Abstract
We have investigated the role of tyrosine phosphorylation of the cyclin-dependent kinase (cdk) inhibitor p27Kip1 using the acute promyelocytic leukemia cell line NB4 together with granulocyte colony-stimulating factor (G-CSF). Short-term G-CSF stimulation resulted in a rapid tyrosine dephosphorylation of p27Kip1 accompanied by a change in its binding preferences to cdks. On G-CSF stimulation, p27Kip1 dissociated from cdk4 and associated with cdk2. Binding assays with recombinant p27Kip1 confirmed that tyrosine-phosphorylated p27Kip1 preferentially bound to cdk4, whereas unphosphorylated protein preferentially associated with cdk2. In addition, studies with p27Kip1 point mutations revealed a decisive role of Tyr88 and Tyr89 in binding to cdk4. Furthermore, phosphorylation of Tyr88 and Tyr89 was accompanied by strong nuclear translocation of p27Kip1. Taken together, this report provides the first evidence that tyrosine phosphorylation of p27Kip1 plays a crucial role in binding to cdks and its subcellular localization. Moreover, both effects are mediated by application of G-CSF.
Introduction
p27Kip1 is an important regulator of the cell cycle, acting as a potent inhibitor of cyclin E– and A–dependent kinase cdk2, and as positive regulator of cyclin D-dependent kinases like cdk4.1 However, recent data indicate that p27Kip1 is not required for the formation of active cyclin D-cdk4 complexes, yet the complexes are more stable when bound to p27Kip1.2 A major mechanism in the regulation of p27Kip1 is phosphorylation on threonine and serine residues, whereas tyrosine phosphorylation remains elusive. Phosphorylation of Thr157 causes accumulation of p27Kip1 in the cytoplasm and prevents G1 arrest.3-5 Thr187 and Ser10 are other phosphorylation sites essential for controlling its protein level.6-8 A decrease in p27Kip1 induces quiescent cells to proliferate, whereas an exit from the cell cycle is associated with up-regulation of p27Kip1.9 It has been shown that p27Kip1-deficient mice are predisposed to the development of spontaneous tumors, and p27Kip1 is considered a tumor suppressor.10,11 Accumulating evidence suggests another role for p27Kip1 in the regulation of cell migration independent of cyclin-cdk inhibition.12,13 With reference to our own investigations, 2 independent studies reported granulocyte colony-stimulating factor (G-CSF)–mediated up-regulation of p27Kip1.14,15 However, the role of p27Kip1 in G-CSF signaling remains unclear.
We demonstrate here for the first time a biologic role of tyrosine phosphorylation on p27Kip1. Using the human acute promyelocytic leukemia cell line NB4 as a model for studying cell cycle proteins in granulopoiesis, short-term treatment with G-CSF resulted in rapid tyrosine dephosphorylation of p27Kip1. In parallel, a switch in its binding preference to cyclin-dependent kinases was observed; a dramatic release from cdk4 coincided with a significantly increased binding to cdk2. Studies with p27Kip1 point mutations support a decisive role of Tyr88 and Tyr89 in p27Kip1-binding to cdk4. In addition, our data provide evidence that phosphorylation of Tyr88 and Tyr89 contributes to subcellular localization of p27Kip1.
Materials and methods
Expression constructs
Site-directed mutagenesis of human p27Kip1 cDNA was performed in the vector pCS2+-p27Kip1 (gift from Nisar Malek, Hannover, Germany) with the QuickChange site-directed mutagenesis kit (Stratagene, Heidelberg, Germany). For transient expression of p27Kip1 PolyFect Transfection Reagent (Qiagen, Hilden, Germany) was used. To express point-mutated p27Kip1 as GST fusion protein, cDNA fragments (SmaI and XhoI restricted) containing the point mutations were subcloned into SmaI and XhoI-restricted plasmid pGEX-5x-3-p27Kip1 wild type (wt; gift from Nisar Malek). GST fusion proteins were expressed in DH5α and TKX1 (Stratagene) Escherichia coli strains.
Antibodies
For precipitation, blotting, and immunostaining the following antibodies were used: polyclonal antibodies to cdk2 (06-505, Upstate Biotechnology, Hamburg, Germany), cdk4 (06-139, Upstate Biotechnology), cyclin D (sc-753, Santa Cruz Biotechnology, Heidelberg, Germany), Grb2 (sc-255, Santa Cruz Biotechnology), p21Cip1 (sc-397, Santa Cruz Biotechnology), p27Kip1 (sc-528, Santa Cruz Biotechnology, used generally with the exception of experiments shown in Figure 2C, Figure 3E, and Figure 4A), p85 (06-505, Upstate Biotechnology), PU.1 (sc-352, Santa Cruz Biotechnology), and monoclonal antibodies to Abl (8E9, BD Biosciences, Heidelberg, Germany), BrdU (347583, BD Biosciences), cyclin E (554182, BD Biosciences), G-CSF receptor (gift from M. Hadam, Hannover, Germany), Grb2 (G16729, BD Biosciences), p27Kip1 (610242, BD Biosciences, used in experiments shown in Figure 2C, Figure 3E, and Figure 4A), antiphosphotyrosine pY99 (sc-7020, Santa Cruz Biotechnology), and Src (05-184, Upstate Biotechnology).
Cell culture, stimulation, and preparation of cell lysates
The human acute promyelocytic leukemia cell line NB4 (DMSZ, Braunschweig, Germany, all other cells from American Type Culture Collection, Rockville, MD), the human myelomonocytic leukemia cell line U937, human promyelocytic leukemia cells HL60, and the human chronic myelogenous leukemia cell line K562 were cultured in RPMI 1640 supplemented with 10% FBS, 20 mM l-glutamine, 1000 U/L penicillin, and 100 mg/L streptomycin. Human embryo renal cortical cells HEK293 and mouse embryo fibroblast cells NIH/3T3 were cultured in DMEM with 10% FBS. For stimulation with G-CSF (50 ng/mL; Amgen, Thousand Oaks, CA) or treatment with vanadate (100 μM), 8 × 106 NB4 cells were seeded into culture flasks in 20 mL medium and were incubated for 24 hours at 37°C. At the time points indicated, stimulation was stopped by pouring the cell suspension into ice-cold PBS supplemented with 100 μM sodium orthovanadate (Na3VO4). Cell extracts were prepared by solubilization in RIPA buffer (20 mM Tris HCl, pH 7.5, 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) containing protease and phosphatase inhibitors (10 μg/mL aprotinin, 5 μg/mL phenylmethylsulfonyl fluoride, 4 μg/mL leupeptin, 10 μg/mL antipain, 4 μg/mL pepstatin, 50 mM NaF, and 2 mM Na3VO4).
Subcellular fractionation
NB4 cells were washed once with PBS and once with hypotonic lysis buffer (10 mM Tris HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 10 mM KCl, 2 mM MgCl2, 1 mM DTT). The cell pellet was resuspended in an equivalent volume hypotonic lysis buffer containing protease and phosphatase inhibitors, transferred into an ice-cooled Dounce homogenizer, incubated 20 minutes at 4°C, and homogenized by 15 strokes with the homogenizer. After centrifugation of the cell extract at 1600g, the supernatant fraction (cytosolic proteins) was separated from the pellet fraction (nuclear proteins), and the pellet fraction was lysed in RIPA buffer.
Immunoprecipitations and Western blotting
Immunoprecipitations (IPs) from 500 μg total cell lysate were performed by incubation in IP buffer (20 mM Tris HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 5% glycerol, 0.5% Tween 20) containing protease and phosphatase inhibitors, 2 μg rabbit polyclonal or mouse monoclonal antibody, and protein A-Sepharose or protein G-Sepharose overnight at 4°C. The pellets were washed 3 times with RIPA buffer, resuspended in sample buffer, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To dephosphorylate tyrosine residues of precipitated p27Kip1, the washed immunocomplexes were incubated with 10 U LAR protein tyrosine phosphatase (LAR-PTP; New England Biolabs, Frankfurt/Main, Germany) for 30 minutes at 30°C as described in the manufacturer's protocol. Antibodies used for precipitation and blotting are described in detail (see “Antibodies”). For redetection with another antibody the previous antibody was removed completely from the blot by incubation in blot-stripping buffer (60 mM Tris, pH 6.8, 2% SDS, 0.1% β-mercaptoethanol) for 20 minutes at 50°C while shaking gently.
Isoelectric focusing
For isoelectric focusing (IEF) of immunocomplexes, the precipitates were washed 3 times with 1% Triton X-100/Tris-buffered saline (20 mM Tris, pH 7.5, 150 mM NaCl), and lysed in 50 μL IEF lysis buffer (6 M urea, 2 M thiourea, 4% CHAPS, 2% DTT) for 20 minutes at 4°C. Thereafter, proteins from the lysate were purified with Micro Bio-Spin R6 (Bio-Rad, Munich, Germany) chromatography columns that had been buffered with rehydration solution (6 M urea, 2 M thiourea, 2% CHAPS, 0.4% DTT). The eluates were diluted with 250 μL rehydration solution, and the final volume was supplemented with 0.5% IPG buffer (pH 3-10 NL). Loading of the samples onto immobiline drystrip gels (pH 3-10 NL, 13 cm) and focusing in an IPGphor IEF system (Pharmacia, Freiburg, Germany) were performed following the manufacturer's instructions. Analysis of the separated proteins was accomplished on a Macintosh computer using the public domain National Institutes of Health Image software (http://rsb.info.nih.gov/nih-image/).
Phosphoamino acid analysis
NB4 cells (4 × 106/10 mL) were seeded into culture flasks (see “Cell culture, stimulation, and preparation of cell lysates”) and were incubated overnight. Thereafter, the cells were washed once with phosphate-free medium, preincubated in 5 mL phosphate-free medium (3% FCS, 20 mM l-glutamine, 1000 U/L penicillin, 100 mg/L streptomycin) for 1 hour, and labeled with 1 mCi (37 MBq)/mL [32P] phosphoric acid for 4 hours. Cell extracts and anti-p27Kip1 immunoprecipitations were prepared as depicted above. Phosphoamino acid analysis was performed as described previously.16
In vitro binding of p27Kip1
In vitro binding of p27Kip1 to cdk2 and cdk4 was carried out with 500 μg total cell lysate using 2 × lysis buffer (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 200 mM NaCl, 2% NP-40, 10% glycerol). The protein complexes were precipitated by incubation of 1 μg GST fusion protein with glutathione-Sepharose beads in binding buffer (25 mM Tris-HCl, pH 7.5, 1 mM DTT, 30 mM MgCl2, 40 mM NaCl, 0.5% NP-40, protease and phosphatase inhibitors as described) for 15 minutes at 4°C. Precipitates were washed 3 times with RIPA buffer, resuspended in sample buffer, and analyzed by Western blotting.
Immunofluorescence
At 48 hours after transient transfection, cells grown on glass coverslips were incubated with BrdU (10 μM; Sigma, Munich, Germany) for 4 hours. Thereafter, cells were rinsed in PBS and fixed in 4% paraformaldehyde at room temperature for 15 minutes. Coverslips were stored in PBS at 4°C until stained. Cells were permeabilized for 20 minutes in 0.2% Triton X100/PBS and rinsed 3 times in PBS. After the cells were blocked in antibody dilution buffer (3% BSA, 0.1% Tween 20/PBS) for 15 minutes, they were incubated for 1 hour with anti-p27Kip1 antibody (monoclonal, see “Antibodies”) at room temperature. The coverslips were rinsed 3 times in PBS and incubated with rhodamine-conjugated anti–mouse IgG (H+L) antibody (715-295-150, Jackson ImmunoResearch, Hamburg, Germany) for 30 minutes at room temperature. The coverslips were rinsed 3 times in PBS and the cells were treated with DNAse I (50 μg/mL in PBS) for 1 hour at 37°C. After washing 3 times in PBS the coverslips were incubated in antibody dilution buffer supplemented with anti–human IgG1 blocking antibody (1 mg/mL; gift from M. Hadam, Hannover, Germany) and anti-BrdU–FITC antibody for 1 hour at room temperature. The coverslips were rinsed 3 times in PBS and mounted on glass slides. Images were obtained on a Nikon Eclipse TE 300 microscope (Düsseldorf, Germany) with a × 20 objective using a Spot 2 camera (Diagnostic Instruments, Ismaning/Munich, Germany) and Metamorph 4.0 Software (Universal Imaging, Ismaning/Munich, Germany).
Hoechst-33342 dye staining
Morphologic changes of apoptotic cells were determined by fluorescence microscopy. Therefore, cells on coverslips immunostained with anti-p27Kip1 antibody (see “Immunofluorescence”) were rinsed 3 times in PBS, incubated in 0.5 mM Hoechst-33342 dye (Sigma) for 10 minutes at room temperature, and visualized under a fluorescence microscope (see “Immunofluorescence”). The cells with nuclei containing condensed chromatin or cells with fragmented nuclei were defined as apoptotic cells.
Results
p27Kip1 interacts with the G-CSF receptor via Grb2
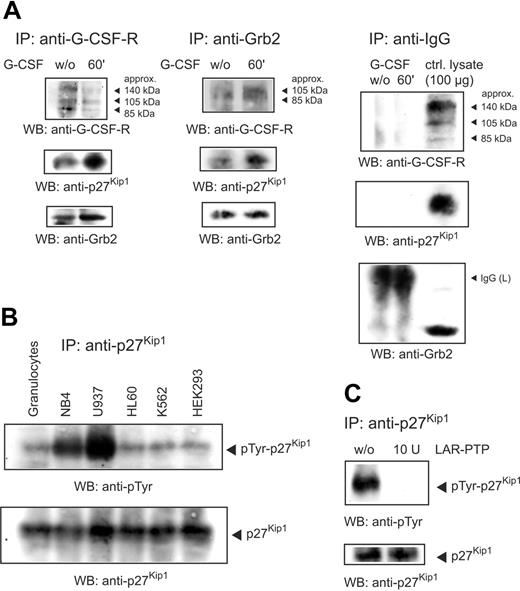
It has been reported that Grb2 docks to the G-CSF receptor17,18 and is associated with p27Kip1.19 To examine the role of p27Kip1 and Grb2 in G-CSF–mediated signaling we performed an anti–G-CSF receptor immunoprecipitation from total lysate of unstimulated and G-CSF–stimulated NB4 cells. Detection of the Western blot with antibodies to p27Kip1 (for control of specificity, see Figure S1A, available on the Blood website; see the Supplemental Figure link at the top of the online article) and Grb2 showed that both proteins were associated with the receptor (Figure 1A left panel). In this analysis 3 major G-CSF receptor isotypes (arrows) were detected, representing different posttranslational modifications. Furthermore, on stimulation with G-CSF for 60 minutes, stronger signals of p27Kip1 and Grb2, as compared to unstimulated cells, indicate an enhanced interaction of both proteins with the receptor. In addition, Grb2 immunoprecipitations confirm an interaction of p27Kip1 with the G-CSF receptor via Grb2 (Figure 1A middle panel). Again, on G-CSF stimulation stronger signals of p27Kip1 and the G-CSF receptor argue for an increased interaction of the proteins, as compared to unstimulated cells. To exclude in these immunoprecipitations the possibility of unspecific binding we performed as a negative control immunoprecipitations with an irrelevant anti–human IgG antibody. As expected, no unspecific binding to our investigated proteins was detected (Figure 1A, right panel).
p27Kip1 interacts with Grb2 and G-CSF receptor and is tyrosine-phosphorylated in NB4 cells. (A) Lysates of NB4 cells untreated or stimulated with G-CSF for 60 minutes were precipitated with antibody to the G-CSF receptor or Grb2 and probed as indicated. Control immunoprecipitations with an antibody to human IgG excluded the possibility of unspecific binding. (B) Lysates of different cell lines were precipitated with anti-p27Kip1 and the Western blot was probed as indicated. (C) Immunoprecipitated p27Kip1 from NB4 cells was incubated with 10 U LAR-PTP for 30 minutes at 30°C. Tyrosine phosphorylation of LAR-treated p27Kip1 was compared to untreated p27Kip1.
p27Kip1 interacts with Grb2 and G-CSF receptor and is tyrosine-phosphorylated in NB4 cells. (A) Lysates of NB4 cells untreated or stimulated with G-CSF for 60 minutes were precipitated with antibody to the G-CSF receptor or Grb2 and probed as indicated. Control immunoprecipitations with an antibody to human IgG excluded the possibility of unspecific binding. (B) Lysates of different cell lines were precipitated with anti-p27Kip1 and the Western blot was probed as indicated. (C) Immunoprecipitated p27Kip1 from NB4 cells was incubated with 10 U LAR-PTP for 30 minutes at 30°C. Tyrosine phosphorylation of LAR-treated p27Kip1 was compared to untreated p27Kip1.
p27Kip1 is tyrosine phosphorylated in NB4 cells
Until now, only phosphorylation of p27Kip1 on threonine and serine residues has been shown to play a physiologic role, whereas tyrosine phosphorylation remains elusive. To investigate tyrosine phosphorylation of p27Kip1 we first performed immunoprecipitations with p27Kip1 from the lysate of different cell lines. Detection of the corresponding Western blots using a monoclonal antibody to phosphotyrosine revealed strong tyrosine phosphorylation in the cell lines NB4 and U937 (Figure 1B). To verify tyrosine phosphorylation of p27Kip1 we incubated p27Kip1 immunoprecipitates with LAR-PTP and probed the Western blots with antiphosphotyrosine antibody. As expected, incubation with LAR-PTP completely removed tyrosine phosphorylation (Figure 1C). In addition, we performed an in vivo labeling of cellular p27Kip1 with [32P] phosphoric acid. Again, we observed a distinct tyrosine phosphorylation of p27Kip1 (Figure S1C). The intensities of phosphoserine, phosphothreonine, and phosphotyrosine approximately mirror the respective occurrence of the residues in the molecule.
G-CSF induces tyrosine dephosphorylation of p27Kip1
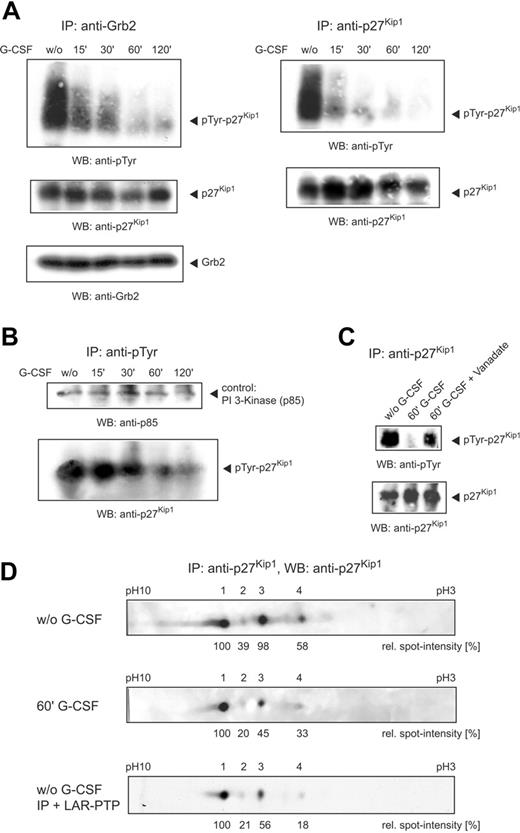
To examine the effect of G-CSF on p27Kip1 tyrosine phosphorylation in NB4 cells, we stimulated cells with G-CSF and harvested them at different time points. Grb2 and p27Kip1 immunoprecipitates performed from the lysate of unstimulated cells and probed with antiphosphotyrosine antibody showed a broad band in the molecular weight region of p27Kip1. To our surprise, however, on stimulation the phosphotyrosine signal rather declined from 15 minutes onward (Figure 2A).
To confirm the G-CSF–mediated tyrosine dephosphorylation of p27Kip1 we performed antiphosphotyrosine immunoprecipitations and probed them with anti-p27Kip1 antibody. The longer the cells were stimulated with G-CSF the less p27Kip1 was precipitable with the antibody (Figure 2B). As a p27Kip1-independent control the Western blot was detected with an antibody to the PI 3-kinase subunit p85, which is also implicated in G-CSF–mediated signaling.20 However, tyrosine phosphorylation of p85 remained unaffected by G-CSF stimulation in NB4 cells.
To further strengthen these findings, we investigated whether sodium orthovanadate, a potent inhibitor of phosphotyrosine-specific phosphatases, alters G-CSF–mediated tyrosine dephosphorylation of p27Kip1. Therefore, we stimulated NB4 cells in parallel with vanadate and G-CSF and performed p27Kip1 immunoprecipitations. As shown in Figure 2C, G-CSF–mediated tyrosine dephosphorylation of p27Kip1 was significantly reduced by vanadate.
The G-CSF–mediated phosphotyrosine shift was analyzed in more detail by IEF of p27Kip1 immunoprecipitates using a nonlinear gradient pH 3 to 10 with increased resolution between pH 5 to 7. IEF of the immunoprecipitate from the lysate of untreated NB4 cells resulted in 4 p27Kip1 spots (Figure 2C, top panel). Densitometric quantitation confirmed 2 equally dominant spots (spots 1 and 3). G-CSF treatment of the cells for 60 minutes resulted in most p27Kip1 migrating as spot 1. The intensity of the other forms, including spot 3, was significantly diminished (Figure 2C, middle panel). Due to a methodologic restriction of IEF, tyrosine phosphorylation could not be assessed on the blots. However, because an increase of the isoelectric point usually is accompanied by a decrease in phosphorylation, dephosphorylation of p27Kip1 after application of G-CSF must be considered the most likely explanation. Therefore, we incubated p27Kip1 immunoprecipitate from lysate of NB4 cells with LAR-PTP. Similar to G-CSF treatment, IEF of the phosphatase-treated immunoprecipitate resulted in most of p27Kip1 migrating as spot 1, whereas the intensity of the other 3 forms was significantly diminished (Figure 2C, bottom panel). Taken together, these data show that treatment of the promyelocytic cell line NB4 with G-CSF for 2 hours resulted in a strong decline of p27Kip1 tyrosine phosphorylation. In addition, IEF confirmed a significant switch in p27Kip1 modification induced by G-CSF.
Application of G-CSF induces tyrosine dephosphorylation of p27Kip1. (A) NB4 cells were stimulated with G-CSF and harvested at different time points. Grb2 and p27Kip1 immunoprecipitations were performed and detected with antibodies as indicated. (B) Lysates of stimulated NB4 cells were precipitated with antiphosphotyrosine antibody and detected with anti-p27Kip1 and anti–PI 3-kinase (p85) antibody. (C) NB4 cells were stimulated with G-CSF or G-CSF/vanadate for 60 minutes. p27Kip1 immunoprecipitations were performed and detected with antibodies as indicated. (D) p27Kip1 immunoprecipitate (incubated with or without 10 U LAR-PTP) from NB4 lysate of untreated cells or cells stimulated with G-CSF for 60 minutes was separated by IEF using a nonlinear gradient pH 3 to 10 and probed with anti-p27Kip1 antibody.
Application of G-CSF induces tyrosine dephosphorylation of p27Kip1. (A) NB4 cells were stimulated with G-CSF and harvested at different time points. Grb2 and p27Kip1 immunoprecipitations were performed and detected with antibodies as indicated. (B) Lysates of stimulated NB4 cells were precipitated with antiphosphotyrosine antibody and detected with anti-p27Kip1 and anti–PI 3-kinase (p85) antibody. (C) NB4 cells were stimulated with G-CSF or G-CSF/vanadate for 60 minutes. p27Kip1 immunoprecipitations were performed and detected with antibodies as indicated. (D) p27Kip1 immunoprecipitate (incubated with or without 10 U LAR-PTP) from NB4 lysate of untreated cells or cells stimulated with G-CSF for 60 minutes was separated by IEF using a nonlinear gradient pH 3 to 10 and probed with anti-p27Kip1 antibody.
Phosphorylation of p27Kip1 on tyrosine residues 88 and 89 modulates binding preference to cdks
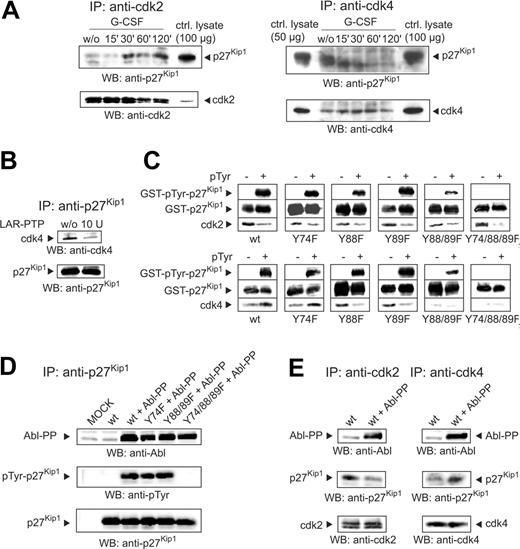
Considering the interaction of p27Kip1 with cyclin E-cdk2 and cyclin D-cdk4 protein complexes we examined the binding of p27Kip1 to cdk2 and cdk4 on G-CSF stimulation. Thus, we performed immunoprecipitations from the lysate of stimulated NB4 cells using anti-cdk2 and anti-cdk4 antibodies. Detection of the cdk2 immunoprecipitates with anti-p27Kip1 antibody demonstrated an elevated binding of p27Kip1 to cdk2, which could be visualized from 30 minutes after stimulation with G-CSF. In contrast, reduced binding to cdk4 was detectable as early as 15 minutes after stimulation (Figure 3A).
Tyrosine dephosphorylation of p27Kip1 alters the binding to cdks. (A) NB4 cells were stimulated with G-CSF and harvested at different time points. Immunoprecipitations with cdk2 and cdk4 were performed and probed by Western blotting with antibodies as indicated. A total of 50 or 100 μg NB4 lysate from untreated cells was also loaded as a control. (B) p27Kip1 immunoprecipitate from NB4 lysate was incubated with LAR-PTP. The Western blot was probed with antibodies to cdk4 and p27Kip1. Binding of cdk4 to p27Kip1 (treated with LAR-PTP or untreated) was detected. (C) GST-p27Kip1 fusion proteins (p27Kip1 wt and tyrosine to phenylalanine mutations Y74F, Y88F, Y89F, Y88/89F, Y74/88/89F) were expressed in E coli strains DH5α (pTyr -: not tyrosine-phosphorylated) and TKX1 (pTyr +: tyrosine-phosphorylated). After incubation of the fusion proteins in NB4 lysate together with GSH agarose for 15 minutes at 4°C, the protein complexes were separated by PAGE and blotted and binding of the p27Kip1 wt or point mutations with cdk2 and cdk4 was evaluated by detection with the appropriate antibodies. (D) Plasmids containing p27Kip1 wt sequence as well as point mutations (with or without Abl-PP sequence) were transiently expressed in NIH/3T3 cells. p27Kip1 immunoprecipitations were performed and probed with antibodies as indicated. As control for Abl-PP expression, a total of 100 μg lysate was also detected. (E) Lysate from transfected NIH/3T3 cells was immunoprecipitated and probed as indicated.
Tyrosine dephosphorylation of p27Kip1 alters the binding to cdks. (A) NB4 cells were stimulated with G-CSF and harvested at different time points. Immunoprecipitations with cdk2 and cdk4 were performed and probed by Western blotting with antibodies as indicated. A total of 50 or 100 μg NB4 lysate from untreated cells was also loaded as a control. (B) p27Kip1 immunoprecipitate from NB4 lysate was incubated with LAR-PTP. The Western blot was probed with antibodies to cdk4 and p27Kip1. Binding of cdk4 to p27Kip1 (treated with LAR-PTP or untreated) was detected. (C) GST-p27Kip1 fusion proteins (p27Kip1 wt and tyrosine to phenylalanine mutations Y74F, Y88F, Y89F, Y88/89F, Y74/88/89F) were expressed in E coli strains DH5α (pTyr -: not tyrosine-phosphorylated) and TKX1 (pTyr +: tyrosine-phosphorylated). After incubation of the fusion proteins in NB4 lysate together with GSH agarose for 15 minutes at 4°C, the protein complexes were separated by PAGE and blotted and binding of the p27Kip1 wt or point mutations with cdk2 and cdk4 was evaluated by detection with the appropriate antibodies. (D) Plasmids containing p27Kip1 wt sequence as well as point mutations (with or without Abl-PP sequence) were transiently expressed in NIH/3T3 cells. p27Kip1 immunoprecipitations were performed and probed with antibodies as indicated. As control for Abl-PP expression, a total of 100 μg lysate was also detected. (E) Lysate from transfected NIH/3T3 cells was immunoprecipitated and probed as indicated.
To verify that tyrosine phosphorylation of p27Kip1 plays a role in cellular signal transduction we tested binding of p27Kip1 to cdk4 by incubation of p27Kip1 immunoprecipitate with LAR-PTP and probed the Western blot with anti-cdk4. Immunoprecipitated p27Kip1 incubated with LAR-PTP was in weak association with cdk4, whereas untreated protein largely bound to cdk4 (Figure 3B). To confirm the binding preference and to determine the corresponding tyrosine phosphorylation sites, we investigated the in vitro binding of GST-p27Kip1 fusion proteins to cdk2 and cdk4. Hence, fusion protein was expressed in E coli strains DH5α and TKX1. TKX1 allows for specific tyrosine phosphorylation of the fusion protein by induction of a tyrosine kinase gene. Within p27Kip1, there are 3 tyrosine residues at positions 74, 88, and 89 (Tyr74, Tyr88 and Tyr89), whereas the GST part of the fusion protein contains no tyrosine residue. Pull-down assays with GST-p27Kip1 fusion proteins incubated in NB4 lysate show that unphosphorylated (pTyr-) wt p27Kip1 preferentially bound to cdk2 (Figure 3C, upper left panel). In contrast, phosphorylated (pTyr+) p27Kip1 wt preferentially associated with cdk4 (Figure 3C, bottom left panel). To determine the tyrosine residues responsible for the binding preference to cdk2 and cdk4, respectively, we performed assays with tyrosine to phenylalanine point mutations (Y74F, Y88F, Y89F, Y88/89F, and Y74/88/89F). Detection of Western blots demonstrated that binding to cdk2 was unaffected by any point mutation (Figure 3C, top panels). In all cases the unphosphorylated protein (pTyr-) displayed a stronger binding to cdk2 when compared to the corresponding phosphorylated (pTyr+) version. In contrast, interaction of GST-p27Kip1 with cdk4 was significantly influenced by mutations of tyrosine residues. Detection of Western blots with anti-cdk4 antibody (Figure 3C, bottom panels) showed that the Y74F mutation, compared to the wild-type protein, induced no change in binding to cdk4, whereas Y88F and Y89F mutations blocked preferential binding of tyrosine-phosphorylated (pTyr+) protein to cdk4. In contrast to wt and Y74F proteins unphosphorylated (pTyr-) Y88F and Y89F mutations displayed a stronger binding to cdk4. Most notably, double mutation of residues 88 and 89 (Y88/89F) resulted in a general blockade of binding to cdk4, illustrating that both residues are essential for binding. As a consequence, the triple mutant (Y74/88/89F) also displayed general failure of binding to cdk4.
Considering the interaction of p27Kip1 with cyclin E-cdk2 and cyclin D-cdk4 protein complexes, we investigated the in vitro binding of GST-p27Kip1 wt to cyclin E and cyclin D. However, neither unphosphorylated nor tyrosine-phosphorylated p27Kip1 showed interaction with cyclin E or cyclin D (Figure S1B).
To examine the binding preference of tyrosine-phosphorylated p27Kip1 to cdk2 and cdk4 in vivo we transiently expressed wt, Y74F, Y88/89F, and Y74/88/89F p27Kip1 in NIH/3T3 fibroblasts. To ensure tyrosine phosphorylation of transient p27Kip1 we cotransfected the cells with a plasmid containing the sequence of constitutive active c-Abl kinase (Abl-PP).21 To confirm Abl-PP–induced tyrosine phosphorylation of p27Kip1 we performed from lysate of transfected cells immunoprecipitates to p27Kip1 and detected the corresponding Western blot with antiphosphotyrosine antibody. As expected, with the exception of the mutant lacking all tyrosine residues (Y74/88/89F) cotransfection of Abl-PP resulted in tyrosine phosphorylation of p27Kip1, whereas in the absence of Abl-PP no tyrosine phosphorylation on wt p27Kip1 was detectable (Figure 3D). In cells not cotransfected with Abl-PP immunoprecipitated cdk2 was found predominantly in association with transient p27Kip1. In contrast, immunoprecipitated cdk4 preferentially bound to p27Kip1 phosphorylated by Abl-PP (Figure 3E). In summary, these results mirror the in vitro data presented and support the importance of p27Kip1 tyrosine phosphorylation.
Tyrosine phosphorylation of p27Kip1 contributes to nuclear translocation
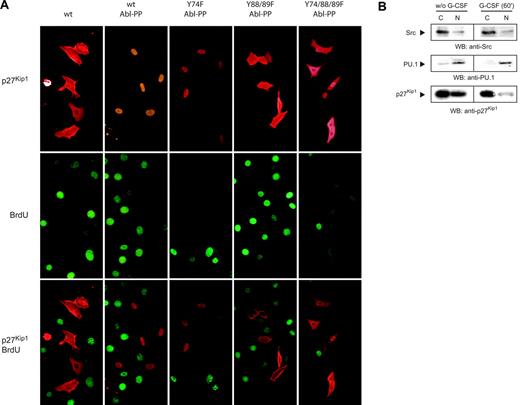
To investigate the biologic role of p27Kip1 tyrosine phosphorylation, we examined subcellular localization of transiently expressed p27Kip1 by immunostains with antibody to p27Kip1. Fluorescence microscopy showed equal distribution of unphosphorylated wt p27Kip1 in the cytoplasm and nucleus (Figure 4A, top left panel), whereas tyrosine phosphorylation of wt and Y74F p27Kip1 induced by Abl-PP cotransfection resulted in strong nuclear translocation. Y88/89F and Y74/88/89F p27Kip1 cotransfected with Abl-PP failed to translocate to the nucleus (Figure 4A, top panels). To investigate proliferation we incubated transfected cells with BrdU for 4 hours. In all transfection assays the overall number of BrdU+ cells and efficiency of p27Kip1 expression were comparable. Immunostaining with anti-BrdU antibody showed that p27Kip1-overexpressing cells did not incorporate BrdU. In contrast, cells with lack of transient p27Kip1 displayed BrdU incorporation (Figure 4A, middle and bottom panels).
To rule out apoptosis as an explanation for failed active cycling of transiently expressing cells, we stained nuclei with Hoechst-33342 dye. However, cells expressing transient protein did not significantly display morphologic features of apoptosis, such as cell shrinkage, chromatin condensation, and nuclear pyknosis (Table 1).
Detection of apoptotic NIH/3T3 cells by fluorescence microscopy
Transfected cells . | p27Kip1wt . | p27Kipl wt c-Abl-PP . | p27Kipl Y74F c-Abl-PP . | p27Kipl Y88/89F c-Abl-PP . | p27Kipl Y74/88/89F c-Abl-PP . |
|---|---|---|---|---|---|
| p27Kip1 positive | |||||
| Counted, no. | 216 | 238 | 168 | 206 | 222 |
| Apoptotic, no. | 4 | 4 | 4 | 6 | 6 |
| Apoptotic, % | 1.85 | 1.68 | 2.38 | 2.91 | 2.70 |
| p27Kip1 negative | |||||
| Counted, no. | 286 | 228 | ND | ND | ND |
| Apoptotic, no. | 6 | 4 | ND | ND | ND |
| Apoptotic, % | 2.09 | 1.75 | ND | ND | ND |
Transfected cells . | p27Kip1wt . | p27Kipl wt c-Abl-PP . | p27Kipl Y74F c-Abl-PP . | p27Kipl Y88/89F c-Abl-PP . | p27Kipl Y74/88/89F c-Abl-PP . |
|---|---|---|---|---|---|
| p27Kip1 positive | |||||
| Counted, no. | 216 | 238 | 168 | 206 | 222 |
| Apoptotic, no. | 4 | 4 | 4 | 6 | 6 |
| Apoptotic, % | 1.85 | 1.68 | 2.38 | 2.91 | 2.70 |
| p27Kip1 negative | |||||
| Counted, no. | 286 | 228 | ND | ND | ND |
| Apoptotic, no. | 6 | 4 | ND | ND | ND |
| Apoptotic, % | 2.09 | 1.75 | ND | ND | ND |
At least 2 independent transfection assays were scored.
p27Kip1 positive indicates transfected cells overexpressing p27Kip1; p27Kip1 negative, transfected cells without p27Kip1 overexpression; ND, not determined.
Taken together, these findings show that phosphorylation of both Tyr88 and Tyr89 modulates nuclear translocation. However, overexpression of p27Kip1 independently of tyrosine phosphorylation resulted in a general blockade of proliferation, whereas apoptosis was not affected.
To relate our data on NIH/3T3 fibroblasts to NB4 cells, we examined subcellular distribution of p27Kip1 in untreated and G-CSF–stimulated NB4 cells by cell fractionation. Western blotting showed (Figure 4B) that p27Kip1 was equally distributed between cytosolic fraction and nuclear fraction in untreated cells. On stimulation with G-CSF for 60 minutes, however, the p27Kip1 signal in the nuclear fraction strongly declined. Because our data document G-CSF–mediated tyrosine dephosphorylation of p27Kip1, nuclear translocation of tyrosine-phosphorylated protein must be considered the most likely explanation.
Discussion
In this study we investigated the cyclin-dependent kinase (cdk) inhibitor p27Kip1 in G-CSF receptor–mediated signaling using the acute promyelocytic leukemia cell line NB4. To examine the link between receptor and p27Kip1 in NB4, we analyzed the role of growth factor receptor-bound protein 2 (Grb2). It has been reported that Grb2 docks to the G-CSF receptor17,18 and is associated with p27Kip1.19 In this regard, our data suggest that p27Kip1 contributes to G-CSF–mediated signaling via interaction with Grb2.
A major mechanism in the regulation of p27Kip1 is phosphorylation on threonine and serine residues, whereas tyrosine phosphorylation remains elusive. Thus, we analyzed p27Kip1 tyrosine phosphorylation of different cell lines and detected strong phosphorylation in G-CSF receptor–expressing leukemic cell lines NB4 and U937. Furthermore, in both cell lines a rapid tyrosine dephosphorylation of p27Kip1 was observed on G-CSF stimulation (for U937 data not shown). The phosphotyrosine shift was analyzed in more detail by IEF of p27Kip1 immunoprecipitates. IEF of p27Kip1 from the lysate of untreated cells resulted in 4 spots. The intensity of 3 spots, representing differently modified p27Kip1, was significantly diminished on G-CSF stimulation. Comparable results of assays with tyrosine-specific LAR-PTP and G-CSF strongly suggest that G-CSF stimulation mediates tyrosine dephosphorylation of p27Kip1.In view of these data, it is believable that the phosphorylation status of p27Kip1 confers a different mobility. This could also explain the broad phosphotyrosine band of p27Kip1 detected in our Western blots.
Investigating the interaction of p27Kip1 with cdks, we found that tyrosine phosphorylation of p27Kip1 plays a crucial role in binding to cdk2 and cdk4. On G-CSF stimulation rapid tyrosine dephosphorylation of p27Kip1 was accompanied by a dramatic release from cdk4, coinciding with a significantly increased binding to cdk2.
Although our results are restricted to NB4 cells stimulated by G-CSF, similar observations may be envisaged when assaying other cell line/growth factor combinations. A comparable growth factor–mediated switch in binding of p27Kip1 to cyclin-dependent kinases has been reported earlier.22 The authors suggested a model in which TGF-β modulates p27Kip1 phosphorylation from its cyclin D1–bound assembly phospho form to an alternate form that binds tightly to inhibit cyclin E-cdk2. In their experiments, precipitated p27Kip1 was treated with intestinal alkaline phosphatase and characterized by 2-dimensional IEF, and after phosphatase treatment, most of p27Kip1 from the lysate of TGF-β–sensitive cells migrated at the highest IEF point, at pH 6.5. Likewise, in our experiments with G-CSF–treated NB4 cells, most p27Kip1 migrated as a spot with similar isoelectric point. However, by using intestinal alkaline phosphatase the authors were unable to provide information about the type of amino acid residue dephosphorylated upon TGF-β stimulation.22 In contrast, in our studies tyrosine-specific LAR-PTP was used, which allowed the demonstration of tyrosine-specific phosphorylation on p27Kip1. Furthermore, we demonstrated that immunoprecipitated p27Kip1 incubated with LAR phosphatase was in weak association with cdk4, whereas untreated protein largely bound to cdk4. This observation together with our studies on GST-p27Kip1 fusion proteins and transiently expressed p27Kip1 strengthen the suggestion that tyrosine phosphorylation plays a crucial role for binding to cdk4.
Tyrosine phosphorylation of p27Kip1 contributes to nuclear translocation. (A) NIH/3T3 cells were incubated with BrdU for 4 hours. p27Kip1 distribution and BrdU incorporation were detected with the appropriate antibodies. (B) NB4 cells (with or without stimulation with G-CSF for 60 minutes) were fractionated, lysate (100 μg) from cytosolic fraction C and nuclear fraction N was probed by Western blotting as indicated. Transcription factor PU.1 and Src kinase were used as markers for nuclear and cytosolic fraction, respectively.
Tyrosine phosphorylation of p27Kip1 contributes to nuclear translocation. (A) NIH/3T3 cells were incubated with BrdU for 4 hours. p27Kip1 distribution and BrdU incorporation were detected with the appropriate antibodies. (B) NB4 cells (with or without stimulation with G-CSF for 60 minutes) were fractionated, lysate (100 μg) from cytosolic fraction C and nuclear fraction N was probed by Western blotting as indicated. Transcription factor PU.1 and Src kinase were used as markers for nuclear and cytosolic fraction, respectively.
To determine the tyrosine residues responsible for the binding preferences to cdk2 and cdk4, respectively, we performed assays with tyrosine to phenylalanine point mutations. Y88/89F and Y74/88/89F mutations blocked preferential binding of p27Kip1 to cdk4. Furthermore, in contrast to p27Kip1 wt and Y74F proteins, unphosphorylated Y88F or Y89F mutation displayed a stronger binding to cdk4 than their tyrosine-phosphorylated version. This divergence could be explained by loss of increased binding of cdk4 to phosphorylated p27Kip1. However, this would require unequal exposure of the different binding assays, which is unlikely because of standardized assay procedures. It is tempting to speculate that mutations of 2 adjacent tyrosine residues reveal divergent binding properties due to unknown steric effects. In our opinion, the data on binding of Y88/89F and Y74/88/89F to cdk4 are not disputed by the divergent behavior of Y88F and Y89F mutants.
Based on these studies we suggest that tyrosine residues at position 88 and 89 play a decisive role for binding to cdk4, whereas association with cdk2 appears not to be influenced by mutation of any tyrosine residue. This may be consistent with recent kinetic data on p27Kip1 binding to the cdk2-cyclin A complex, which suggest a complex sequential mechanism regulated by segments of p27Kip1 that are highly conserved within the family of cyclin-dependent kinase inhibitors.23 However, kinetic experiments on binding to cdk4 were not performed in this study. We thus present here a novel binding mechanism of p27Kip1 to cdk4 via phosphorylation of p27Kip1 on Tyr88 and Tyr89.
Considering the regulation of cdk2 by cyclin E and cdk4 by cyclin D, we investigated the interaction of GST-p27Kip1 wt with cyclin E and cyclin D. Neither unphosphorylated nor tyrosine-phosphorylated p27Kip1 displayed interaction with cyclin E or cyclin D. In part, these findings did not surprise us because it is well documented that p27Kip1 acts as potent inhibitor of cyclin E–dependent kinase cdk2,1 resulting in a release of cyclin E from cdk2. According to our studies unphosphorylated protein binds preferentially to cdk2, whereas phosphorylated protein associates preferentially with cdk4. In this context, we expected interaction of tyrosine-phosphorylated GST-p27Kip1 wt with cyclin D because p27Kip1 is considered as positive regulator of cyclin D–dependent kinases like cdk4.1 However, failure of interaction with cyclin D in our experimental settings mirrors recent data indicating that p27Kip1 is not required for the formation of active cyclin D-cdk4 complexes.2
To investigate the biologic role of p27Kip1 tyrosine phosphorylation we examined subcellular localization of transiently expressed p27Kip1 in NIH/3T3 cells. To ensure tyrosine phosphorylation of transient p27Kip1 we cotransfected a plasmid containing the sequence of constitutive active c-Abl tyrosine kinase (Abl-PP). In this regard 2 arguments decided in favor of Abl-PP. First, c-Abl is generally located in the cytoplasm as well in the nucleus,24 a prerequisite for studying the role of p27Kip1 tyrosine phosphorylation on subcellular localization. Second, studies on the myelomonocytic leukemia cell line U937 attested interaction of p27Kip1 with c-Abl (data not shown). We assume that NB4 cells reflect this interaction.
Our findings indicate that phosphorylation of both Tyr88 and Tyr89 contributes to nuclear translocation. Because there are no data on interference of both tyrosine residues with the nuclear localization signal (amino acid residues 153-166) documented,25 it is tempting to speculate that phosphorylation of both tyrosine residues may interfere with nuclear export.
To relate the data on NIH/3T3 cells to untreated and G-CSF–stimulated NB4 cells we investigated subcellular distribution of p27Kip1 by cell fractionation and observed a strong decline of p27Kip1 in the nuclear fraction on G-CSF stimulation. Because our data document G-CSF–mediated tyrosine dephosphorylation of p27Kip1, nuclear translocation of tyrosine-phosphorylated protein must be considered the most likely explanation.
In parallel, we analyzed the influence of tyrosine point mutations on proliferation and observed independently of tyrosine phosphorylation a general blockade of proliferation of p27Kip1 overexpressing cells. We expected this finding only in cells expressing unphosphorylated wt p27Kip1. According to the data presented, we suggest that unphosphorylated p27Kip1 binds to cdk2 and, thus, inhibits proliferation. However, for cells expressing tyrosine-phosphorylated p27Kip1, it remains elusive whether nonphysiologic levels of transient overexpression may have overlapped possible nuances in proliferation that have been modulated by tyrosine phosphorylation. However, in regard to our data on NB4 cells we postulate a role for p27Kip1 tyrosine phosphorylation in promoting the transforming potential of leukemic blasts blocked in granulopoiesis. Thus, G-CSF–mediated tyrosine dephosphorylation of p27Kip1 may result in sequestration of cell cycle proteins in favor of cellular events like differentiation or migration. This could correlate with a recently published study indicating that differentiation of NB4 cells and other acute promyelocytic leukemia blasts from clinical samples treated with all-trans-retinoic acid (ATRA) was significantly enhanced by application of G-CSF.26 It is well known that the clinical potential of ATRA in differentiation of acute promyelocytic leukemia blasts is intensified by myeloid growth factors like G-CSF.27,28 However, the underlying molecular mechanisms induced by growth factors are unclear and will have to be investigated by further work.
Supported by grant SFB566 of the Deutsche Forschungsgemeinschaft (DFG). C.K. conceived, designed, and performed research and wrote paper; M.D. performed research and wrote paper; A. Kardinal performed research; A. Koch provided analytical tools; D.T.B. reviewed and discussed research; T.T. designed and discussed research; and K.W. was involved in the conception and discussion of the research.
The online version of the article contains a data supplement.
Prepublished online as Blood First Edition Paper, September 29, 2005; DOI 10.1182/blood-2005-05-1771.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal