood (PB) CD4+ and CD8+ T cells display surface BLT1
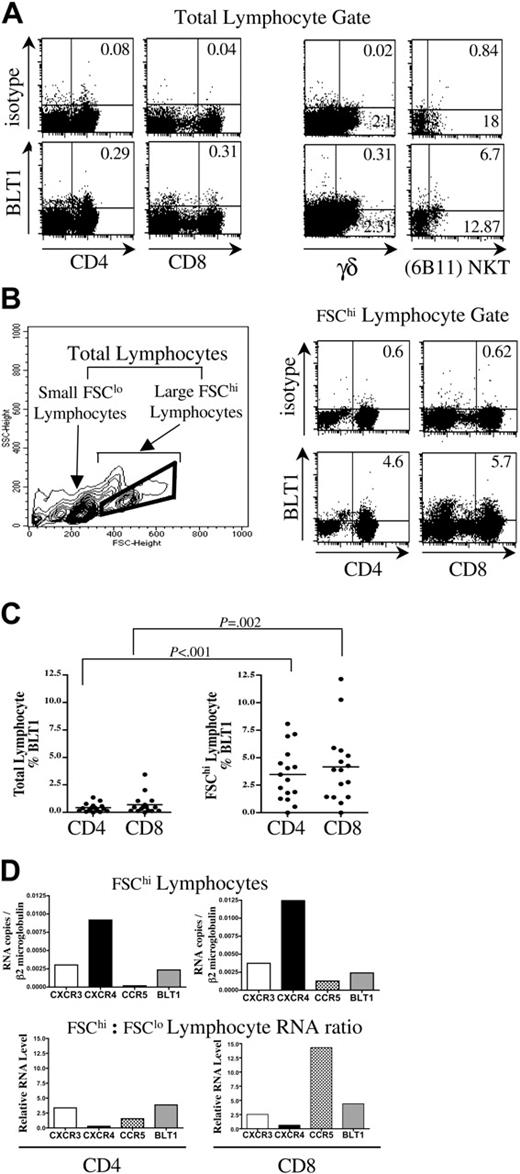
We pursued a strategy of ex vivo characterization to more closely approximate the phenotype and function of BLT1+ human PB CD4+ and CD8+ T cells. Using recently available monoclonal antibodies,15 we examined BLT1 surface expression on freshly isolated peripheral blood leukocytes (PBLs) from 15 healthy donors by flow cytometry. BLT1 was present on a small fraction of total PB CD3+CD4+ T cells (0.42% ± 0.49% SD), and CD3+CD8+ T cells (0.69% ± 0.44% SD) at baseline (Figure 1A).
A substantial fraction of PB NKT and γδ T cells display surface BLT1 in healthy donors
We also characterized BLT1 expression on innate lymphocytes. In contrast to conventional αβ T cells, a much larger fraction of these T cells displays surface BLT1 in healthy individuals (Figure 1A). Of PB γδ T cells, 5% ± 4% SD (range 0.9%-11.1%, n = 7) display surface BLT1. Rare CD1d-restricted NKT cells were identified directly from PBMCs by dual staining of a canonical TCR rearrangement using antibodies specific for the TCR Vα24 chain and the invariant Vα24JαQ (6B11). We found that 13% ± 22% SD (range, 0%-64%, n = 10) of PB NKT cells express surface BLT1.
BLT1 expression is significantly enriched on circulating FSChi large CD4+ and CD8+ lymphocytes. Representative data showing BLT1 expression as percent of quadrant on different T-cell types. (A) Cells were simultaneously gated on total lymphocyte and CD3 gates to characterize CD4+, CD8+, and γδ cells; and total lymphocyte and CD4 gates to characterize NKT cells. (B) Left panel: contour density plot of PBMC defining small FSClo, large FSChi, and total lymphocyte gates. Right panel: FACS plot demonstrating enrichment of surface BLT1 on FSChi large lymphocyte gated CD3+CD4+ and CD3+CD8+ T cells. (C) BLT1 expression is significantly enriched on FSChi CD4+ and CD8+ T cells compared with total CD4+ and CD8+ T cells in freshly isolated PBMCs (n = 15). (D) FSChi CD4+ and CD8+ T cells express increased amounts of BLT1 mRNA by quantitative real-time PCR. These data are representative of 3 separate experiments.
BLT1 expression is significantly enriched on circulating FSChi large CD4+ and CD8+ lymphocytes. Representative data showing BLT1 expression as percent of quadrant on different T-cell types. (A) Cells were simultaneously gated on total lymphocyte and CD3 gates to characterize CD4+, CD8+, and γδ cells; and total lymphocyte and CD4 gates to characterize NKT cells. (B) Left panel: contour density plot of PBMC defining small FSClo, large FSChi, and total lymphocyte gates. Right panel: FACS plot demonstrating enrichment of surface BLT1 on FSChi large lymphocyte gated CD3+CD4+ and CD3+CD8+ T cells. (C) BLT1 expression is significantly enriched on FSChi CD4+ and CD8+ T cells compared with total CD4+ and CD8+ T cells in freshly isolated PBMCs (n = 15). (D) FSChi CD4+ and CD8+ T cells express increased amounts of BLT1 mRNA by quantitative real-time PCR. These data are representative of 3 separate experiments.
Surface BLT1 expression is enriched on FSChi CD4+ and CD8+ T lymphocytes
We noted a striking enrichment of BLT1 surface expression on freshly isolated FSChi large lymphocytes (Figure 1B, total lymphocyte gate = FSClo + FSChi lymphocyte gate). These lymphocytes are SSClo (side scatter)lo as they have low granularity, but have 2 to 3 times the diameter or FSC of small lymphocytes. They are not very recently activated as they are CD69– CD25– (data not shown). As shown in Figure 1B-C, 16-fold more FSChi gated CD3+ CD4+ T cells express surface BLT1 and almost 11-fold more FSChi CD3+ CD8+ T cells express surface BLT1 (P < .002). We were intrigued by this observation, as other investigators have reported a circulating CD62Lhi CD27+ resting memory CD4+ T-cell population with similar SSClo and FSChi scatter properties that have important roles in immune surveillance and recall responses.35
PB FSChi T lymphocytes of healthy donors are enriched for BLT1 mRNA
To confirm that BLT1 expression is increased on FSChi lymphocytes, we FACS-sorted freshly isolated PBMCs for FSChi and FSClo CD3+ CD4+ and CD3+ CD8+ cells and performed quantitative mRNA analysis by real-time PCR. As shown in Figure 1D, FSChi CD4+ T cells express BLT1 mRNA at levels on par with CXCR3, while FSChi CD8+ T cells express BLT1 mRNA at levels on par with CXCR3 and CCR5. Compared with FSClo lymphocytes, FSChi CD4+ and CD8+ T cells were enriched at the mRNA level for CXCR3, CCR5, and BLT1, consistent with a more differentiated profile.
BLT1 is preferentially expressed on preterminally differentiated antigen-primed PB T cells in health
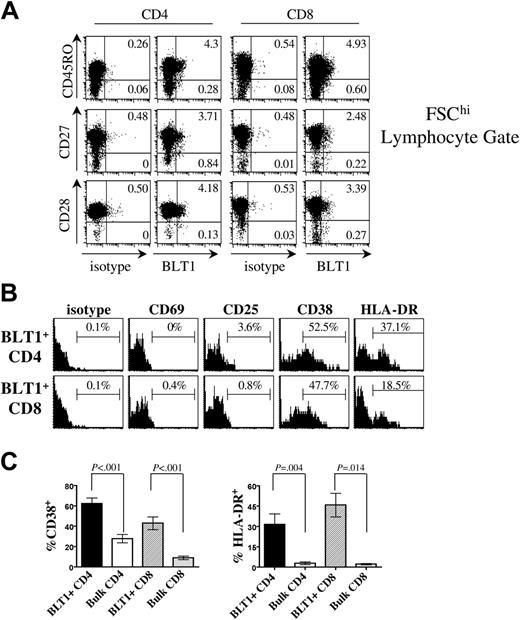
To further characterize this BLT1-enriched population, we examined cell surface markers associated with memory and effector T-cell differentiation. We find that PB BLT1+ T cells are mostly antigen-experienced CD45RO+ cells (Figure 2A) that are not terminally differentiated and have not lost expression of CD27 or CD28. Compared with bulk T cells, BLT1 is preferentially expressed on CD45RO+ memory T cells (P < .005). The majority of BLT1+ T cells are also CD62Lhi (data not shown), consistent with a preterminally differentiated lymphocyte population and a central memory phenotype.20
PB BLT1+ T cells are enriched for markers of late activation in healthy donors
When we characterized PB BLT1+ T cells according to markers of activation (Figure 2B), we found that BLT1+ T cells are CD69– and predominantly CD25–, thus they have not been recently activated. Surprisingly, we found that the activation markers CD38 and HLA-DR are more commonly expressed by CD4+ and CD8+ BLT1+ T cells compared with bulk T cells (Figure 2B and 2C, P < .001 and P < .02, respectively). CD38 and HLA-DR are markers of late activation, and have been reliably associated with antigen-specific memory CD8+ T-cell populations.36,37 However, recently a novel role for CD38 in leukocyte migration has been elucidated,38,39 which demonstrated that CD38 enhances chemotactic responsiveness and inflammatory tissue entry of dendritic cells.39
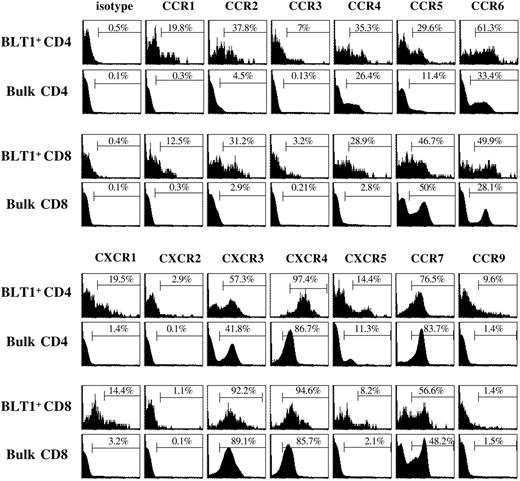
PB BLT1+ T cells are enriched for a subset of inflammatory chemokine receptors associated with memory/effector T cells in healthy donors
We next studied surface chemokine receptor coexpression to glean insight into tissue localization and cellular interactions of BLT1+ T cells and the temporal patterns in which they might execute effector functions (Figure 3; Table 1). CD4+ BLT1+ T cells express significantly higher levels of the Th1-associated chemokine receptors, CXCR3 (58%) and CCR5 (30%), compared with bulk CD4+ T cells (P < .01). In contrast, CD8+ BLT1+ T cells do not express CXCR3 (88%) or CCR5 (41%) at levels significantly different from bulk CD8+ T cells. However, CD8+ BLT1+ T cells express CCR4 (P = .05) and CCR6 (P = .047) at higher levels than bulk, consistent with a less differentiated central memory phenotype.40 Furthermore, 73% of CD4+ BLT1+ T cells and 54% of CD8+ BLT1+ T cells are CCR7+, suggesting an ability to home to lymphoid organs, also consistent with a central memory phenotype.20
Human BLT1+ T cells from blood are enriched for early inflammatory chemokine receptors
. | CCR1 . | CXCR1 . | CCR2 . | CXCR3 . | CCR4 . | CXCR4 . | CCR5 . | CCR6 . | CCR7 . |
|---|---|---|---|---|---|---|---|---|---|
| CD4 | |||||||||
| BLT1+, mean ± SD, % | 30.4 ± 11.1 | 33.95 ± 17.1 | 36.5 ± 6.1 | 58.3 ± 12 | 33.2 ± 15.4 | 94.3 ± 6.4 | 29.5 ± 12.8 | 51.4 ± 11.1 | 73.5 ± 13.3 |
| Total, mean ± SD, % | 0.74 ± 0.89 | 1.00 ± 0.47 | 1.5 ± 1.7 | 31.7 ± 8.0 | 25.8 ± 9.2 | 88.9 ± 11.7 | 10.4 ± 2.9 | 28.5 ± 14.9 | 72.6 ± 11.5 |
| P | .001 | <.002 | <.001 | .003 | .34 | .39 | .005 | .025 | .914 |
| Fold enrichment, mean ± SE | 145.2 ± 74.1 | 36.8 ± 8.6 | 90.8 ± 42.1 | 1.9 ± 0.3 | NA | NA | 3.1 ± 0.7 | 4.6 ± 3.1 | NA |
| CD8 | |||||||||
| BLT1+, mean ± SD, % | 17.8 ± 5.4 | 19.7 ± 15.0 | 26.6 ± 6.2 | 87.6 ± 7.5 | 17.4 ± 12.1 | 97.23 ± 2.6 | 40.9 ± 14.3 | 39.2 ± 13.4 | 56.6 ± 7.8 |
| Total, mean ± SD, % | 0.85 ± 1.02 | 2.0 ± 1.4 | 1.6 ± 1.8 | 75.9 ± 9.9 | 5.6 ± 4.5 | 92.3 ± 6.3 | 30.1 ± 9.8 | 21.4 ± 10.4 | 54.3 ± 10.6 |
| P | .001 | .03 | <.001 | .07 | .047 | .15 | .15 | .047 | .7 |
| Fold enrichment, mean ± SE | 79.5 ± 39.2 | 10.7 ± 2.2 | 83.3 ± 38.0 | 1.2 ± 0.1 | 3.8 ± 1.2 | NA | NA | 2.7 ± 1.1 | NA |
. | CCR1 . | CXCR1 . | CCR2 . | CXCR3 . | CCR4 . | CXCR4 . | CCR5 . | CCR6 . | CCR7 . |
|---|---|---|---|---|---|---|---|---|---|
| CD4 | |||||||||
| BLT1+, mean ± SD, % | 30.4 ± 11.1 | 33.95 ± 17.1 | 36.5 ± 6.1 | 58.3 ± 12 | 33.2 ± 15.4 | 94.3 ± 6.4 | 29.5 ± 12.8 | 51.4 ± 11.1 | 73.5 ± 13.3 |
| Total, mean ± SD, % | 0.74 ± 0.89 | 1.00 ± 0.47 | 1.5 ± 1.7 | 31.7 ± 8.0 | 25.8 ± 9.2 | 88.9 ± 11.7 | 10.4 ± 2.9 | 28.5 ± 14.9 | 72.6 ± 11.5 |
| P | .001 | <.002 | <.001 | .003 | .34 | .39 | .005 | .025 | .914 |
| Fold enrichment, mean ± SE | 145.2 ± 74.1 | 36.8 ± 8.6 | 90.8 ± 42.1 | 1.9 ± 0.3 | NA | NA | 3.1 ± 0.7 | 4.6 ± 3.1 | NA |
| CD8 | |||||||||
| BLT1+, mean ± SD, % | 17.8 ± 5.4 | 19.7 ± 15.0 | 26.6 ± 6.2 | 87.6 ± 7.5 | 17.4 ± 12.1 | 97.23 ± 2.6 | 40.9 ± 14.3 | 39.2 ± 13.4 | 56.6 ± 7.8 |
| Total, mean ± SD, % | 0.85 ± 1.02 | 2.0 ± 1.4 | 1.6 ± 1.8 | 75.9 ± 9.9 | 5.6 ± 4.5 | 92.3 ± 6.3 | 30.1 ± 9.8 | 21.4 ± 10.4 | 54.3 ± 10.6 |
| P | .001 | .03 | <.001 | .07 | .047 | .15 | .15 | .047 | .7 |
| Fold enrichment, mean ± SE | 79.5 ± 39.2 | 10.7 ± 2.2 | 83.3 ± 38.0 | 1.2 ± 0.1 | 3.8 ± 1.2 | NA | NA | 2.7 ± 1.1 | NA |
Freshly isolated BLT1+ T cells from blood were analyzed for coexpression of chemokine receptors by flow cytometry. These data are from 5 to 6 independent experiments. NA indicates not applicable.
Circulating BLT1+ T cells are CD27+ preterminally differentiated memory T cells that express CD38 and HLA-DR. (A) BLT1+ T-cell coexpression of differentiation markers. Approximately 92% of circulating BLT1+CD4+ T cells and 86% of BLT1+ CD8+ T cells coexpress CD45RO. The majority of BLT1+ CD4+ and CD8+ T cells are CD27+ (90% of CD4 and 89% of CD8) and CD28+ (97% CD4 and 76% CD8), comparable with levels of CD27 and CD28 expressed by bulk T cells. Representative data are shown gated on FSChi and CD3+ cells; these data are representative of 7 (CD45RO and CD27) and 4 (CD28) different experiments. Numbers represent percentages of gated population. (B) Coexpression of activation markers on total BLT1+ CD3+ CD4+ and CD3+ CD8+ T cells. Cells were simultaneously gated on the total lymphocyte gate, CD3+ CD4 or CD8 gate, and the BLT1+ gate, to define the frequency of BLT1+ CD4+ or CD8+ cells that coexpress the activation marker of interest. These data are representative of 4 to 10 separate donors. (C) BLT1+ T cells are significantly enriched for CD38 (n = 10) and HLA-DR (n = 6) compared with bulk. Error bars represent SEM.
Circulating BLT1+ T cells are CD27+ preterminally differentiated memory T cells that express CD38 and HLA-DR. (A) BLT1+ T-cell coexpression of differentiation markers. Approximately 92% of circulating BLT1+CD4+ T cells and 86% of BLT1+ CD8+ T cells coexpress CD45RO. The majority of BLT1+ CD4+ and CD8+ T cells are CD27+ (90% of CD4 and 89% of CD8) and CD28+ (97% CD4 and 76% CD8), comparable with levels of CD27 and CD28 expressed by bulk T cells. Representative data are shown gated on FSChi and CD3+ cells; these data are representative of 7 (CD45RO and CD27) and 4 (CD28) different experiments. Numbers represent percentages of gated population. (B) Coexpression of activation markers on total BLT1+ CD3+ CD4+ and CD3+ CD8+ T cells. Cells were simultaneously gated on the total lymphocyte gate, CD3+ CD4 or CD8 gate, and the BLT1+ gate, to define the frequency of BLT1+ CD4+ or CD8+ cells that coexpress the activation marker of interest. These data are representative of 4 to 10 separate donors. (C) BLT1+ T cells are significantly enriched for CD38 (n = 10) and HLA-DR (n = 6) compared with bulk. Error bars represent SEM.
Notably, the inflammatory chemokine receptors, CCR1, CXCR1, CCR2, and CCR6, are expressed at significantly higher levels on both CD4+ and CD8+ BLT1+ T-cell populations compared with bulk T cells (Figure 3; Table 1). Their ligands are induced at the onset of the immune response in inflamed tissue by resident cells, activated innate cells, or activated mature dendritic cells.41-44 The fold enrichment of CCR1 and CCR2 on CD4+ BLT1+ T cells (145 ± 74-fold and 91 ± 42-fold, respectively) and CD8+ BLT1+ T cells (80 ± 39-fold and 83 ± 38-fold, respectively) was exceptionally dramatic. Although it has been reported that CCR2 can be expressed at relatively high levels on circulating memory CD4+ cells,33 we and others have noted minimal CCR2 expression on freshly isolated T cells.32,45 In fact, other investigators have reported high-level CCR1 and CCR2 expression only on CD45RO+ memory cells in the presence of IL-2 in vitro.45 In this respect BLT1, CCR1, and CCR2 are similar as they are expressed at very low levels on PB T cells in the absence of ongoing inflammation.
BLT1+ CD4+ T cells are more polarized than BLT1– CD45RA– CD4+ T cells in PB
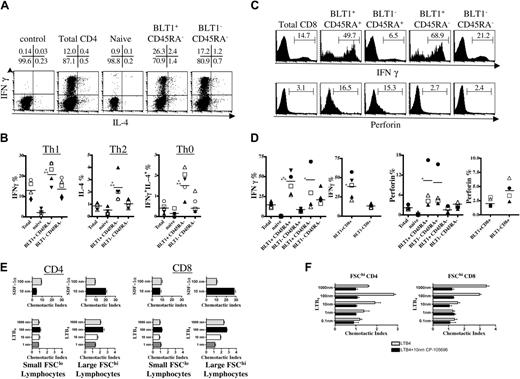
We determined the effector functions of memory/effector BLT1+ CD4+ T cells in healthy donors. Fresh negatively selected CD4+ T cells were activated with PMA and ionomycin to determine polarizing effector cytokine production by intracytoplasmic staining. BLT1+ CD45RA– CD4+ T cells contain a significantly larger fraction of IFNγ-producing Th1 cells, IL-4–producing Th2 cells, and simultaneous IFNγ- and IL-4–producing Th0 cells than BLT1– CD45RA– CD4+ T cells (Figure 4A-B). Although BLT1+ Th1 cytokine–producing cells were the highest in overall frequency among all BLT1+ CD4+ T cells, Th0 cytokine–producing cells were comparatively more increased among BLT1+ T cells than BLT1– CD45RA– T cells.
IFNγ-producing cells are more frequent among BLT1+ CD8+ T cells than memory/effector BLT1– CD8+ T cells in PB
When we investigated effector functions of BLT1+ CD8+ T cells (Figure 4C-D), IFNγ-producing cells were significantly more common among BLT1+ CD8+ T cells than memory/effector BLT1– CD8+ T cells after activation. IFNγ-producing BLT1+ CD8+ T cells were present in both the CD45RA– and the CD45RA re-expressing memory/effector subset. Fresh, negatively selected CD8+ T cells were activated with PMA and ionomycin. After activation, BLT1+ CD45RA+ CCR7– CD8+ T cells contained a striking 5 ± 2-fold larger fraction of IFNγ-producing cells than did BLT1– CD45RA+ CCR7– CD8+ T cells (P = .001).
We then studied the cytotoxic potential of freshly isolated unstimulated peripheral blood BLT1+ CD8+ T cells by intracellular staining for perforin.40,46 BLT1+ CD8+ T cells did not contain significantly different amounts of intracellular perforin compared with BLT1– CD8+ T cells (Figure 4C-D). We also characterized CD57 expression, a marker generally associated with CCR7– terminally differentiated cytotoxic T lymphocytes (CTLs).47 Only a minority of BLT1+ CD8+ T cells express CD57, indicating that these cells are not terminally differentiated CTLs (data not shown). Thus, PB BLT1+ CD8+ T cells, a high frequency of which express CCR7 (Figure 3; Table 1), can rapidly secrete the effector cytokine IFNγ when activated, but lack the potent cytolytic potential of more differentiated CTLs.
FSChi T lymphocytes are functionally responsive to LTB4
In chemotaxis assays (Figure 4E), FSClo CD4+ and CD8+ T cells migrated to SDF-1α, but not to LTB4. In contrast, despite BLT1+ T cells being a minor population of all FSChi T cells (Figure 1B), FSChi CD4+ and CD8+ T cells still migrated to LTB4 with peak chemotactic indices of 2 to 3. These cells also chemotaxed to 10 and 100 nm SDF-1α, but more robustly than FSClo cells at these concentrations. Furthermore, the specific LTB4 receptor antagonist CP-10569634 inhibited chemotaxis of FSChi T cells to LTB4 (Figure 4F). Cells used in these assays chemotaxed similarly to 100 nm SDF-1α in the absence and presence of CP-105696 (data not shown). Thus, FSChi T cells are enriched for BLT1 by surface protein and are functionally responsive to LTB4.
Ex vivo characterization of chemokine receptor coexpression on circulating BLT1+ T cells. Representative example of chemokine receptor coexpression on total BLT1+ T cells and bulk T cells. Gating strategy used is as described in Figure 2B. These data are representative of 5 to 6 separate donors.
Ex vivo characterization of chemokine receptor coexpression on circulating BLT1+ T cells. Representative example of chemokine receptor coexpression on total BLT1+ T cells and bulk T cells. Gating strategy used is as described in Figure 2B. These data are representative of 5 to 6 separate donors.
In vitro–primed effector T cells transiently up-regulate surface BLT1
As only a small fraction of PB T cells express BLT1 during health, we wanted to determine if BLT1 expression could be induced during active inflammation. We found that BLT1 is most robustly induced by priming naive (CD45RO–CD25–) T cells with alloactivated dendritic cells in vitro. Under these conditions, activated T-cell surface BLT1 is induced at day 6, when the majority of T cells are CD45RO+ CD25+ FSChi blasts (Figure 5A). Induction of lymphocyte chemokine receptor expression is developmentally regulated, and other investigators have noted CCR1, CCR2, CCR5, and CXCR6 induction on in vitro–activated T cells on or after day 6, as opposed to CXCR5, CXCR3, or CCR4, which are expressed earlier.30
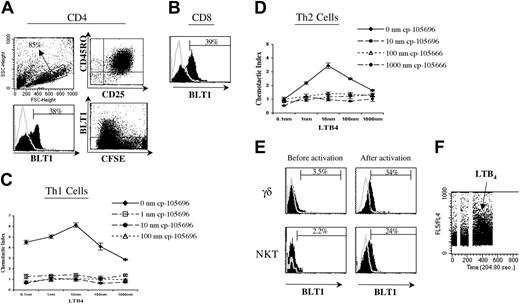
In a representative example in Figure 5, a striking 38% of in vitro–activated effector CD4+ T cells (Figure 5A, bottom left panel) and 39% of effector CD8+ T cells were induced to express surface BLT1 (Figure 5B). In vitro–activated effector T cells also chemotax to LTB4, and this chemotaxis was abrogated by the specific LTB4 receptor antagonist CP-105696 in a dose-dependent fashion (Figure 5C-D). Cells used in these assays chemotaxed similarly to 10 nm SDF-1α in the absence and presence of CP-105696 (data not shown).
BLT1 is not stably expressed on in vitro–activated T cells. It is transiently expressed between days 6 to 10 of activation, peaking at day 8, and subsequently undergoes down-regulation. Furthermore, we failed to see BLT1 surface expression or ligand responsiveness on several T-helper and CTL lines or clones maintained in long-term culture by several rounds of restimulation (data not shown).
In vitro–activated innate T cells up-regulate surface BLT1
We found when activated in vitro, BLT1 surface expression is also dramatically up-regulated on innate γδ T cells and NKT cells (Figure 5E). When Miltenyi Biotec bead–purified γδ and Vα24 cells were activated with PHA, BLT1 surface expression started to increase by day 4. Furthermore, BLT1 expressed on restimulated NKT clones demonstrated functional responsiveness to LTB4 by calcium flux (Figure 5F), as well as chemotaxis (data not shown).
Ex vivo functional characterization of circulating BLT1+ T cells. (A) Representative FACS plots of IFNγ and IL-4 production by different CD4+ T-cell subsets. Percentage of gated populations is shown in quadrants. (B) Circulating BLT1+ CD4+ memory T cells are more polarized than BLT1– CD4+ memory T cells. Negatively selected magnetic bead–purified peripheral blood CD4+ T cells were prelabeled and then activated for 4 hours with PMA and ionomycin before fixation and permeabilization for cytokine analysis. BLT1+ CD45RA– CD4+ T cells were 1.6 ± 0.2-fold more enriched for IFN-γ–producing Th1 cells (P = .036; n = 5), 2.3 ± 0.6-fold more enriched for IL-4–producing Th2 cells (P = .04; n = 5), and 4.1 ± 2.8-fold more enriched for both IFNγ- and IL-4–producing Th0 cells (P = .012; n = 5) compared with BLT1– CD45RA– CD4+ memory T cells. *P < .05 compared with total CD4+ T cells, and **P < .05 compared with BLT1– CD45RA– CD4+ T cells. Symbols represent individual study subjects. (C) Representative histogram plots of IFNγ production and perforin expression by BLT1+ and BLT1– CD8+ T-cell subsets. (D) A large percentage of circulating BLT1+ CD8+ memory T cells rapidly produce IFNγ but lack significant perforin expression. As described above, purified peripheral blood CD8+ T cells were prelabeled and then activated for 4 hours before analysis for IFNγ production, or freshly isolated unactivated PBMCs were stained for intracellular perforin. After stimulation, total BLT1+ CD8+ T cells contained 2.9 ± 1.1-fold more IFNγ-producing cells than did BLT1– CD8+ T cells (P = .007; n = 5), and BLT1+ CD45RA+ CD8+ T cells contained 5 ± 2-fold more IFNγ-producing cells than did BLT1– CD45RA+ CD8+ T cells (P = .001; n = 5). *P < .05 compared with total CD8+, and **P < .05 compared with BLT1– RA+ CD8+ memory T cells. Symbols represent individual study subjects. (E) Sorted FSChi CD4+ and CD8+ T-cell lymphocytes chemotax to LTB4, but FSClo CD4+ and CD8+ T cells do not. These data are representative of 3 to 6 separate experiments. (F) CP-105696 (10 nm) inhibits LTB4-mediated chemotaxis of FSChi CD4+ and CD8+ T cells. These data are representative of at least 3 separate experiments.
Ex vivo functional characterization of circulating BLT1+ T cells. (A) Representative FACS plots of IFNγ and IL-4 production by different CD4+ T-cell subsets. Percentage of gated populations is shown in quadrants. (B) Circulating BLT1+ CD4+ memory T cells are more polarized than BLT1– CD4+ memory T cells. Negatively selected magnetic bead–purified peripheral blood CD4+ T cells were prelabeled and then activated for 4 hours with PMA and ionomycin before fixation and permeabilization for cytokine analysis. BLT1+ CD45RA– CD4+ T cells were 1.6 ± 0.2-fold more enriched for IFN-γ–producing Th1 cells (P = .036; n = 5), 2.3 ± 0.6-fold more enriched for IL-4–producing Th2 cells (P = .04; n = 5), and 4.1 ± 2.8-fold more enriched for both IFNγ- and IL-4–producing Th0 cells (P = .012; n = 5) compared with BLT1– CD45RA– CD4+ memory T cells. *P < .05 compared with total CD4+ T cells, and **P < .05 compared with BLT1– CD45RA– CD4+ T cells. Symbols represent individual study subjects. (C) Representative histogram plots of IFNγ production and perforin expression by BLT1+ and BLT1– CD8+ T-cell subsets. (D) A large percentage of circulating BLT1+ CD8+ memory T cells rapidly produce IFNγ but lack significant perforin expression. As described above, purified peripheral blood CD8+ T cells were prelabeled and then activated for 4 hours before analysis for IFNγ production, or freshly isolated unactivated PBMCs were stained for intracellular perforin. After stimulation, total BLT1+ CD8+ T cells contained 2.9 ± 1.1-fold more IFNγ-producing cells than did BLT1– CD8+ T cells (P = .007; n = 5), and BLT1+ CD45RA+ CD8+ T cells contained 5 ± 2-fold more IFNγ-producing cells than did BLT1– CD45RA+ CD8+ T cells (P = .001; n = 5). *P < .05 compared with total CD8+, and **P < .05 compared with BLT1– RA+ CD8+ memory T cells. Symbols represent individual study subjects. (E) Sorted FSChi CD4+ and CD8+ T-cell lymphocytes chemotax to LTB4, but FSClo CD4+ and CD8+ T cells do not. These data are representative of 3 to 6 separate experiments. (F) CP-105696 (10 nm) inhibits LTB4-mediated chemotaxis of FSChi CD4+ and CD8+ T cells. These data are representative of at least 3 separate experiments.
BLT1+ memory/effector T cells are present in the lungs of asymptomatic asthmatics
The ability to rapidly secrete T-cell effector cytokines when activated and the marked coexpression of inflammatory chemokine receptors suggest that PB BLT1+ T cells may migrate into peripheral tissues to perform effector functions. We studied surface BLT1 expression on tissue-infiltrating lymphocytes freshly isolated from BAL samples obtained from asymptomatic asthmatics (Figure 6). BLT1+ T cells were enriched in the BAL compartment compared with simultaneously obtained blood samples in these subjects. Figure 6A is a representative example in which blood and BAL T-cell BLT1 expression are compared. Blood and BAL total T-cell and BLT1+ T-cell phenotype are compared in Figure 6B and demonstrate that BAL resident T cells are mostly memory in phenotype. BLT1 surface expression was significantly increased on both BAL memory CD4+ T cells (P = .042) and CD8+ T cells (P = .034) compared with blood T cells (Figure 6C).
It is intriguing to note that within 24 hours of segmental allergen challenge, BAL resident CD8+ T cells completely lost surface BLT1 expression (S.Y.T., C.M.L., A.D.L., manuscript in preparation). Only 2 other chemoattractant receptors, CXCR3 and CCR6, lost surface expression at this early time point after allergen challenge. Furthermore, 4 of 5 study subjects completely lost surface BLT1 expression on blood FSChi CD4+ T cells within 24 hours of allergen challenge.
Compared with atopic asthmatic individuals, BAL resident T cells from 2 healthy control subjects did not express increased surface BLT1 on repeat measurements (n = 4) (data not shown). Furthermore, BAL T cells from lung transplant recipients with evidence of chronic rejection express increased BLT1, while BAL T cells from transplant recipients without histologic evidence of rejection do not express BLT1.48 These data suggest that BLT1+ T cells traffic more into inflamed tissue compared with noninflamed tissue.
In vitro activation up-regulates BLT1 surface expression on T cells. (A) Dendritic cell (DC) primed naive CD4+ T cells up-regulate surface BLT1 expression, and this expression is first seen on day 6 of activation. Representative data showing scatter plot (top left panel), differentiation and activation markers (top right panel), histogram plot of BLT1 expression (bottom left panel), and CFSE labeling demonstrating BLT1 expression on divided cells at day 6 (bottom right panel). These data are representative of 3 to 6 different experiments. (B) DC primed CD8+ T cells also up-regulate surface BLT1 expression; shown is 1 representative experiment of 4. (C-D) In vitro–activated human effector T cells chemotax to LTB4 and are inhibited by CP-105696. These data are representative of at least 3 separate experiments. (E) PHA-activated γδ and Vα24 cells up-regulate surface BLT1 expression. γδ and Vα24 cells were isolated from PBMCs and activated with PHA as described in “Patients, materials, and methods” (top and bottom rows, respectively). Representative examples of preactivation ex vivo BLT1 surface expression in PBMCs (panels), and postactivation in vitro BLT1 surface expression (right panels) are shown. These data are representative of 4 (γδ) and 6 (Vα24) separate experiments. (F) NKT cell clones flux calcium when stimulated with LTB4. These data are representative of experiments on 4 separate NKT cell clones.
In vitro activation up-regulates BLT1 surface expression on T cells. (A) Dendritic cell (DC) primed naive CD4+ T cells up-regulate surface BLT1 expression, and this expression is first seen on day 6 of activation. Representative data showing scatter plot (top left panel), differentiation and activation markers (top right panel), histogram plot of BLT1 expression (bottom left panel), and CFSE labeling demonstrating BLT1 expression on divided cells at day 6 (bottom right panel). These data are representative of 3 to 6 different experiments. (B) DC primed CD8+ T cells also up-regulate surface BLT1 expression; shown is 1 representative experiment of 4. (C-D) In vitro–activated human effector T cells chemotax to LTB4 and are inhibited by CP-105696. These data are representative of at least 3 separate experiments. (E) PHA-activated γδ and Vα24 cells up-regulate surface BLT1 expression. γδ and Vα24 cells were isolated from PBMCs and activated with PHA as described in “Patients, materials, and methods” (top and bottom rows, respectively). Representative examples of preactivation ex vivo BLT1 surface expression in PBMCs (panels), and postactivation in vitro BLT1 surface expression (right panels) are shown. These data are representative of 4 (γδ) and 6 (Vα24) separate experiments. (F) NKT cell clones flux calcium when stimulated with LTB4. These data are representative of experiments on 4 separate NKT cell clones.
During acute EBV infection, BLT1 surface expression is dramatically increased on activated tetramer+ CD8+ T cells
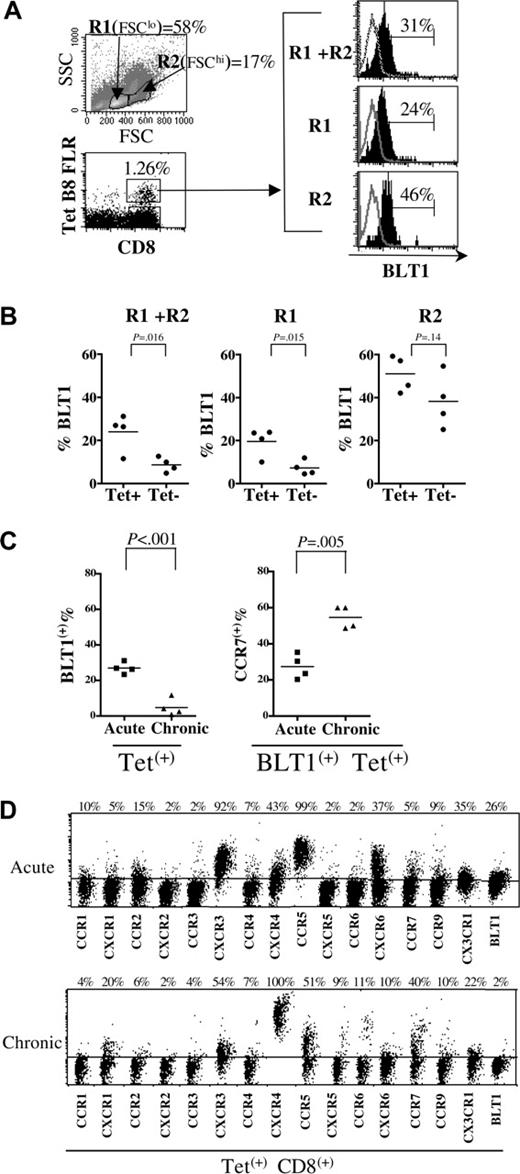
To further define the role of BLT1 during inflammation in humans, we studied surface BLT1 expression on antigen-specific effector CD8+ T cells generated during the primary immune response to EBV (Figure 7). In individuals with acute EBV infection, unlike in healthy individuals, a substantial number of activated blasts are present in the FSChi gate in PB (Figure 7A). In Figure 7A, using the HLA-B8–restricted latent epitope, FLR-specific CD8 tetramer in a representative example, we show that 31% of total circulating FLR-specific Tet+ CD8+ T cells express surface BLT1, while 24% of FSClo and 46% of FSChi FLR-specific Tet+ CD8+ T cells express BLT1. Compared with the Tet– CD8+ T-cell population for each specific tetramer studied in 4 different individuals with acute EBV infection (Figure 7B), BLT1 was significantly enriched in the Tet+ CD8+ T-cell population, in both the total lymphocyte and FSClo gates (P < .02).
When Tet+ CD8+ T-cell responses are compared, surface BLT1 expression is dramatically increased on activated antigen-specific PB CD8+ T cells during the acute phase of infection (P = .001) (Figure 7C) compared with the chronic phase. Consistent with the higher levels of CCR7 expression observed on BLT1+ CD8+ T cells in health (Figure 3), more Tet+ CD8+ BLT1+ T cells re-express CCR7 in chronic compared with acute infection (Figure 7C). When BLT1 expression on Tet+ CD8+ T cells was compared with other chemokine receptors, we noted that the relative increase in BLT1 expression was on par with that of other inflammatory chemokine receptors in the acute setting (Figure 7D). In a representative example during acute infection, inflammatory chemokine receptors such as CXCR3, CCR5, CXCR6, and CX3CR1 are increased on antigen-specific CD8+ T cells, as is BLT1, while chemokine receptors such as CXCR1, CCR4, CXCR4, CCR6, and CCR7 are decreased. After resolution of acute infection, BLT1 is expressed at much lower levels on PB Tet+ CD8+ T cells. Thus in the absence of acute inflammatory stimuli, BLT1 expression on T cells is subsequently down-regulated, consistent with our observation of transient expression on in vitro–activated effectors.
Discussion
Here, we present novel findings defining the phenotype and function of peripheral blood BLT1+ T cells, which are a rare population of antigen-primed T cells in healthy individuals. Our studies also establish that in vitro priming dramatically, though transiently, up-regulates ligand-responsive BLT1 expression on human effector T cells. We thus provide new insights into LTB4-BLT1–mediated homing in humans during health and inflammation.
We extend mouse studies demonstrating BLT1-mediated effector T-cell trafficking by providing novel insights into unique features central to BLT1 expression on human effector T cells.8-10 Despite BLT1+ T cells being an extremely rare peripheral blood population in health, we have determined that BLT1 is markedly inducible on in vitro–primed cells and during acute infection in vivo. Furthermore, BLT1+ T cells are increased in the airways, but not blood, of asymptomatic asthmatics. Our data suggest that BLT1 plays a role in human effector T-cell inflammatory homing in the periphery. Moreover, investigators in our lab have recently shown that BLT1 mediates effector T-cell trafficking into the lung during rejection of the airways in a mouse model of lung transplant rejection.48 Additionally, BLT1 was up-regulated on human T lymphocytes infiltrating the grafts of lung transplant recipients undergoing chronic rejection, further supporting a role for BLT1-mediated peripheral effector trafficking in humans.
BLT1+ memory T cells traffic into the airways of asymptomatic atopic asthmatics. (A) Representative example of freshly isolated blood and bronchoalveolar lavage (BAL) total lymphocyte BLT1 expression in one study subject. (B) Representative phenotypic characterization of blood, BAL, and BLT1+ blood and BAL lymphocytes in a study subject. (C) BLT1+ memory T cells are enriched in the BAL compared with blood in 5 atopic asymptomatic study subjects. Symbols represent individual study subjects.
BLT1+ memory T cells traffic into the airways of asymptomatic atopic asthmatics. (A) Representative example of freshly isolated blood and bronchoalveolar lavage (BAL) total lymphocyte BLT1 expression in one study subject. (B) Representative phenotypic characterization of blood, BAL, and BLT1+ blood and BAL lymphocytes in a study subject. (C) BLT1+ memory T cells are enriched in the BAL compared with blood in 5 atopic asymptomatic study subjects. Symbols represent individual study subjects.
Our study highlights a unique feature of the LTB4-BLT1 pathway in adaptive immunity, in that high-level BLT1 expression on effector T cells is transiently expressed and tightly regulated by antigen and inflammatory stimuli. Thus, in conditions of health, BLT1 is expressed only on a very rare PB T-cell population. This inflammation-regulated expression of BLT1 parallels that of inflammatory chemokine receptors such as CCR1 and CCR2 on T cells, and is distinct from that of other inflammatory chemokine receptors such as CXCR3 and CCR5, which are readily detected at high levels on PB T cells.
BLT1 surface expression is increased on EBV-specific CD8+ T cells during acute EBV infection. (A) Scatter and lymphocyte gates showing increased numbers of circulating activated blasts (top left panel) and increased surface BLT1 expression on tetramer+ EBV-specific CD8+ T cells (bottom left panel) during acute EBV infection in R1 (FSClo), R2 (FSChi), and total (R1+R2) lymphocyte gates (right panel) in a representative sample. (B) BLT1 surface expression is significantly enriched on total and FSClo small lymphocyte-gated EBV-specific tetramer-positive CD8+ T cells (n = 4). (C) BLT1 surface expression is increased on tetramer+ EBV-specific CD8+ T cells during acute symptomatic EBV infection compared with the chronic phase of infection in healthy asymptomatic donors (P < .001); more Tet+ CD8+ BLT1+ T cells re-express CCR7 in chronic compared with acute infection. (D) Tetramer+ EBV-specific CD8+ T cells were gated on to compare surface chemoattractant receptor expression profile of EBV-specific HLA-B8–restricted QAK tetramer+ CD8+ T cells during acute infection (top panel), and EBV-specific HLA-B8–restricted RAK tetramer+ CD8+ T cells during the latent or chronic phase of infection (bottom panel). These data are representative of at least 3 donors.
BLT1 surface expression is increased on EBV-specific CD8+ T cells during acute EBV infection. (A) Scatter and lymphocyte gates showing increased numbers of circulating activated blasts (top left panel) and increased surface BLT1 expression on tetramer+ EBV-specific CD8+ T cells (bottom left panel) during acute EBV infection in R1 (FSClo), R2 (FSChi), and total (R1+R2) lymphocyte gates (right panel) in a representative sample. (B) BLT1 surface expression is significantly enriched on total and FSClo small lymphocyte-gated EBV-specific tetramer-positive CD8+ T cells (n = 4). (C) BLT1 surface expression is increased on tetramer+ EBV-specific CD8+ T cells during acute symptomatic EBV infection compared with the chronic phase of infection in healthy asymptomatic donors (P < .001); more Tet+ CD8+ BLT1+ T cells re-express CCR7 in chronic compared with acute infection. (D) Tetramer+ EBV-specific CD8+ T cells were gated on to compare surface chemoattractant receptor expression profile of EBV-specific HLA-B8–restricted QAK tetramer+ CD8+ T cells during acute infection (top panel), and EBV-specific HLA-B8–restricted RAK tetramer+ CD8+ T cells during the latent or chronic phase of infection (bottom panel). These data are representative of at least 3 donors.
Furthermore, in health we determined that peripheral blood BLT1+ T cells are antigen-primed memory/effector T cells by defining their phenotype and function. By doing so, we demonstrate that these BLT1+ T cells possess migratory, phenotypic, and effector properties consistent with those of immunomodulatory T cells that may rapidly initiate secondary immune responses.
With regard to migratory features, blood BLT1+ T cells are dramatically enriched for CD38 (P < .001) compared with bulk T cells. Given recent data on CD38-deficient mice,39 these data suggest that circulating memory BLT1+ T cells may be equipped to rapidly migrate into inflamed peripheral tissue from blood during the earliest phases of inflammation. It has been proposed that CD38 may act as a sensor of tissue damage and potentiates leukocyte chemotaxis.39 Thus, CD38 may also be a marker of blood T cells endowed with enhanced chemotactic responsiveness to chemokine ligands for which they bear receptors.
With regard to chemokine receptor–mediated homing, it is striking that inflammatory chemokine receptors, CCR1 (P < .001), CXCR1 (P < .05), CCR2 (P < .001), and CCR6 (P < .05), are significantly enriched on peripheral blood CD4+ and CD8+ BLT1+ T cells. It has been reported that CCL2-CCR2 and LTB4-BLT1 act synergistically to recruit monocytes.49 Other investigators report that LTB4 strongly induces CCL2 production by human monocytes,50 supporting a likelihood of cross-talk between these mediators and their receptors.51 Therefore, BLT1 may work in combination or synergistically with strongly coexpressed chemokine receptors, such as CCR1, CXCR1, CCR2, and CCR6, to augment peripheral blood T-lymphocyte extravasation into inflamed tissue.
Phenotypically, circulating BLT1+ T cells are mostly SSClo, FSChi, CD62Lhi, CCR7+, and CD27+, consistent with phenotypic features of memory T cells involved in immune surveillance35 and central memory cells.20 Murine studies have led to the proposal that by preventing activation-induced cell death, CD27 promotes long-term survival of memory T cells.52 Human studies have also shown that long-lived memory CD4+53 and CD8+ T cells do not differentiate to lose expression of CD27.54 With regard to central memory cells, recently it has been shown that the chemokine receptors CXCR3, CCR4, and CXCR5 identify functional subsets within the human CD4+ central memory pool.24 BLT1+ T cells are not enriched for CXCR5 (data not shown), which identifies the least differentiated CD4+ central memory24 subset. As Th1 cytokine–producing cells are most frequent among BLT1+ CD4+ T cells, it is not surprising that total BLT1+ CD4+ T cells are significantly enriched for CXCR3 and CCR5, the Th1-associated chemokine receptors, compared with bulk CD4+ T cells (P < .01). However, as CCR2 and CCR1 are most strikingly enriched on all BLT1+ CD4+ T cells, and BLT1 is expressed not only on Th1 cells, but also on Th2 and Th0 functional subsets, it is likely that BLT1 defines a unique subset common to the more functionally differentiated central memory T-helper subsets described by Rivino et al.24
Functionally, a larger fraction of blood BLT1+ T cells rapidly secretes polarizing effector cytokines compared with BLT1– memory/effector T cells. CD4+ BLT1+ T cells are particularly enriched for IFNγ+ IL-4+ Th0 cells. Notably, NKT cells, a very rare population in circulating blood (range, 0.021%-0.051%),32 possess a Th0 subset important for immune regulation.55 Although rare in peripheral blood, NKT and γδ cells critically modulate the adaptive immune response by early secretion of IFNγ, TNFα, or IL-4.56,57 Recently, it has been shown that early secretion of IFNγ by NK cells can also influence Th1 polarization.58 Polarizing cytokines critically induce and stabilize the generation of their own T-helper subset, while inhibiting development of the opposing subsets at the onset of the immune response by promoting autoamplification signaling loops.59 Unlike immunomodulatory cytokines secreted by innate lymphocytes such as NKT and γδ T cells in response to noncognate inflammatory stimuli, effector cytokines secreted by tissue-infiltrating BLT1+ T cells would be in response to cognate antigen rechallenge in secondary responses.
A significant fraction of BLT1+ T cells express CCR7, suggesting that they can migrate to draining lymph nodes from either blood or tissue, as has been shown for CCR7+ memory/effector cells by investigators in our lab.60 There are also data on the expression of 5-lipoxygenase in dendritic cells in human lymph nodes,61 suggesting that they can synthesize LTB4. Furthermore, expression of CCR1 and CCR2 on lymph node–homing BLT1+ T cells could facilitate rapid interactions with lymph node–resident mature DCs, as the ligands for these receptors, CCL2, CCL3, and CCL5, are also synthesized by activated mature DCs.62 This may help amplify polarized immune responses by promoting interactions between DCs and T cells, as has been described for CXCL16 and CCL22.63,64 Thus, BLT1+ T cells may interact with activated tissue resident or lymph node resident antigen-presenting cells to enable rapid local or systemic initiation and progression of the secondary immune response.
We thus provide novel insights into antigen-primed BLT1+ T cells in peripheral blood during health and suggest a mechanism by which the LTB4-BLT1 chemoattractant pathway may link innate and adaptive immunity. We also provide new insights into LTB4-BLT1–mediated effector T-cell peripheral homing during active inflammation.
Prepublished online as Blood First Edition Paper, September 22, 2005; DOI 10.1182/blood-2005-06-2362.
Supported by grants from the National Institutes of Health (KO8 AI055663 to S.A.I., RO1 AI050892 to A.D.L.), Infectious Diseases Society of America Young Investigator Award (S.A.I.), and the Dana Foundation (A.D.L.).
S.A.I. designed research, performed research, analyzed data, and wrote the paper. S.Y.T. designed research, performed research, and helped write the paper. C.H. designed research, performed research, and helped write the paper. B.D.M. designed research, performed research, and provided donor samples. T.K.M. designed and performed research. C.B. designed research and provided donor samples. C.M.L. designed research and provided donor samples. A.M.T. designed research and helped write the paper. A.D.L. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Shannon Bromley for critical review of the paper.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal