Abstract
Local vasodilation in response to hypoxia is a fundamental physiologic response ensuring oxygen delivery to tissues under metabolic stress. Recent studies identify a role for the red blood cell (RBC), with hemoglobin the hypoxic sensor. Herein, we investigate the mechanisms regulating this process and explore the relative roles of adenosine triphosphate, S-nitrosohemoglobin, and nitrite as effectors. We provide evidence that hypoxic RBCs mediate vasodilation by reducing nitrite to nitric oxide (NO) and ATP release. NO dependence for nitrite-mediated vasodilation was evidenced by NO gas formation, stimulation of cGMP production, and inhibition of mitochondrial respiration in a process sensitive to the NO scavenger C-PTIO. The nitrite reductase activity of hemoglobin is modulated by heme deoxygenation and heme redox potential, with maximal activity observed at 50% hemoglobin oxygenation (P50). Concomitantly, vasodilation is initiated at the P50, suggesting that oxygen sensing by hemoglobin is mechanistically linked to nitrite reduction and stimulation of vasodilation. Mutation of the conserved β93cys residue decreases the heme redox potential (ie, decreases E1/2), an effect that increases nitrite reductase activity and vasodilation at any given hemoglobin saturation. These data support a function for RBC hemoglobin as an allosterically and redox-regulated nitrite reductase whose “enzyme activity” couples hypoxia to increased NO-dependent blood flow.
Introduction
Local vasodilation in response to tissue hypoxia is a fundamental physiologic response that ensures delivery of oxygen to respiring tissues under metabolic stress.1 The red blood cell (RBC) itself contributes to this process, with hemoglobin (Hb) acting as the hypoxic sensor that couples decreasing oxygen tension to increased blood flow. How RBCs mediate this function is less clear; proposed mechanisms include ATP release,1-3 S-nitrosohemoglobin (SNOHb)–dependent vasodilation,4,5 and a nitrite-reductase activity of deoxyhemoglobin.6-8
Nitrite, a biologic metabolite of nitric oxide (NO), is present in a variety of foods, is used in meat-curing procedures, and is a pharmacologic agent for the treatment of cyanide poisoning.9 Nitrite has been appreciated as an inflammatory mediator of nitration reactions10 and a precursor for NO under acidic or ischemic conditions.11,12 At physiologic pH, relatively high nitrite concentrations vasodilate aortic rings through the activation of soluble guanylate cyclase (sGC).13-15 However, in the presence of ascorbate or acidosis (pH 6.6), nitrite is a more potent vasodilator and suggests a biologic function.16 Furthermore, with the development of methodologies that can measure nitrite in biologic matrices, the possibility for specific vascular functions for nitrite has emerged. Nitrite is present in the plasma and RBCs at approximately 120 nM and 290 nM, respectively, in healthy subjects.17 Plasma nitrite levels correlate with endothelial nitric oxide synthase (eNOS) activity,18 are tightly controlled between species,19 are increased upon NO inhalation,20 and decrease in tissues and blood on exposure to hypoxiaor exercise-induced stress.20-22
Recent studies propose a model in which oxygen sensing by the RBC is coupled to the transformation of nitrite to NO by a deoxyhemoglobin-mediated reduction reaction.6,7,23 Moreover, we have shown that this process occurs through an allosterically controlled mechanism.24 Herein, we explore how this model fits into existing paradigms of hypoxic vasodilation and elucidate molecular mechanisms that regulate this activity. Presented data suggest that the vasoactivity of nitrite and Hb occurs by a nitrite reductase activity resulting in the ultimate formation of NO via mechanisms that are distinct from ATP-dependent vasodilation, does not require SNOHb as an intermediate, and is modulated by oxygen, heme redox potential, and the conserved cysteine β93 residue, with maximal reductase activity occurring at Hb P50 (the oxygen tension at which 50% of Hb is oxygenated).
Materials and methods
Materials
All reagents were purchased from Sigma Chemical (St Louis, MO). cGMP EIA kit was from Cayman Chemical (Ann Arbor, MI). Recombinant hemoglobin in which the β93cys was replaced with either Ala, Gly, or Lys was synthesized as previously described.25 Human RBCs were obtained from venous blood after collection in heparin or EDTA and washing with PBS (3 ×). Normal human adult hemoglobin (HbA) was prepared by hypotonic lysis of washed RBCs. Hemoglobins were stored in the CO (carbon monoxide)–bound form at –80°C and were converted to oxyhemoglobin as previously described.25 NEM (N-ethylmaleimide)–modified HbA was prepared by incubating 200 μM HbA with 10 mM NEM in PBS at pH 7.4 and 20°C for 1 hour. Excess NEM was removed using a Sephadex G-25 column. HBOC-201 (Hemopure) was a kind gift from Biopure (Cambridge, MA). All animal and human studies were performed according to protocols approved by University of Alabama at Birmingham Institutional Animal Care and Use Committee and Institutional Review Board, respectively.
Evaluating nitrite reductase kinetics
Cell-free Hb. Hemoglobin and sodium nitrite were suspended in PBS and deoxygenated independently by purging with helium using a tonometer with a glass cuvette attached. Visible spectra were periodically taken during deoxygenation and analyzed by spectral deconvolution that involved the spectra of oxyHb, deoxyHb, and metHb as the base spectra. Deoxygenated nitrite solution was added to deoxyHb at 37°C using a gas-tight Hamilton syringe. Final reactant concentrations were nitrite (2.5 mM, to stimulate pseudo–first order conditions) and deoxyHb (50 μM). Solutions were mixed by hand first and then by constant stirring using a magnetic stir-bar. Constant positive pressure was applied with helium to ensure an anaerobic environment and to prevent oxygen leaking into the system. Spectra were taken every 10 seconds, and changes in absorbance at 630 nm were recorded as a function of time after the subtraction of absorbance at 800 nm. Concentrations of HbNO, metHb, and metHb-nitrite in the final spectrum were calculated by spectral deconvolution. Hemoglobin P50 was determined using tonometry or open-flow respirometry, as described in “Red blood cells.”
Red blood cells. Optical spectra of hemoglobin in intact human RBCs were acquired with a recording spectrophotometer (Cary model 14; AVIV Associates, Lakewood, NJ) equipped with a scattered transmission accessory. A dilute suspension of whole milk in the reference cuvette was used to attenuate the reference light beam and to balance light scattering caused by RBCs. The thermostated cuvette, serving as the respirometry chamber, was a cylindrical quartz tube with a 16-mm light path, containing 5 mL stirred (460 rpm) sample with a 5-mL gas head-space. The floor of the chamber was a polarographic O2 sensor (2110; Orbisphere, Geneva, Switzerland). To maintain steady-state solution oxygen tensions and hence hemoglobin fractional saturations, the composition of the gas phase was modulated by mixing dry air and O2-free N2 using a digital mass flow controller (Tylan, Carson, CA), humidifying the gas, and passing it through the cuvette head-space at a flow rate of 100 mL/min. All experiments were performed at 37°C. In a typical experiment, buffer oxygen tension would first be altered to reach the desired level by altering oxygen content of the gas phase and then slowly injecting RBCs to reach 0.3% HCT final concentration (approximately 60-70 μM heme). On re-equilibration of solution oxygen, nitrite (500 μM) was added, and spectra were accumulated every 2 minutes for 1 hour. Solution was stirred from the top using a motorized turbine (460 rpm) that resulted in minimal (less than 0.5%) hemolysis. The latter was assessed at the end of each experiment by centrifugation of solution (1000g, 15 minutes, 10°C) and measurement of hemoglobin in the supernatant. Kinetics of nitrite reaction with RBC hemoglobin was evaluated by deconvolution of acquired spectra using least-squares analysis and assuming the presence of oxyhemoglobin, deoxyhemoglobin, methemoglobin, and nitrosylhemoglobin, which are ligation/oxidation species that might have been populated during the reaction.26 Base spectra for these species were collected as follows: oxyhemoglobin, RBCs incubated at 21% O2; deoxyhemoglobin, RBCs incubated at 0% O2, 100% N2; methemoglobin, oxygenated RBCs treated with 500 mM nitrite for 10 minutes; nitrosylhemoglobin, 100 μM DEA-nonoate added to deoxygenated RBCs (0.3% HCT) for 10 minutes. Loss of deoxyhemoglobin was used as a specific index to follow the RBC nitrite reductase activity. Importantly, no consumption of oxygen was observed (not shown) over the duration of the experiments, suggesting that loss of deoxyhemoglobin does not occur through heme oxygenation but as a result of direct nitrite-dependent reactions. For NO gas formation studies, 200 μL head-space gas was collected and directly injected into an NO-chemiluminescence analyzer (Antek, Houston, TX). NO concentrations were determined using a standard curve–generated I3-dependent reduction of nitrite, as described previously.27
Vessel relaxation studies
Isometric tension in isolated endothelium-intact rat or rabbit thoracic aortic ring segments were measured according to 2 protocols, using phenylephrine and L-NAME–contracted vessels in custom-made chambers fitted with oxygen sensors, as described previously.7 The first protocol involved a classic pharmacologic approach. After tension development reached a plateau (approximately 70%-80% of maximal), RBCs and then cumulative additions of nitrite were added to the vessel bath, and effects on tension were monitored. Vessel tension experiments at low oxygen tensions were performed using 0%, 1%, and 5% oxygen gas mixtures. This resulted in measured oxygen tensions of 15, 25, and 60 mmHg, respectively. Because of oxygen back-diffusion from the atmosphere, solution oxygen tensions do not reflect tensions in perfused gas. For these studies, vessels either were precontracted with phenylephrine and L-NAME before the equilibration of vessels at a given oxygen tension or were equilibrated at a given oxygen tension followed by precontraction. No differences in nitrite/RBC responses were observed, though the latter protocol resulted in greater contractile tone. In all cases, vessels were allowed to equilibrate at a given oxygen tension for 10 minutes before test compounds were added. In the second protocol, to probe the oxygen dependence of RBC-nitrite–dependent vasodilation, vessel tension and oxygen tension were monitored simultaneously as oxygen was removed from the vessel bath by perfusion with 95% N2/5% CO2. Based on data collected from these simultaneous measurements, the oxygen tension at which dilation started could be calculated in 2 ways, as reported28 and as indicated in Figure 3A. In the example shown (Figure 3A), rates of deoxygenation in the 2 chambers were similar, but, because of potential differences in tubing lengths, pressures, and other factors that supply gas to individual chambers, the rate of deoxygenation could differ significantly. It was critical, therefore, to measure oxygen tension simultaneously within the same bioassay chamber. Although tissue oxygen was not directly measured, the low tissue volume compared with bath volume ensured rapid equilibration. Two protocols were necessary because rat thoracic aorta spontaneously dilate as oxygen tension is decreased below approximately 10 to 20 mmHg,7 a response that represents compromised vessel ATP production from respiration as oxygen tensions are lowered.28 Performing vasodilation experiments at tensions lower than 20 mmHg oxygen is complicated by a continually changing baseline. Previous studies from our laboratory showed an effect of RBCs alone on this parameter,7 but this was a consequence of rapid oxygen off-loading from RBCs as they became deoxygenated with the released oxygen serving as a buffer. In the current study, this effect was eliminated by slowing the rate of deoxygenation. For the first protocol, we chose to investigate rat RBCs because they have a higher P50 than human RBCs (35-40 mmHg vs 28 mmHg, respectively), allowing for a larger experimental window in which oxygen tensions can be altered to modulate the hemoglobin oxygenation state without affecting basal vessel tone. Rat and human RBCs were investigated using the second protocol; n corresponded to individual experiments conducted using different vessel and RBC preparations, and Y was calculated using the equation Y = (n log P50) – (n log pO2), where n corresponded to the Hill coefficient and pO2 was the oxygen tension at which vasodilation was initiated. RBCs at a concentration of 0.3% HCT (approximately 60 μM heme) were used for these studies. Adding cellular or other biomolecules with significant hydrophobic content into bioassay chambers resulted in excessive foaming that increased noise on tension measurements and RBC lysis. These factors limited the concentration of RBCs that could be administered. Typically, less than 1% HCT can be tolerated in bioassay chambers; however, we found 0.3% HCT resulted in reproducibly low (less than 0.1%) hemolysis without compromising signal-noise ratios on vessel tension measurements. Within a given experiment, each condition was repeated 2 to 4 times, and each experiment was repeated 3 or more times.
Measurement of cGMP. cGMP was measured by competition ELISA using IBMX-pretreated vessels, as described previously.27
Measurement of mitochondrial respiration
Rat liver mitochondria were isolated by standard differential centrifugation techniques in buffer composed of sucrose (250 mM), Tris (10 mM), and EGTA (1 mM), pH 7. All steps were performed at 4°C, and mitochondria were stored on ice and used within 4 hours of isolation. Protein was determined using the Folin-phenol reagent against a standard curve constructed using bovine serum albumin. Oxygen tension was measured using a Clark-type oxygen sensor (Instech, Plymouth Meeting, PA) in a 1-mL sealed chamber, magnetically stirred at 37°C. Mitochondria (2 mg/mL) were incubated in respiration buffer (120 mM KCl, 10 mM HEPES, 1 mM EGTA, 25 mM sucrose, 5 mM MgCl2, 5 mM KH2PO4, pH 7.4). State 3 respiration was stimulated by the addition of succinate (15 mM) and ADP (0.5 mM) in the presence or absence of RBCs (0.3 HCT), sodium nitrite (20 μM), and C-PTIO (200 μM), and oxygen concentration was recorded using a digital recording device (Dataq, Akron, OH) connected to a personal computer. Oxygen off-loading from RBCs as they deoxygenate was determined using a dual-chamber respirometer (Oroboros, Innsbruck, Austria). Oxygen tensions were lowered at a constant rate using ascorbate/ascorbate oxidase in the absence and presence of RBCs (0.3% HCT). Using this approach, as oxygen tensions reached the RBC P50, oxygen was off-loaded into solution, as indicated by a slowing in the rate of oxygen consumption (not shown).
Statistical analysis
All results are reported as the mean plus or minus SEM. Statistical analysis was performed using Origin Software. Differences between the groups were assessed by Student t test.
Results
RBCs, hypoxia, and nitrite constitute an integrated system for hypoxic vasodilation
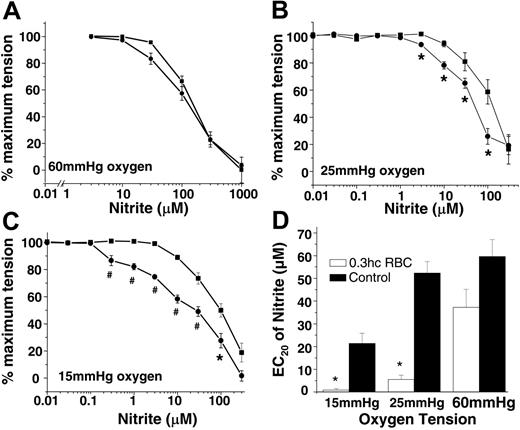
In the first series of experiments, aortic rings were equilibrated with fixed oxygen tensions of 15, 25, and 60 mmHg and then were exposed to increasing concentrations of nitrite in the absence or presence of RBCs. Figure 1A-C shows that RBCs had no effect on nitrite-dependent vasodilation at 60 mmHg oxygen. However, at 25 mmHg and 15 mmHg, nitrite was a more potent vasodilator in the presence of RBCs (indicated by the leftward shift in dose-response curves) such that at the lower oxygen tension, as little as 200 nM nitrite produced vasodilation (note that blood flow is controlled to the 4th power of vessel radius and, thus, a change in tension of a few percentage points would equate to significant changes in blood flow in vivo). To illustrate this potentiating effect of RBCs, Figure 1D shows that the EC20 for nitrite-induced vasodilation is decreased in the presence of RBCs at 15 and 25 mmHg oxygen.
Nitrite-dependent hypoxic vasodilation by RBCs is NO dependent
Previous studies have shown NO production from the reaction of nitrite with deoxygenated cell-free Hb or RBCs, as follows (equation 1)7,24,26,29-31 : Hb2+ + NO2– → Hb3+ + NO + OH–.
We tested the hypothesis that the formation of NO through this reaction mediates the relaxation shown in Figure 1. Figure 2A shows that nitrite-dependent hypoxic vasorelaxation was inhibited by the cell-impermeable NO scavenger C-PTIO (Figure 2A). Moreover, consistent with the NO-dependent activation of sGC, the incubation of nitrite and deoxygenated RBCs resulted in the accumulation of vessel cGMP, which was also inhibited by C-PTIO (Figure 2B). Control experiments showed that C-PTIO did not affect Hb oxidation or the ligation state (data not shown), further suggesting scavenging of NO as the mechanistic basis for its inhibitory effects. Importantly, no increase in cGMP was observed with nitrite or RBCs alone or when they were used together under oxygenated conditions. Although the yield of bioactive NO over 10 minutes was relatively small—estimated to be 20 nM based on comparison with a NO donor standard curve (Figure 2B, inset)—under these experimental conditions it was clearly sufficient to stimulate NO-dependent signaling and vasodilation.
Hypoxia, RBCs, and nitrite stimulate vasodilation. Nitrite-dependent vasodilation alone (▪) or in the presence of RBCs (0.3% HCT) (•) at 60 mmHg (A), 25 mmHg (B), and 15 mmHg (C) oxygen. Using a P50 value of 35 mmHg for rat RBCs and a Hill coefficient of 2.5,33,34 at 15 mmHg, 25 mmHg, and 60 mmHg oxygen, Hb saturation corresponded to 11%, 30%, and 82%, respectively, encompassing physiologic oxygen saturations. Data are mean ± SEM (n = 3). *P < .05; #P < .01 relative to the corresponding nitrite dose without RBCs. (D) EC20 (nitrite concentration that stimulated 20% dilation) values (determined from 3 independent experiments) of nitrite (▪) and nitrite + 0.3% HCT rat RBCs (□) at 15 mmHg, 25 mmHg, and 60 mmHg oxygen. *P < .01 relative to corresponding controls (n = 3).
Hypoxia, RBCs, and nitrite stimulate vasodilation. Nitrite-dependent vasodilation alone (▪) or in the presence of RBCs (0.3% HCT) (•) at 60 mmHg (A), 25 mmHg (B), and 15 mmHg (C) oxygen. Using a P50 value of 35 mmHg for rat RBCs and a Hill coefficient of 2.5,33,34 at 15 mmHg, 25 mmHg, and 60 mmHg oxygen, Hb saturation corresponded to 11%, 30%, and 82%, respectively, encompassing physiologic oxygen saturations. Data are mean ± SEM (n = 3). *P < .05; #P < .01 relative to the corresponding nitrite dose without RBCs. (D) EC20 (nitrite concentration that stimulated 20% dilation) values (determined from 3 independent experiments) of nitrite (▪) and nitrite + 0.3% HCT rat RBCs (□) at 15 mmHg, 25 mmHg, and 60 mmHg oxygen. *P < .01 relative to corresponding controls (n = 3).
A role for NO formation was further tested using a third biologically relevant sensor for NO production that is independent of cGMP: NO-dependent inhibition of mitochondrial respiration.32 Using isolated mitochondria as a model for probing NO production, Figure 2C-D shows that deoxygenating RBCs and nitrite inhibit state 3 respiration through a mechanism inhibited by C-PTIO. With mitochondria alone or in the presence of nitrite (20 μM), oxygen consumption was linear until completely anoxic. With RBCs, a small inhibition was observed at oxygen tensions lower than 40 mmHg (approximately 20%), a property attributed to oxygen off-loading by hemoglobin as it becomes deoxygenated. In the presence of RBCs and nitrite, a further inhibition of respiration was observed. Importantly, the onset of inhibition occurred at oxygen tensions lower than the rat RBC P50 (35-40 mmHg33,34 ), consistent with the data presented in Figure 3.
Oxygen-dependent control of vasodilatory activity of RBCs and nitrite
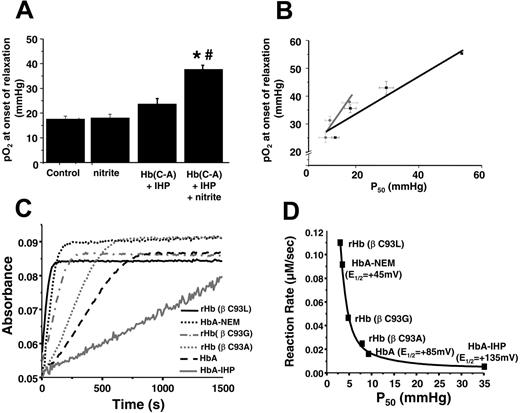
Our data suggest a model in which RBCs mediate NO-dependent vasodilation through a nitrite reductase activity that is regulated by oxygen. To more clearly define the oxygen dependence of this process, we used an alternative “kinetic” bioassay protocol that simultaneously monitors vessel tension and oxygen tension as the system is deoxygenated. As shown in Figure 3A, the intersection in regression lines of vessel tension before and after the initiation of relaxation is used to determine the oxygen tension at which RBCs and nitrite begin to vasodilate. Vessels alone dilate spontaneously as the oxygen tension reaches 15 to 20 mmHg (Figure 3B),7,28 an effect caused by limiting substrate oxygen availability for mitochondrial ATP production.28 Importantly, the presence of RBCs or nitrite alone has no effect on this process (Figure 3B). However, in the presence of RBCs and nitrite, vessel dilation starts at significantly higher oxygen tensions. The lack of effect of RBCs or nitrite alone suggests that the oxygen tension at which vessels begin to dilate provides insight into how oxygen sensing by the RBCs is coupled to nitrite-dependent vasodilation and reflects the oxygen tension at which the nitrite reductase activity of the RBC achieves a threshold to initiate NO-dependent signal transduction and relaxation. To further investigate how oxygen sensing by RBCs is linked to nitrite-mediated vasodilation, we used Hb preparations with a range of P50 that included human RBCs (P50 = 28 mmHg), rat RBCs (P50 = 35-40 mmHg), cell-free HbA (varying P50), and a cell-free crosslinked Hb designed as a blood substitute (HBOC-201, P50 = 38 mmHg). Figure 3B shows that compared with control, neither nitrite, RBCs, HbA, nor HBOC-201 alone affected the oxygen tension–vessel tension relationship. However, the combination of nitrite and either RBCs, HbA, or HBOC-201 initiated vasodilation at higher oxygen tensions.7 Interestingly, the oxygen tension at which vasodilation was appreciated was directly proportional to the intrinsic P50 (Figure 3C), resulting in the initiation of nitrite-dependent vasodilation between 50% and 60% hemoglobin fractional oxygen saturation (Figure 3C, inset).
Nitrite metabolism by deoxygenated RBCs stimulates NO-dependent vasodilation and inhibition of mitochondrial respiration. (A) Representative vessel tension traces showing rat RBCs (0.3% HCT) and nitrite-dependent vasodilation at 25 mmHg oxygen in the presence (black) and absence (gray) of 200 μM C-PTIO. Arrows indicate the times and concentrations of nitrite added. (B) Changes in cGMP in rat thoracic aorta treated as indicated for 10 minutes at 25 mmHg oxygen (▪) or 600 mmHg oxygen (□). Final concentrations were nitrite 3 μM, rat RBCs 0.3% HCT, and C-PTIO 200 μM. *P < .01 relative to control and nitrite alone; *P < .05 relative to RBCs alone (n = 3). (inset) To estimate the amount of NO produced by RBC/nitrite, a calibration curve was determined for increased vessel cGMP in response to increasing concentrations of the NO donor, DeaNonoate, at 25 mmHg oxygen. In a concentration-dependent manner, NO increased cGMP, which was inhibited by C-PTIO (○). Specifically, 100 nM DeaNonoate increased cGMP from 526.3 ± 46 pmol/g to 5602.6 ± 1340 pmol/g in the absence of C-PTIO and to 653 ± 97.9 nM in the presence of C-PTIO (values represent mean ± SEM). Using this curve, an estimated 20 nM NO was produced by RBCs (0.3% HCT) and nitrite (3 μM) over 10 minutes. (C) Representative traces of oxygen concentration as a function of time for mitochondria in state 3 respiration (black line) in the presence of 20 μM nitrite (gray line), 0.3% HCT RBCs (dashed line), or RBCs and nitrite (dotted line) are shown. Respiratory substrates were added to oxygen electrode chamber containing mitochondria and RBCs. The arrow indicates the time of nitrite addition. (D) Respiration rate measured at 30 μM oxygen. Inhibition of respiration by RBCs + nitrite was completely reversed by C-PTIO, consistent with NO formation. C-PTIO had no effect alone (not shown), and neither did RBCs alone. *P < .001 relative to RBCs alone. #P < .002 relative to RBCs + nitrite + PTIO (n = 3).
Nitrite metabolism by deoxygenated RBCs stimulates NO-dependent vasodilation and inhibition of mitochondrial respiration. (A) Representative vessel tension traces showing rat RBCs (0.3% HCT) and nitrite-dependent vasodilation at 25 mmHg oxygen in the presence (black) and absence (gray) of 200 μM C-PTIO. Arrows indicate the times and concentrations of nitrite added. (B) Changes in cGMP in rat thoracic aorta treated as indicated for 10 minutes at 25 mmHg oxygen (▪) or 600 mmHg oxygen (□). Final concentrations were nitrite 3 μM, rat RBCs 0.3% HCT, and C-PTIO 200 μM. *P < .01 relative to control and nitrite alone; *P < .05 relative to RBCs alone (n = 3). (inset) To estimate the amount of NO produced by RBC/nitrite, a calibration curve was determined for increased vessel cGMP in response to increasing concentrations of the NO donor, DeaNonoate, at 25 mmHg oxygen. In a concentration-dependent manner, NO increased cGMP, which was inhibited by C-PTIO (○). Specifically, 100 nM DeaNonoate increased cGMP from 526.3 ± 46 pmol/g to 5602.6 ± 1340 pmol/g in the absence of C-PTIO and to 653 ± 97.9 nM in the presence of C-PTIO (values represent mean ± SEM). Using this curve, an estimated 20 nM NO was produced by RBCs (0.3% HCT) and nitrite (3 μM) over 10 minutes. (C) Representative traces of oxygen concentration as a function of time for mitochondria in state 3 respiration (black line) in the presence of 20 μM nitrite (gray line), 0.3% HCT RBCs (dashed line), or RBCs and nitrite (dotted line) are shown. Respiratory substrates were added to oxygen electrode chamber containing mitochondria and RBCs. The arrow indicates the time of nitrite addition. (D) Respiration rate measured at 30 μM oxygen. Inhibition of respiration by RBCs + nitrite was completely reversed by C-PTIO, consistent with NO formation. C-PTIO had no effect alone (not shown), and neither did RBCs alone. *P < .001 relative to RBCs alone. #P < .002 relative to RBCs + nitrite + PTIO (n = 3).
RBC nitrite reductase kinetics are maximal at 50% oxygen saturation (P50)
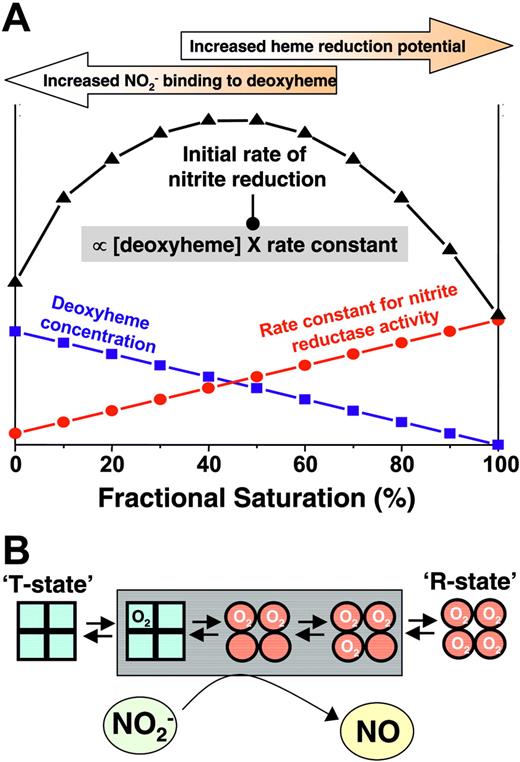
Our recent biochemical studies illustrate that deoxyHb is an allosterically regulated nitrite reductase with a maximal activity at 50% oxygen saturation.24 We have proposed that this peak activity at P50 occurs secondarily to a balance between the availability of deoxyhemes for nitrite binding, which is maximal for fully deoxygenated or T-state Hb, and the ability of the heme to donate an electron to nitrite (more negative heme redox potential), which is maximal for R-state Hb. Thus, the rate of the reaction, which is a product of the deoxyheme concentration and the biomolecular rate constant for heme-mediated nitrite reduction (greatest in R-state), peaks around the P50. To test whether similar effects occurred with intact RBCs, we monitored Hb visible absorption spectra after adding nitrite to intact RBCs at varying Hb oxygen saturations between 0% and 100% and determined the nitrite reductase activities at each saturation. Using the measured rate constants, Figure 3D shows the predicted initial rate of reaction between nitrite and RBC deoxyhemoglobin as a function of fractional saturation and demonstrates a maximal reductase reaction rate between 40% and 50% saturation, a result consistent with observations of a threshold for vasodilation at the Hb P50.
RBC P50regulates nitrite-dependent vasodilation. (A) Representative traces of simultaneously measured vessel tension and oxygen tension as a function of time with RBCs alone (gray trace) or RBCs + nitrite (black trace). (B) Nitrite-dependent dilation starts at higher oxygen tensions in response to human RBCs (hRBCs), HBOC-201, or HbA and IHP. Final concentrations: nitrite (2 μM), human RBCs (0.3% HCT), HBOC-201 (25 μM), HbA (25 μM), and IHP (625 μM). †P < .03 relative to nitrite and P < .01 relative to hRBCs alone (n = 3). *P < .001 relative to nitrite alone. #P < .01 relative to Hb + IHP. Data represent mean ± SEM (n = 3-5). (C) Relationship of Hb P50 and the oxygen tension at the initiation of vessel dilation in the presence of nitrite (2 μM). Data from rat RBCs, hRBCs, HBOC-201, and cell-free HbA (in which P50 was modulated by the addition of varying concentrations of IHP, resulting in heme/IHP ratios ranging from 1:0.25 to 1:25) are shown. A linear relationship (y = 19.6 + 0.62x; r = 0.89; note intercept is not zero because of basal dilatory responses of vessels to hypoxia) between P50 and oxygen tension at onset of relaxation was observed. (inset) Calculated values for Hb fractional saturation (Y) at the oxygen tension at which nitrite-dependent vasodilation was initiated. (D) Using the calculated rate constants for nitrite reactions with RBC deoxyHb, the predicted velocity or initial rate of nitrite reaction with deoxyHb under physiologic conditions (total Hb = 20 mM in heme; circulating nitrite concentrations = 500 nM19 ) and as a function of Hb fractional saturation are shown and indicate a maximal rate of reaction around the Hb P50. Data were collected using RBCs from 3 different preparations. (inset) Representative traces showing NO formation (measured by NO chemiluminescence) in the head-space of the reaction between RBC (0.3% hc) and nitrite (500 μM) after 2 minutes in the open-flow respirometer (head-space volume and reaction solution volume 5 mL each with 100 mL/min gas flow). Traces show NO production after injection of 200 μL of [A] air alone or head-space from [B] nitrite in PBS at 28 mmHg oxygen or nitrite + RBC at [C] 0 mmHg (0% fractional saturation), [D] 28 mmHg oxygen (50% fractional saturation), and [E] 155 mmHg (100% fractional saturation).
RBC P50regulates nitrite-dependent vasodilation. (A) Representative traces of simultaneously measured vessel tension and oxygen tension as a function of time with RBCs alone (gray trace) or RBCs + nitrite (black trace). (B) Nitrite-dependent dilation starts at higher oxygen tensions in response to human RBCs (hRBCs), HBOC-201, or HbA and IHP. Final concentrations: nitrite (2 μM), human RBCs (0.3% HCT), HBOC-201 (25 μM), HbA (25 μM), and IHP (625 μM). †P < .03 relative to nitrite and P < .01 relative to hRBCs alone (n = 3). *P < .001 relative to nitrite alone. #P < .01 relative to Hb + IHP. Data represent mean ± SEM (n = 3-5). (C) Relationship of Hb P50 and the oxygen tension at the initiation of vessel dilation in the presence of nitrite (2 μM). Data from rat RBCs, hRBCs, HBOC-201, and cell-free HbA (in which P50 was modulated by the addition of varying concentrations of IHP, resulting in heme/IHP ratios ranging from 1:0.25 to 1:25) are shown. A linear relationship (y = 19.6 + 0.62x; r = 0.89; note intercept is not zero because of basal dilatory responses of vessels to hypoxia) between P50 and oxygen tension at onset of relaxation was observed. (inset) Calculated values for Hb fractional saturation (Y) at the oxygen tension at which nitrite-dependent vasodilation was initiated. (D) Using the calculated rate constants for nitrite reactions with RBC deoxyHb, the predicted velocity or initial rate of nitrite reaction with deoxyHb under physiologic conditions (total Hb = 20 mM in heme; circulating nitrite concentrations = 500 nM19 ) and as a function of Hb fractional saturation are shown and indicate a maximal rate of reaction around the Hb P50. Data were collected using RBCs from 3 different preparations. (inset) Representative traces showing NO formation (measured by NO chemiluminescence) in the head-space of the reaction between RBC (0.3% hc) and nitrite (500 μM) after 2 minutes in the open-flow respirometer (head-space volume and reaction solution volume 5 mL each with 100 mL/min gas flow). Traces show NO production after injection of 200 μL of [A] air alone or head-space from [B] nitrite in PBS at 28 mmHg oxygen or nitrite + RBC at [C] 0 mmHg (0% fractional saturation), [D] 28 mmHg oxygen (50% fractional saturation), and [E] 155 mmHg (100% fractional saturation).
Finally, to directly probe for NO production and to examine the oxygen dependence, head-space gas was collected using gas-tight syringes and was injected directly into a NO analyzer. Figure 3D (inset) shows increased NO production at 50% compared with 0% or 100% fractional saturation, consistent with the maximal nitrite reductase activity at intermediate Hb oxygen saturations. This was a qualitative assessment of NO formation because several factors determine the precise concentration of NO in the gas head-space, including rates of NO production, partition coefficients between gas and aqueous phases, gas-flow rate compared with mixing rates, and NO reactions with oxygen and Hb.
Role of conserved β-globin cysteine 93 in nitrite-dependent hypoxic vasodilation
It is known that the β93cys residue regulates Hb oxygen affinity and heme redox potential.35-38 Moreover, a role for cysteine 93 in transporting NO as an S-nitrosothiol has been proposed to mediate hypoxic vasodilation by RBCs.4,39 Because SNOHb is formed to some extent from the reaction of nitrite with Hb7,30 and β93cys modulated heme redox potential,37,38,40 the role of β93cys in the nitrite reductase and vasodilator activity of Hb was evaluated. First, experiments similar to those described for Figure 3A-B were performed with a recombinant Hb in which the β93cys residue was replaced with an alanine (HbC→A). Oxygen affinity was modulated by IHP, and nitrite dependent vasodilation was tested. Figure 4A shows that Hb(C→A) increased the oxygen tension at which nitrite-dependent relaxation occurs, precluding a requirement for SNOHb formation as an intermediate in this process. Both HbA and Hb(C→A) potentiate nitrite-dependent vasodilation in a manner proportionate with each respective P50 but, compared with HbA, vasodilation with Hb(C→A) begins at oxygen tensions higher than the respective Hb P50 (Figure 4B). Furthermore, deoxyRBC-nitrite–dependent relaxation was not affected by ascorbate (a reductant that reduces S-nitrosothiols in a metal-dependent manner) or glutathione (GSH) (which modulates S-nitrosated RBC– and cell-free SNOHb–dependent vasodilation27 ) (Table 1), suggesting no role for an S-nitrosothiol intermediate in this process.
Effect of 8-SPT (adenosine receptor antagonist), ascorbate, and glutathione (GSH) on the potentiation of nitrite-dependent vasodilation by deoxygenated RBCs
. | EC20 for nitrite-dependent vasodilation, μM . |
|---|---|
| RBCs | 5.4 ± 1.8 |
| RBCs + 100 μM glutathione | 7.1 ± 2.1 |
| RBCs + 100 μM ascorbate | 6.3 ± 0.3 |
| RBCs + 15 μM 8-SPT | 5.3 ± 0.6 |
. | EC20 for nitrite-dependent vasodilation, μM . |
|---|---|
| RBCs | 5.4 ± 1.8 |
| RBCs + 100 μM glutathione | 7.1 ± 2.1 |
| RBCs + 100 μM ascorbate | 6.3 ± 0.3 |
| RBCs + 15 μM 8-SPT | 5.3 ± 0.6 |
Experiments were performed with rat RBCs at 25 mmHg. Data represent mean ± SEM (n = 3). Final concentration of RBCs was 0.3% HCT.
Kinetic evaluation of the nitrite reductase activity revealed insight into the mechanisms through which the β93cys residue modulates the nitrite reductase activity. Alkylation (by NEM) of the β93cys decreased the heme redox potential (E1/2) from +85 mV to +45 mV,38,40 suggesting this derivative would more efficiently reduce nitrite. Consistent with this proposal, previous data show that the nitrite reductase activity of NEM-treated deoxyHb was increased approximately 6-fold41 (Figure 4C). The opposite effect is observed with Hb treated with IHP, which increases the E1/2 to 135 mV.24 Figure 4C also shows that the rate of nitrite reduction was faster with recombinant deoxyHb in which the β93cys residue was substituted with either Ala, Leu, or Gly, consistent with each increasing R-state character,25 and which may underlie data that these recombinant Hbs stimulated vasodilation at oxygen tensions higher than each respective P50 compared with HbA.
β93cys residue controls nitrite reductase activity of Hb. (A) Hb(C→A) potentiates nitrite-dependent relaxation. Final concentrations: nitrite (2 μM), Hb(C→A) (25 μM), and IHP (625 μM). *P < .001 relative to nitrite alone. #P < .01 relative to Hb(C→A) + IHP. Data represent mean ± SEM (n = 3-5). (B) Relationship between P50 of HbA (▪) or Hb (C→A) (⬡) and the oxygen tension at the initiation of vessel dilation in the presence of nitrite (2 μM). Data for HbA are taken from Figure 3C. P50 was modulated by the addition of varying amounts of IHP and was determined under identical buffer, pH, temperature, and PCO2 conditions as for vessel dilation studies. A linear relationship between P50 and oxygen tension at the onset of relaxation was observed for both Hbs (black line: y = 20.7 + 0.67x; r = 0.96; gray line: y = 11.6 + 1.48x; r = 0.94). Data represent mean ± SEM (n = 3-6). (C) Representative reaction traces for metHb formation from the reaction between nitrite and various deoxyHbs (experiments performed under anoxic conditions). (D) Relationship between Hb P50 and rate of nitrite reduction. Shown are the best fit line (R 2 = 0.99) and the average redox potentials (E1/2) from values in the literature.37,40,42
β93cys residue controls nitrite reductase activity of Hb. (A) Hb(C→A) potentiates nitrite-dependent relaxation. Final concentrations: nitrite (2 μM), Hb(C→A) (25 μM), and IHP (625 μM). *P < .001 relative to nitrite alone. #P < .01 relative to Hb(C→A) + IHP. Data represent mean ± SEM (n = 3-5). (B) Relationship between P50 of HbA (▪) or Hb (C→A) (⬡) and the oxygen tension at the initiation of vessel dilation in the presence of nitrite (2 μM). Data for HbA are taken from Figure 3C. P50 was modulated by the addition of varying amounts of IHP and was determined under identical buffer, pH, temperature, and PCO2 conditions as for vessel dilation studies. A linear relationship between P50 and oxygen tension at the onset of relaxation was observed for both Hbs (black line: y = 20.7 + 0.67x; r = 0.96; gray line: y = 11.6 + 1.48x; r = 0.94). Data represent mean ± SEM (n = 3-6). (C) Representative reaction traces for metHb formation from the reaction between nitrite and various deoxyHbs (experiments performed under anoxic conditions). (D) Relationship between Hb P50 and rate of nitrite reduction. Shown are the best fit line (R 2 = 0.99) and the average redox potentials (E1/2) from values in the literature.37,40,42
Although deoxygenation of Hb (ie, T-state Hb) appears to be required for nitrite reduction, R-state Hb (oxygenated conformation) has more negative redox potential and is expected, therefore, to be a more efficient nitrite reductant.40,42 In support of this thesis that both the concentration of deoxy heme and heme redox potential are critical regulators of the nitrite reductase activity, Figure 4D shows the correlation between the nitrite reductase rates and both Hb P50 and E1/2. Indeed, R-state stabilizing conditions, such as cysteine alkylation and mutation, both reduce E1/2 (making the heme a more efficient electron donor) and increase the rate of nitrite reduction.
Hypoxic vasodilation mediated by RBCs occurs through nitrite- or ATP-dependent mechanisms
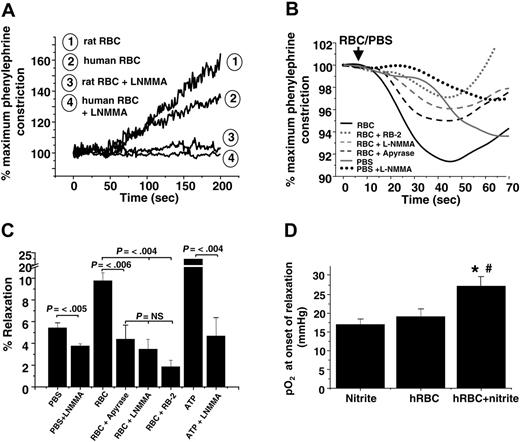
Previous studies have shown a vasodilatory effect of RBCs added to rabbit thoracic aorta at low oxygen tensions (approximately 10 mmHg),39,43 a response that has been interpreted in favor of the hypothesis that SNOHb is a modulator of hypoxic vasodilation. However, deoxygenation of RBCs also results in ATP release, which stimulates vasodilation by activation of P2Y (purinergic) receptors and eNOS.2,3,44 The role of RBCs and nitrite was evaluated in the context of these proposed mechanisms. Human RBCs failed to vasodilate rat aortic ring preparations in the absence of nitrite (Figure 5A). In the absence of L-NMMA, nitrite-free RBCs promoted vasoconstriction consistent with scavenging of endothelial-derived NO. These data are in contrast to previous studies showing vasodilatory effects of RBCs added to rabbit aortic preparations under hypoxic conditions.39,43 To directly compare our results with those of these studies, nitrite-free RBCs were added to hypoxic rabbit thoracic aorta preparations. Interestingly, using this vascular preparation, RBCs alone induced an initial and brief 10% relaxation that was followed by vasoconstriction (Figure 5B). Although this effect has been proposed to be mediated by SNOHb, we found it was dependent on 2 alternative mechanisms: (1) oxygen-dependent stimulation of eNOS, which is supported by the observation that adding PBS (containing dissolved oxygen) stimulated vasodilation inhibitable by L-NMMA and (2) hypoxic release of ATP from RBCs, the latter blocked by inhibiting ATP-dependent signaling, which was achieved by catalyzing ATP degradation with apyrase, blocking the ATP-purinergic receptor with RB2, and inhibiting eNOS with L-NMMA (Figure 5C). The lack of RBC ATP–dependent vasodilation of rat aorta (Figure 5A) may be explained by the lower (2- to 3-fold) sensitivity of these vessels to ATP-induced relaxation compared with rabbit aorta (data not shown).
Given the sensitivity of rabbit aorta to vasodilatory effects of ATP, this experimental system was used to evaluate nitrite-dependent vasorelaxation. As shown in Figure 5D, vasodilation elicited by deoxygenated RBCs and nitrite was not inhibited by incubation with L-NMMA, suggesting an ATP-independent mechanism. Moreover, adenosine-mediated pathways, another putative mechanism through which RBCs may affect vascular tone, were not involved in nitrite-dependent vasodilation (Table 1). Importantly, nitrite and deoxygenated RBCs stimulated vasodilation in both vessel types.
Discussion
Understanding how RBCs regulate NO metabolism during hypoxia is a central question in vascular physiology. This function has largely been attributed to SNOHb and ATP-dependent vasodilation, but recent studies have suggested a role for nitrite.6,7,23 Our data support a model in which the oxygen-sensing function of RBCs is linked to nitrite reduction with the subsequent formation of NO and stimulation of vasodilation.
A biochemical rationale for NO production from nitrite is shown in equation 1. Our data show that deoxygenating RBCs and nitrite produce NO (measured in the head space) and stimulate various NO-dependent processes, including vasodilation, cGMP formation, and inhibition of mitochondrial respiration, which are sensitive to C-PTIO. Recently, this putative NO scavenger was also shown to react with nitrogen dioxide.45 However, nitrogen dioxide does not stimulate vasodilation, does not activate sGC, and does not inhibit mitochondrial respiration. Together with the cell-impermeable nature of C-PTIO, our data indicate a functional role for NO produced outside the RBC and from nitrite reactions with deoxyHb. However, a NO-dependent mechanism for relaxation appears untenable considering the rapid inactivation reactions of NO with intracellular Hb that typically inhibit vessel relaxation.46,47 Two models that may overcome these apparent limitations have been discussed recently: direct NO release from RBCs into the extracellular compartment or formation of NO outside the RBC through an intermediate that is produced from the nitrite-deoxyHb reaction and that can escape the RBC.8,26,46,48 Clearly, these models remain speculative, and reconciling how NO can escape the RBC and avoid rapid scavenging reactions with Hb remains a challenge requiring further investigation. Interestingly, hypoxia decreases the threshold NO concentration required to elicit signaling and vasodilation.49,50 Moreover, metabolic acidosis will also stimulate nitrite reduction to NO by hemoglobin-dependent24 and -independent processes.16 These data support an integrated model in which hypoxia induces blood flow by RBC- and vessel wall–dependent mechanisms.
Other potential mechanisms that reduce nitrite to NO include xanthine oxidase and mitochondria.12,51 Although these may play a role in the tissue metabolism of nitrite,22 in all the models used to determine NO bioactivity herein, hypoxic RBCs and nitrite were absolutely required. In a recent study,31 no dilatory effect of the combination of cell-free Hb, IHP, or nitrite was observed. The nature of these discrepant results is unclear, but the discrepancy might have arisen because of different experimental conditions and because IHP alone induced significant relaxation, which might have masked any dilatory effects of Hb and nitrite. We used IHP controls in all our experiments and observed no IHP-dependent vasodilation.
ATP and nitrite represent independent mediators of RBC vasodilation. (A) Representative vessel tension traces (at 25 mmHg oxygen) showing that human and rat RBCs induce contraction or have no vasoactive effect on rat thoracic aorta in the absence or presence, respectively, of L-NMMA. (B) Representative vessel tension traces and (C) percentages of relaxation stimulated by the addition of human RBCs (0.3% HCT) or ATP (1 μM) to rabbit thoracic aorta at 15 mmHg oxygen tension alone or after preincubation with 8 U/mL apyrase, 100 μM reactive blue-2 (RB-2), or 100 μM L-NMMA. To control for the addition of oxygen and oxygen off-loading from RBCs, an equivalent volume of ice-cold PBS saturated with 95% oxygen was added that also resulted in relaxation in an L-NMMA–inhibitable manner. Values are mean ± SEM (n = 3-6). P values are shown. NS = not significant. (D) Human RBCs (0.3% HCT) and nitrite (2 μM) were added to rabbit thoracic aorta incubated with L-NMMA (100 μM) to inhibit ATP-dependent relaxation. Dilation was initiated in a manner similar to that observed with rat vessels (Figure 3) at an oxygen tension of approximately 27 mmHg. Data represent mean ± SEM (n = 4-5). *P < .04 relative to RBCs alone. #P < .01 relative to nitrite alone.
ATP and nitrite represent independent mediators of RBC vasodilation. (A) Representative vessel tension traces (at 25 mmHg oxygen) showing that human and rat RBCs induce contraction or have no vasoactive effect on rat thoracic aorta in the absence or presence, respectively, of L-NMMA. (B) Representative vessel tension traces and (C) percentages of relaxation stimulated by the addition of human RBCs (0.3% HCT) or ATP (1 μM) to rabbit thoracic aorta at 15 mmHg oxygen tension alone or after preincubation with 8 U/mL apyrase, 100 μM reactive blue-2 (RB-2), or 100 μM L-NMMA. To control for the addition of oxygen and oxygen off-loading from RBCs, an equivalent volume of ice-cold PBS saturated with 95% oxygen was added that also resulted in relaxation in an L-NMMA–inhibitable manner. Values are mean ± SEM (n = 3-6). P values are shown. NS = not significant. (D) Human RBCs (0.3% HCT) and nitrite (2 μM) were added to rabbit thoracic aorta incubated with L-NMMA (100 μM) to inhibit ATP-dependent relaxation. Dilation was initiated in a manner similar to that observed with rat vessels (Figure 3) at an oxygen tension of approximately 27 mmHg. Data represent mean ± SEM (n = 4-5). *P < .04 relative to RBCs alone. #P < .01 relative to nitrite alone.
Our data also reveal a key role for the Hb P50 and the β93cys residues in regulating nitrite reductase activity, with sufficient NO (to stimulate vasodilation) formed as RBCs reach between 50% and 60% fractional saturation. In addition, kinetic studies showed a bell-shaped dependence on fractional saturation, with maximal RBC nitrite reductase activity occurring at approximately 40% to 60% hemoglobin-oxygen saturation. It is important to note that the former measurement reflects an integrated response of NO formation from the RBC and of vascular response to NO, each of which is modulated by hypoxia. Given the requirement for cell signaling stimuli to reach a certain threshold concentration and for multiple hypoxia-dependent pathways that regulate NO metabolism, our data suggest that the stimulation of nitrite-dependent vasodilation by RBCs will be restricted to vascular beds in which Hb fractional saturations decrease to approximately 40% to 60%. Consistent with this concept, recent studies show that physiologic oxygen tensions between the A3 and A4 arterioles, where blood pressure and oxygen delivery are controlled, are between 25 and 30 mmHg,52,53 supporting a mechanism for a P50-regulated nitrite reductase activity of Hb in hypoxic vasodilation. Because of practical limitations, we performed our studies at nitrite/RBC ratios higher than those in vivo. However, nitrite has previously been administered into the human forearm at a level that more closely mimics basal in vivo ratios and significantly increased blood flow in an oxygenation-sensitive manner,7 suggesting that the mechanisms described herein operate at varying nitrite/RBC ratios that encompass physiologic and potentially therapeutic conditions.
Our recent studies suggest that the nitrite reductase reaction depends on the availability of deoxyheme sites for nitrite binding and the potential of heme redox. These requirements are met at the opposing spectra of Hb conformational states, namely, more deoxyhemes in the T-state and more negative redox potential in the R-state. However, we propose that the opposing physicochemical requirement (that the heme be deoxygenated to allow nitrite binding though the rate of reduction reaction is greatest with R-state Hb) results in optimal conformation for nitrite reduction at the Hb P50 (Figure 6). Importantly, the precise heme redox potential will vary and is modulated by many factors, including allosteric effectors (eg, inorganic phosphates, pH) and chain heterogeneity. How these factors are integrated to regulate nitrite reduction under biologic conditions remains to be elucidated and is critical to better assess the role of nitrite–RBC interaction as a mechanism for hypoxic vasodilation.
Proposed model for allosteric regulation of nitrite reduction by deoxyhemoglobin. The rate of nitrite reduction by deoxyhemoglobin is proportional to the product of the deoxyheme concentration and the rate constant, the latter of which is determined by heme reduction potential being greater and having a more negative E1/2. Deoxyheme concentration and heme reduction potential/rate constant are maximal at low and high oxygen fractional saturations, respectively, resulting in a maximal initial rate of nitrite reduction at approximately 50% fractional saturation (ie, P50). Note that the precise relationship between heme redox potential and fractional saturation is not known. In the model, a linear dependence is approximated based on observations that the rate constant for nitrite-reduction increases linearly with fractional saturation. The points shown on figure are theoretical and are presented to illustrate the concept that the product of rate constant and deoxyhemoglobin concentration determine the initial rate. Also illustrated are hemoglobin conformations (using the symmetry model; for the sake of simplicity, conformations adhering to the sequential model are not shown and R-T transition is denoted as occurring between first and second oxygen binding steps) that are populated as a function of fractional saturation. Conformations highlighted in the gray box denote the proposed intermediates populated at intermediate fractional saturations that have increased nitrite reductase activities and available deoxyheme binding sites to maximally reduce nitrite to NO and stimulate vasodilation.
Proposed model for allosteric regulation of nitrite reduction by deoxyhemoglobin. The rate of nitrite reduction by deoxyhemoglobin is proportional to the product of the deoxyheme concentration and the rate constant, the latter of which is determined by heme reduction potential being greater and having a more negative E1/2. Deoxyheme concentration and heme reduction potential/rate constant are maximal at low and high oxygen fractional saturations, respectively, resulting in a maximal initial rate of nitrite reduction at approximately 50% fractional saturation (ie, P50). Note that the precise relationship between heme redox potential and fractional saturation is not known. In the model, a linear dependence is approximated based on observations that the rate constant for nitrite-reduction increases linearly with fractional saturation. The points shown on figure are theoretical and are presented to illustrate the concept that the product of rate constant and deoxyhemoglobin concentration determine the initial rate. Also illustrated are hemoglobin conformations (using the symmetry model; for the sake of simplicity, conformations adhering to the sequential model are not shown and R-T transition is denoted as occurring between first and second oxygen binding steps) that are populated as a function of fractional saturation. Conformations highlighted in the gray box denote the proposed intermediates populated at intermediate fractional saturations that have increased nitrite reductase activities and available deoxyheme binding sites to maximally reduce nitrite to NO and stimulate vasodilation.
Because we used 2 protocols for vessel bioassay experiments, we were able to observe distinct kinetics for vasodilation induced by RBCs and nitrite. With the use of a classic pharmacologic approach, at defined steady-state oxygen tensions that promoted Hb deoxygenation, nitrite and RBCs stimulated vasodilation. However, this response was relatively slow and required 2 to 7 minutes, depending on nitrite dose, for completion of the relaxation response (Figure 2A). When experiments were performed in which oxygen decreased as a function of time, RBC- and nitrite-dependent vasodilation occurred significantly more quickly. For example, Figure 3A shows that when P50 was reached, the time taken for RBC and nitrite (3 μM) to decrease tension by 10% was approximately 10 to 15 seconds. These data suggest different rate limits on the nitrite reductase reaction at steady-state and non–steady-state oxygen tensions. We speculate that the distinct kinetics reflect the concentrations of Hb present in a configuration required for maximal nitrite reduction, as discussed. For example, at equilibrium using carbon monoxide at 50% Hb saturation, approximately 80% of the Hb would be either fully unligated or fully ligated with CO, with the sum of all other intermediates (singly, doubly, or triply ligated states) accounting for only 20%.54 The proposed allosteric model suggests that deoxygenated hemes on R-state tetramers have the greatest nitrite reductase activity, and we speculate that these are present at higher concentrations during nonequilibrium conditions. These concepts are being tested in our laboratory and are illustrated in Figure 6.
Our data also suggest that the β93cys residue is not obligatory in the reactions between Hb and nitrite that lead to vasodilation but that it does modulate this process. Cysteine 93 substitutions increase the rate of nitrite reduction, consistent with the known effects of β93cys alkylation in decreasing the heme redox potential. This may also explain the uncoupling effect described by Figure 4B and may support a role for the Hb redox potential in modulating nitrite reduction in a predictable manner. In addition, these data exclude the requirement of SNOHb as an intermediate in the observed vasodilatory effects.
In addition to the effect on nitrite reduction, our data support that ATP release is a contributing mechanism for RBC-dependent hypoxic vasodilation. Several factors are likely to regulate the relative roles of ATP- or nitrite-dependent pathways, including the activity of transporters that regulate ATP release or nitrite uptake, enzymes that regulate ATP concentrations (eg, ectonucleotidases), and expression pattern, of purinergic receptors. Interestingly, recent data show that NO inhibits ATP release from RBCs55 and that, though our data suggest nitrite-dependent pathways are independent of ATP release, nitrite-derived NO may play a role in regulating ATP release. Further studies are required to examine this interplay and to more clearly define mechanisms of RBC hypoxic sensing and modulation of blood flow in vivo.
In summary, we propose a model whereby, upon deoxygenation of RBCs to their P50, nitrite is reduced to NO, which increases blood flow. This process is contrary to the reaction of nitrite with oxygenated Hb by which nitrite is oxidized to nitrate (nitrite oxidase activity). Therefore, a physiologic function for hemoglobin as allosterically regulated nitrite oxidase and reductase is proposed in the context of hypoxic vasodilation. Given that oxygen transport by hemoglobin is thermally and sterically regulated by the P50, this proposed mechanism for nitrite reduction to NO appears ideally suited for the regulation of hypoxic vasodilation under varied physiologic and pathologic conditions. Coupled with other reports demonstrating differential nitrite tissue compartmentalization,56 nitrite use during hypoxia, and hypoxic conversion of nitrite to nitrosating intermediates,7,22 we propose that nitrite reaction with deoxygenated ferrous heme to produce NO represents a common mechanism through which the biologic functions of NO during hypoxia are regulated.
Prepublished online as Blood First Edition Paper, September 29, 2005; DOI 10.1182/blood-2005-07-2668.
Supported by National Institutes of Health (NIH) grants HL70146 (R.P.P.), HL-24525 (C.H.), MSTP T32 GM08361 (J.H.C.); a Cardiovascular Pathophysiology Training Fellowship (T.S.I.); American Heart Association grant 0455296B (D.K.); and NIH Intramural Research Division funds.
J.H.C. and T.S.I. contributed equally to this work.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 3. RBC P50 regulates nitrite-dependent vasodilation. (A) Representative traces of simultaneously measured vessel tension and oxygen tension as a function of time with RBCs alone (gray trace) or RBCs + nitrite (black trace). (B) Nitrite-dependent dilation starts at higher oxygen tensions in response to human RBCs (hRBCs), HBOC-201, or HbA and IHP. Final concentrations: nitrite (2 μM), human RBCs (0.3% HCT), HBOC-201 (25 μM), HbA (25 μM), and IHP (625 μM). †P < .03 relative to nitrite and P < .01 relative to hRBCs alone (n = 3). *P < .001 relative to nitrite alone. #P < .01 relative to Hb + IHP. Data represent mean ± SEM (n = 3-5). (C) Relationship of Hb P50 and the oxygen tension at the initiation of vessel dilation in the presence of nitrite (2 μM). Data from rat RBCs, hRBCs, HBOC-201, and cell-free HbA (in which P50 was modulated by the addition of varying concentrations of IHP, resulting in heme/IHP ratios ranging from 1:0.25 to 1:25) are shown. A linear relationship (y = 19.6 + 0.62x; r = 0.89; note intercept is not zero because of basal dilatory responses of vessels to hypoxia) between P50 and oxygen tension at onset of relaxation was observed. (inset) Calculated values for Hb fractional saturation (Y) at the oxygen tension at which nitrite-dependent vasodilation was initiated. (D) Using the calculated rate constants for nitrite reactions with RBC deoxyHb, the predicted velocity or initial rate of nitrite reaction with deoxyHb under physiologic conditions (total Hb = 20 mM in heme; circulating nitrite concentrations = 500 nM19) and as a function of Hb fractional saturation are shown and indicate a maximal rate of reaction around the Hb P50. Data were collected using RBCs from 3 different preparations. (inset) Representative traces showing NO formation (measured by NO chemiluminescence) in the head-space of the reaction between RBC (0.3% hc) and nitrite (500 μM) after 2 minutes in the open-flow respirometer (head-space volume and reaction solution volume 5 mL each with 100 mL/min gas flow). Traces show NO production after injection of 200 μL of [A] air alone or head-space from [B] nitrite in PBS at 28 mmHg oxygen or nitrite + RBC at [C] 0 mmHg (0% fractional saturation), [D] 28 mmHg oxygen (50% fractional saturation), and [E] 155 mmHg (100% fractional saturation).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/2/10.1182_blood-2005-07-2668/4/m_zh80020689370003.jpeg?Expires=1767710275&Signature=vlN5zCsxGu3vSHkV0FwJ0-DW6u6Kpn6zd6TWyqLO9LU4PQrIk-F4yBB~lbJ9Beq~b50GcKhKzNoMYbZpkv6iFiabwdJmUYeEC5J4gPO-19BiD9mdtDuMmdANddSl5xNyMMRtot12DgMkrP7mIwu15A2MMDrvuPpRSqUzMJrn5ItUMvgI-8-ImV2-9R5Bxn~Ksos4AUO4QyJhpAi~AwvSN2grt9T-PVRNzcs-~zovz0mr2JIcK2Bwkd-4XZ3O7sLVnby5FSwpiMuiQsYkxGHXN3rMbAumlsVBvOqRr5bUkeqqjFW81-CV9c6LygI-NA7nqaXTHXF-7SQa6K-iJdedEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal