Abstract
Combination chemotherapy with fludarabine plus cyclophosphamide (FC) was compared with the standard regimen of fludarabine monotherapy in first-line treatment of younger patients with chronic lymphocytic leukemia (CLL). Between 1999 and 2003, a total of 375 patients younger than 66 years who predominantly had advanced CLL were randomly assigned to receive either fludarabine (25 mg/m2 for 5 days intravenously, repeated every 28 days) or FC combination therapy (fludarabine 30 mg/m2 plus cyclophosphamide 250 mg/m2 for 3 days intravenously, repeated every 28 days). Both regimens were administered to a maximum of 6 courses. FC combination chemotherapy resulted in significantly higher complete remission rate (24%) and overall response rate (94%) compared with fludarabine alone (7% and 83%; P < .001 and P = .001). FC treatment also resulted in longer median progression-free survival (48 vs 20 months; P = .001) and longer treatment-free survival (37 vs 25 months; P < .001). Thus far, no difference in median overall survival has been observed. FC caused significantly more thrombocytopenia and leukocytopenia but did not increase the number of severe infections. In summary, first-line treatment with FC increases the response rates and the treatment-free interval in younger patients with advanced CLL.
Introduction
The purine analogues cladribine and fludarabine are the most potent cytotoxic drugs available for the treatment of B-cell chronic lymphocytic leukemia (CLL). Three phase 3 studies have shown that fludarabine is superior to chlorambucil and to a combination of cyclophosphamide, adriamycin, and prednisone (CAP) regarding the number of complete remissions and the duration of remission in first- and second-line treatment of CLL.1-3 However, none of these studies could demonstrate a benefit on median overall survival. Moreover, the relatively short progression-free survival time of 25 to 32 months after first-line treatment with fludarabine is still unsatisfactory, especially for younger patients.2,3
In vitro data showed that the exposure of CLL cells to fludarabine and cyclophosphamide resulted in an increased, synergistic cytotoxicity.4,5 DNA repair mechanisms in CLL cells, which are initiated in response to cyclophosphamide exposition, are inhibited by fludarabine.6 This observation was later translated into clinical trials. Results of phase 2 studies evaluating the combination of fludarabine plus cyclophosphamide (FC) showed promising efficacy, with response rates exceeding 90% in previously untreated and pretreated patients.7-11
In 1999 the German CLL Study Group (GCLLSG) initiated a prospective multicenter phase 3 trial, comparing fludarabine alone with the combination FC in first-line therapy of CLL patients younger than 66 years. Here we report results on the efficacy and safety of the 2 regimens.
Patients, materials, and methods
Criteria for eligibility
The diagnosis of CLL was based on the criteria established by the National Cancer Institute (NCI)-Sponsored Working Group in 1996.12 Disease stage was assessed according to the Binet and Rai classifications,13,14 and treatment requirement was assessed according to the NCI criteria.12 Patients were eligible in Binet stage C and stage B if they had rapid disease progression or symptoms as evidenced by enlarged lymph nodes and organs or if they had severe B symptoms. Patients in Binet stage A were included if they had B symptoms. All patients between 18 and 65 years of age without previous treatment of CLL were allowed to enter the trial. Patients had to have a life expectancy of more than 6 months and an Eastern Cooperative Oncology Group performance status of 0, 1, or 2. Patients were excluded if they had severe organ dysfunction, concomitant or previous neoplasms, or autoimmune hemolytic anemia or thrombocytopenia.
The protocol for this study was approved by the institutional review board of the University of Munich. All patients had signed an informed consent form before inclusion in the trial.
Randomization and treatment schedule
Randomization was performed by the Institute of Medical Statistics and Epidemiology, Technical University (Munich, Germany). Patients were randomly assigned to receive either fludarabine alone at a dose of 25 mg/m2, administered intravenously daily over 30 minutes for 5 days, or fludarabine dosed at 30 mg/m2, administered intravenously daily over 30 minutes for 3 days, plus cyclophosphamide dosed at 250 mg/m2, administered intravenously daily over 30 minutes for 3 days. Both regimens were repeated every 28 days; a maximum of 6 courses was administered in each arm. Routine antibiotic, antiviral prophylaxis, or growth factors were not administered.
Treatment was stopped if a life-threatening adverse effect occurred. Patients who had stable or progressive disease after 3 courses of treatment were stopped on treatment within this study. No crossover was planned in case of nonresponse.
Response assessment
After every course of therapy, patients were evaluated by clinical examination and blood count. After 3 and 6 courses of chemotherapy, response assessment by clinical examination, blood count, serum chemistry profile, ultrasound examination or computed tomography (CT), and bone marrow biopsy for confirmation of complete response was performed. Patients who still had signs of toxicity after 3 courses of treatment were reassessed 3 months later. During follow-up, response was assessed every 3 months by clinical examination, blood count, and, if clinically indicated, ultrasound examination.
Clinical response was defined according to the guidelines of the NCI-sponsored workshop.12 Complete remission was defined as normal findings on physical examination, disappearance of all symptoms and normal blood count, defined as lymphocyte count lower than 4 × 109/L, neutrophil count higher than 1.5 × 109/L, platelet count higher than 100 × 109/L, hemoglobin (untransfused) level higher than 110 g/L, and bone marrow lymphocyte percentage less than 30% on aspiration and biopsy. Moreover, we evaluated complete remission (CR) with imaging diagnostics (CR with imaging), which had to show negative findings for lymph node enlargement (enlargement was defined as greater than 1 cm12), splenomegaly (defined by greater than 12-cm diameter), or hepatomegaly (more than 1 cm below costal arch or greater than 14 cm medioclavicular). Unconfirmed complete remission (uCR) was defined as complete remission in a patient for whom a bone marrow biopsy was missing for confirmation of complete response.12 Partial remission was defined as 50% reduction of all measurable disease manifestations in physical and imaging examination and more than 50% improvement of all abnormal blood counts. Progressive disease was defined as 50% or greater enlargement of lymph nodes, splenomegaly, or hepatomegaly, appearance of new lymph nodes, more than 50% increase of circulating lymphocytes, or transformation to a more aggressive histology. Patients who did not fulfill the criteria for partial remission or progressive disease were classified as having stable disease.
Dose modifications
Toxic effects of treatment were evaluated according to the Common Toxicity Criteria (CTC 1.0). Any patient with a CTC grade 3 infection, a neutrophil count lower than 1 × 109/L, or thrombocytopenia between 20 × 109/L and 50 × 109/L with concurrent bleeding complications received a dose-reduced regimen to 75% for the next course. Any patient who had a neutrophil count lower than 0.5 × 109/L or thrombocytopenia lower than 20 × 109/L received a 50% dose reduction for the next treatment cycle.
Statistical analysis
The study was initiated in July 1999, and recruitment was completed in July 2003. A total of 375 patients were enrolled. Statistical analysis was performed on an intent-to-treat basis and included the eligible patients. The analysis presented here was based on the data collected by December 7, 2004.
Time to event was estimated using the Kaplan-Meier method, and treatment comparison was tested with the log-rank test. Overall survival was calculated from the randomization time point to death, and progression-free survival was calculated from randomization to the time of disease progression or death. Treatment-free survival was measured from the end of therapy to the time point of second-line treatment or death. Response rates were calculated for all patients with at least one cycle of therapy. Treatment arms were compared by the χ2 test. Myelotoxicity was also assessed according to the NCI-sponsored Workshop guidelines.12 All statistical tests were 2-sided. Statistical significance was defined as P below .05. Analysis was performed using SPSS version 12.0 (SPSS, Chicago, IL).
Results
Baseline characteristics of patients
Three hundred seventy-five patients were randomly assigned within this study. Thirteen patients had to be excluded because of violations of inclusion criteria (4 patients because of wrong diagnoses, 3 because of missing consent forms, and 6 because of concomitant disease). One hundred eighty-two patients were randomly assigned to receive fludarabine alone, and 180 were randomly assigned to receive FC. Eleven patients were lost to follow-up. Survival data were available in 351 patients, response data in 328 patients, and toxicity data in 346 patients.
Comparison of patients in the 2 treatment arms indicated no significant difference regarding the main clinical features and the risk categories (Table 1). A median number of 6 courses were administered in both treatment arms: 70.7% and 64% of the patients completed 6 courses in the fludarabine and the FC arm, respectively. Major reasons for earlier treatment withdrawal in 51 patients from the fludarabine arm were nonresponse (33%), autoimmune hemolysis (23%), and toxicity (14%), including infections, myelotoxicity, and skin reactions. FC therapy was stopped earlier in 63 patients because of toxicity (30%), partial or complete response (13%), or nonresponse (9%).
Baseline characteristics of eligible patients according to treatment
Characteristics . | Fludarabine . | FC . |
|---|---|---|
| No. patients | 182 | 180 |
| Age, y (range) | 59 (43-65) | 58 (42-64) |
| Age group, % | ||
| 30-39 | 3.8 | 2.8 |
| 40-49 | 12.6 | 16.7 |
| 50-59 | 39.6 | 41.1 |
| 60-65 | 44.0 | 39.4 |
| Binet stage, % | ||
| A | 11.2 | 7.4 |
| B | 53.6 | 57.6 |
| C | 35.2 | 35.0 |
| Rai stage, % | ||
| 0 | 2.4 | 3.1 |
| I or II | 56.6 | 58.2 |
| III or IV | 41.0 | 38.7 |
| Male sex, % | 70.4 | 75.0 |
| ECOG performance status, % | ||
| 0 | 52.6 | 53.9 |
| 1 | 45.0 | 42.5 |
| 2 | 2.4 | 3.6 |
| Leukocyte count, × 109/L (range) | 70.4 (9.1-270.0) | 65.8 (7.9-294.1) |
| Hemoglobin level, g/L (range) | 127 (83-159) | 132 (76-158) |
| Thrombocyte count, × 109/L (range) | 148 (58-281) | 145 (52-292) |
| Thymidine kinase, U/L (range) | 18.5 (4.5-80.8) | 18.4 (5.0-72.5) |
| β2-microglobulin, nmol/L (range) | 275 (129-568) | 275 (103-507) |
Characteristics . | Fludarabine . | FC . |
|---|---|---|
| No. patients | 182 | 180 |
| Age, y (range) | 59 (43-65) | 58 (42-64) |
| Age group, % | ||
| 30-39 | 3.8 | 2.8 |
| 40-49 | 12.6 | 16.7 |
| 50-59 | 39.6 | 41.1 |
| 60-65 | 44.0 | 39.4 |
| Binet stage, % | ||
| A | 11.2 | 7.4 |
| B | 53.6 | 57.6 |
| C | 35.2 | 35.0 |
| Rai stage, % | ||
| 0 | 2.4 | 3.1 |
| I or II | 56.6 | 58.2 |
| III or IV | 41.0 | 38.7 |
| Male sex, % | 70.4 | 75.0 |
| ECOG performance status, % | ||
| 0 | 52.6 | 53.9 |
| 1 | 45.0 | 42.5 |
| 2 | 2.4 | 3.6 |
| Leukocyte count, × 109/L (range) | 70.4 (9.1-270.0) | 65.8 (7.9-294.1) |
| Hemoglobin level, g/L (range) | 127 (83-159) | 132 (76-158) |
| Thrombocyte count, × 109/L (range) | 148 (58-281) | 145 (52-292) |
| Thymidine kinase, U/L (range) | 18.5 (4.5-80.8) | 18.4 (5.0-72.5) |
| β2-microglobulin, nmol/L (range) | 275 (129-568) | 275 (103-507) |
Median values are given for continuous variables (5th–95th percentiles). Percentages are given for categorical values. Rai stage 0 indicates low risk; I or II, intermediate risk; and III or IV, high risk. ECOG indicates Eastern Cooperative Oncology Group.
Response to treatment
Response data were available for 164 patients from each treatment arm. FC treatment induced more complete remissions (P < .001) and a higher rate of overall responses (P = .001) than did fludarabine treatment (Table 2). We also assessed complete response rates with imaging as defined by blood count, bone marrow biopsy, and negative imaging techniques. FC resulted in a complete remission rate with imaging of 16.5% compared with 4.9% with fludarabine alone (P = .001). When including the number of uCRs, the FC regimen yielded a uCR rate of 36% compared with 18.3% for fludarabine (P < .001). The superiority of the FC treatment was most prominent for Binet stage C patients, resulting in a 96.2% overall response rate with 13.2% complete remissions compared with 76.8% overall response with fludarabine and without any complete response (Table 2). Univariate analysis showed that sex, age, elevated serum thymidine kinase (s-TK) levels greater than 10 U/L, and elevated serum β2-microglobulin levels (cutoff level, 301 nmol/L) did not have any impact on response rates (data not shown).
NCI criteria–defined response rates according to treatment arm
. | Fludarabine . | FC . | P . |
|---|---|---|---|
| All patients, no. (%) | 164 (100.0) | 164 (100.0) | |
| CR | 11 (6.7) | 39 (23.8) | < .001 |
| CR with imaging | 8 (4.9) | 27 (16.5) | .001 |
| CR + uCR | 30 (18.3) | 58 (35.4) | < .001 |
| Partial response | 128 (78.0) | 128 (78.0) | >.999 |
| Overall response | 136 (82.9) | 155 (94.5) | .001 |
| Nonresponse | 28 (17.1) | 9 (5.5) | .001 |
| Binet stage, no. (%) | |||
| A | |||
| CR | 4 (21.1) | 6 (46.2) | .24 |
| Overall response | 17 (89.5) | 12 (92.3) | .79 |
| B | |||
| CR | 7 (7.9) | 25 (25.5) | .001 |
| Overall response | 76 (85.4) | 92 (93.9) | .05 |
| C | |||
| CR | 0 (0) | 8 (15.1) | .002 |
| Overall response | 43 (76.8) | 51 (96.2) | .003 |
. | Fludarabine . | FC . | P . |
|---|---|---|---|
| All patients, no. (%) | 164 (100.0) | 164 (100.0) | |
| CR | 11 (6.7) | 39 (23.8) | < .001 |
| CR with imaging | 8 (4.9) | 27 (16.5) | .001 |
| CR + uCR | 30 (18.3) | 58 (35.4) | < .001 |
| Partial response | 128 (78.0) | 128 (78.0) | >.999 |
| Overall response | 136 (82.9) | 155 (94.5) | .001 |
| Nonresponse | 28 (17.1) | 9 (5.5) | .001 |
| Binet stage, no. (%) | |||
| A | |||
| CR | 4 (21.1) | 6 (46.2) | .24 |
| Overall response | 17 (89.5) | 12 (92.3) | .79 |
| B | |||
| CR | 7 (7.9) | 25 (25.5) | .001 |
| Overall response | 76 (85.4) | 92 (93.9) | .05 |
| C | |||
| CR | 0 (0) | 8 (15.1) | .002 |
| Overall response | 43 (76.8) | 51 (96.2) | .003 |
CR was defined according to the NCI criteria as normal findings on physical examination, normal blood count, and less than 30% lymphocytes in bone marrow biopsy. CR with imaging was defined as CR and aspiration plus normal findings on CT or ultrasound examination. Finally, uCR was defined as CR, irrespective of the bone marrow biopsy result. Nonresponse was defined as stable disease or disease progression.
Overall survival
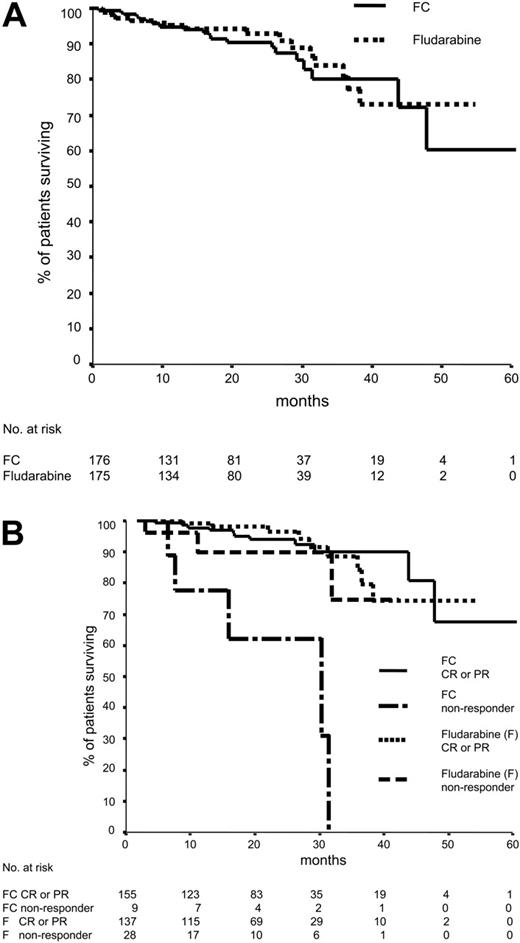
Median time of follow-up was 22 months. There was no significant difference in overall survival between the 2 treatment arms, with 3-year survival rates of 80.7% in the fludarabine arm and 80.3% in the FC arm (Figure 1A). Interestingly, there was also no difference in overall survival between the 2 treatment modalities within the different Binet stages or among the 3 Binet stages, indicating that the median observation time was too short to analyze overall survival.
Overall survival of patients who did not respond to FC chemotherapy was shorter (30 months) than for patients without response to fludarabine (median not reached) or responders to FC or fludarabine (Figure 1B) (P = .006). Interestingly, 5 of 8 patients with nonresponse to FC had a 17p deletion before treatment by fluorescence in situ hybridization (FISH). Six of 7 patients had unmutated immunoglobulin heavy-chain variable-region genes. In contrast, all patients who had achieved complete remission with imaging are alive.
Fifteen of 20 deaths in the FC arm were CLL associated compared with 9 of 17 deaths in the fludarabine arm (P = .51). Three patients in each treatment arm developed secondary aggressive lymphoma (overall incidence rate, 1.7%). Deaths caused by Richter syndrome were documented in 3 fludarabine-treated patients, and one FC-treated patient died. In total, 5 therapy-associated deaths occurred.
Overall survival. (A) Overall survival according to randomization. One hundred seventy-five patients assigned to fludarabine and 176 patients assigned to FC were evaluable for overall survival. Seventeen patients treated with fludarabine and 20 patients treated with FC died (P = .74). Median overall survival has not been reached in either arm. (B) Overall survival according to randomization and response. One hundred sixty-four patients assigned to fludarabine and 164 patients assigned to FC were evaluable for overall survival and response. Of the 28 patients without response to fludarabine therapy (stable disease or progressive disease), 3 have died. In the FC group, 9 patients had no response, and 5 of these patients died (P = .006). Median overall survival in the FC nonresponder group was 30 months; in the fludarabine arm, median overall survival has not yet been reached.
Overall survival. (A) Overall survival according to randomization. One hundred seventy-five patients assigned to fludarabine and 176 patients assigned to FC were evaluable for overall survival. Seventeen patients treated with fludarabine and 20 patients treated with FC died (P = .74). Median overall survival has not been reached in either arm. (B) Overall survival according to randomization and response. One hundred sixty-four patients assigned to fludarabine and 164 patients assigned to FC were evaluable for overall survival and response. Of the 28 patients without response to fludarabine therapy (stable disease or progressive disease), 3 have died. In the FC group, 9 patients had no response, and 5 of these patients died (P = .006). Median overall survival in the FC nonresponder group was 30 months; in the fludarabine arm, median overall survival has not yet been reached.
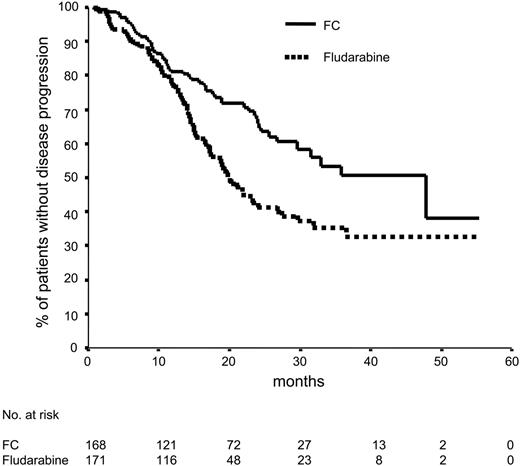
Progression-free survival according to randomization. Seventy-nine (46.2%) of 171 patients treated with fludarabine had progressive disease, according to the NCI criteria, compared with 53 (31.5%) of 168 patients treated with FC, in whom disease progressed. Median progression-free survival was 20 months in the monotherapy arm and 48 months in the combination therapy arm (P = .001).
Progression-free survival according to randomization. Seventy-nine (46.2%) of 171 patients treated with fludarabine had progressive disease, according to the NCI criteria, compared with 53 (31.5%) of 168 patients treated with FC, in whom disease progressed. Median progression-free survival was 20 months in the monotherapy arm and 48 months in the combination therapy arm (P = .001).
Progression-free survival and treatment-free survival
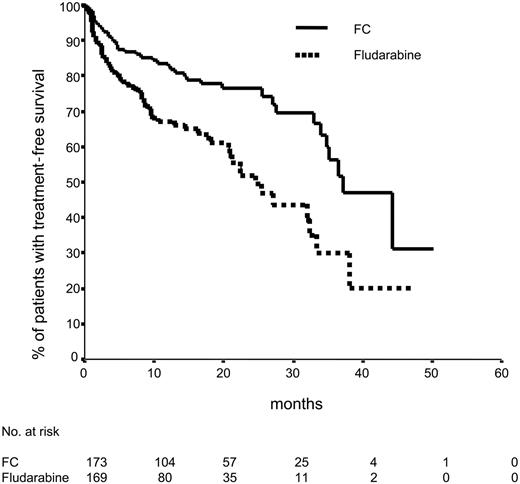
Median progression-free survival was significantly longer in the FC arm (48 months vs 20 months; P = .001) (Figure 2). FC treatment was also superior to fludarabine alone with regard to the progression-free survival in patients in Binet B stage (17 months vs 45 months; P = .001). However, for patients in Binet stages A and C, the progression-free survival in both arms was not different (76 patients with s-TK levels of 10 U/L or lower compared with 190 patients with s-TK levels greater than 10 U/L). Median progression-free survival was 27 months for patients with elevated s-TK levels and was not reached in patients with low s-TK levels (P = .09). No significant difference in progression-free survival was assessed in patients with elevated compared with low serum β2-microglobulin levels (cutoff level, 3.5 nmol/L). Progression-free survival was similar in patients 60 years or older compared with patients younger than 59 years and in male and female patients. FC treatment resulted in a significantly longer treatment-free survival time (37 months) compared with fludarabine (25 months) (Figure 3).
Treatment-free survival according to randomization. Sixty-four (37.6%) of 170 patients treated with fludarabine and 41 (23.4%) of 175 patients treated with FC received second-line treatment or died. Median treatment-free survival time was 25 months compared with 37 months (P < .001).
Treatment-free survival according to randomization. Sixty-four (37.6%) of 170 patients treated with fludarabine and 41 (23.4%) of 175 patients treated with FC received second-line treatment or died. Median treatment-free survival time was 25 months compared with 37 months (P < .001).
Response to second-line treatment
Thirty-two FC-treated and 59 fludarabine-treated patients received relapse treatment. Response data were available in 23 and 54 patients, respectively. The overall response rate to second-line treatment was 69.6% in the primarily FC-treated group and 73.6% in the fludarabine-treated group (P = .72).
Six of 9 patients without response to FC received relapse treatment immediately afterward. Four of them did not respond to relapse treatment, and data on response to second-line treatment were not available in the 2 remaining patients.
Toxicity
Data on toxicities were available from 173 patients in each treatment arm. Treatment-related mortality was recorded in 5 (1.4%) patients. Two patients died of treatment-related adverse effects in the FC arm (one of severe autoimmune hemolytic anemia and thrombocytopenia, one of tumor lysis syndrome), 3 in the fludarabine arm (one of pneumonia with sepsis, one of cerebral bleeding caused by thrombocytopenia, and one of autoimmune hemolytic anemia). Myelotoxicity was the major adverse effect in both arms. Both mild (CTC grades 1 and 2) and severe (CTC grades 3 and 4) myelotoxicity and, in particular, leukocytopenia were significantly more frequent in the FC arm (Table 3). When evaluating treatment-related anemia and thrombocytopenia according to the NCI criteria, severe thrombocytopenia (grades 3 and 4, indicating greater than 50% decrease of platelet counts) was also significantly more frequent in the FC arm (Table 3). The rate of severe infections and the incidence of opportunistic infections were similar in both treatment arms. In each treatment arm, one case of fungal pneumonia occurred; in the FC arm, one case of gastrointestinal tuberculosis was observed. Furthermore, gastrointestinal side effects such as nausea, vomiting, mucositis, and gastritis were more common in the FC arm.
Patients with at least one grade 3 or 4 adverse effect classified by CTC criteria and NCI criteria according to randomization group
. | Fludarabine . | FC . | P . |
|---|---|---|---|
| No. patients | 173 | 173 | |
| CTC grades 3 and 4, % | |||
| All grades 3 and 4 toxicities | 54.0 | 72.6 | .001 |
| Myelotoxicity | 39.3 | 64.2 | .001 |
| Leukocytopenia | 26.0 | 55.5 | < .001 |
| Anemia | 11.6 | 8.1 | .28 |
| AIHA | 3.8 | 2.2 | .37 |
| Thrombocytopenia | 12.7 | 15.6 | .44 |
| Infection | 8.7 | 8.7 | >.999 |
| Gastrointestinal adverse effects | 1.7 | 5.8 | .05 |
| NCI grades 3 and 4, % | |||
| Anemia | 5.2 | 2.4 | .17 |
| Thrombocytopenia | 23.3 | 34.9 | .02 |
. | Fludarabine . | FC . | P . |
|---|---|---|---|
| No. patients | 173 | 173 | |
| CTC grades 3 and 4, % | |||
| All grades 3 and 4 toxicities | 54.0 | 72.6 | .001 |
| Myelotoxicity | 39.3 | 64.2 | .001 |
| Leukocytopenia | 26.0 | 55.5 | < .001 |
| Anemia | 11.6 | 8.1 | .28 |
| AIHA | 3.8 | 2.2 | .37 |
| Thrombocytopenia | 12.7 | 15.6 | .44 |
| Infection | 8.7 | 8.7 | >.999 |
| Gastrointestinal adverse effects | 1.7 | 5.8 | .05 |
| NCI grades 3 and 4, % | |||
| Anemia | 5.2 | 2.4 | .17 |
| Thrombocytopenia | 23.3 | 34.9 | .02 |
Autoimmune hemolytic anemia (AIHA) tended to develop more frequently in the fludarabine arm, but the difference was not statistically significant (7.7% vs 2.8%; P = .06). There was no significant difference in AIHA of CTC grades 3 and 4. At study entry, the Coombs test was positive in 7% compared with 6.8% of patients in the fludarabine and the FC arm, respectively. Thirty-one percent of patients received immunosuppressive agents to treat their AIHA, and 37% required no specific treatment. There was no correlation between initially positive Coombs test findings and subsequent occurrence of AIHA; during treatment with fludarabine or FC, AIHA developed in 2 (11%) of 19 patients who were initially Coombs positive and in 13 (5%) of 262 who were initially Coombs negative.
Discussion
The results of this multicenter phase 3 trial showed that combination therapy consisting of fludarabine plus cyclophosphamide is more efficacious than fludarabine alone in the first-line therapy of CLL. FC treatment improved the overall response rate, the complete remission rate, the progression-free survival time, and the treatment-free survival time.
Preliminary results of an Intergroup trial comparing FC with fludarabine alone showed similar results on the superiority of FC.15 However, the overall response rates in this Intergroup trial were lower than in our study, perhaps because of a higher proportion of elderly patients or the inclusion of a higher proportion of patients at high risk.15
It is noteworthy that in this study the rate of true complete remissions was lower in both treatment arms than in some studies published previously. Complete remission rates of 20% to 40% were reported for fludarabine used as first-line therapy in 2 phase 3 trials.2,3 In our study, the complete remission rate was only 7% in the fludarabine arm. Similarly, higher complete remission rates of 35% to 49% were reported for FC if used as first-line therapy7,9,11 compared with a rate of 24% in our study. One explanation for this difference is that patients with more advanced Binet stage C disease were included in our study (40% vs 33%) compared with the study of the French Cooperative Group on CLL.2 In addition, the French Cooperative Group trial did not use bone marrow biopsy to confirm a complete response.2 Similar results regarding the response rates with fludarabine or FC were observed by the recently presented Intergroup trial.15 These differences in response rates between the phase 2 and the phase 3 trials underscore the need for phase 3 trials and of a systematic evaluation of complete and partial remissions.
We also assessed the complete remission rate, including imaging procedures such as computed tomography (CT) and ultrasound examination, which were performed during the final staging in all patients in this study. Complete remission rates were significantly lower when imaging procedures were used (16% vs 24% and 5% vs 7%), especially with the FC treatment. In the 1996 NCI response criteria, the role of imaging diagnostics was not clearly defined. These criteria state that the “absence of lymphadenopathy by physical examination and appropriate radiographic techniques” is needed for confirmation of complete remission.12 Our results support the need for a revision of these criteria with regard to modern imaging procedures by showing that response rates vary considerably when using CT or ultrasound imaging.
Patients with Binet stage C disease benefited more from the FC regimen than from fludarabine monotherapy. For patients in Binet stage C, no complete remission was achieved by fludarabine treatment alone. This is in agreement with findings of the Intergroup trial, which showed a lower complete response rate for fludarabine treatment of 14% in CLL patients at high risk compared with 25% for those at intermediate risk.3
A 17p deletion, which predicts poor prognosis and resistance to treatment in CLL, was observed in 5 of 9 patients who did not respond to FC.16-18 In the future, alternative treatment strategies are needed (alemtuzumab, allogeneic stem cell transplantation). Cytogenetic analysis of all patients of the CLL4 protocol will show whether response to therapy can be determined by fluorescence in situ hybridization (FISH).
FC treatment resulted in a progression-free survival time of 48 months. Similar results on progression-free survival have been reported.11,15 The difference in time to progression between the 2 treatment arms was 28 months. It remains to be seen how the 2-year progression-free survival rate of 67% obtained with the FC regimen compares with combinations consisting of fludarabine with the antibody rituximab, for which similar progression-free survival rates have been reported.19
The fact that progression-free survival time after FC and fludarabine therapy were not different for patients in Binet stage A can be explained by the small size of the patient group, which reduced statistical power. The lack of difference in progression-free survival in patients with Binet stage C disease might be explained by the short observation period because only 48 (42%) of 115 patients in Binet stage C have had progressive disease or have died thus far.
No difference between fludarabine alone and FC therapy has been observed with regard to overall survival. The median observation time of 22 months was too short to validate a survival difference between fludarabine alone and FC treatment. Similarly, none of the previously published phase 3 trials was able to show a significant survival advantage for any of the first-line therapies.2,3 Because the recurring nature of indolent lymphomas routinely requires subsequent therapies that modify the clinical course of the disease, an overall survival benefit by a more effective first-line treatment strategy is often difficult to prove.
Relapse treatment options in CLL patients range from chlorambucil to allogeneic stem cell transplantation. Inconsistency in relapse strategies is one of the explanations for the lack of survival benefit after FC therapy. Therefore, if possible, future trials should contain specific advice for second- and third-line treatment strategies for patients with CLL. Moreover, and more important, a longer median observation time is needed to determine a survival benefit for any first-line regimen.
A third reason for the missing survival benefit in the FC arm might be an impaired response to second- or third-line treatment. No difference in overall response to second-line therapy was observed between the 2 treatment arms in this trial, but we cannot exclude at the present time that FC treatment selects for more resistant cell clones. In this study, no blood samples for cytogenetic aberration were obtained during follow-up. Therefore, one can only speculate whether FC induces additional genetic aberrations in CLL.
An overall incidence rate of 1.7% for secondary aggressive lymphomas was observed in this trial. Previously published studies reported incidence rates of 3% to 12% for Richter transformation.20-22 Treatment with fludarabine is thought to have a major impact on an increased incidence of transformations, up to 12%, in CLL because of its immunosuppressive effects.22 The rate of Richter transformation in our trial was relatively low, probably related to the short observation period. However, no difference in the rate of secondary aggressive lymphomas was observed between the 2 treatment arms.
The FC combination caused more adverse effects, particularly myelotoxicity. In spite of the higher rate of severe leukocytopenia in the FC arm, the incidence of severe infections was similar in both treatment arms. A possible explanation is that the FC dose was more frequently reduced or delayed than the fludarabine dose. Compared with other studies,9,11 the incidence of infections in patients treated with FC within this trial was relatively low. However, a lower dose of cyclophosphamide was used in this trial, and the patients included in this trial were younger than in previous phase 2 trials using FC.11,15
Another important adverse effect of fludarabine is the occurrence of autoimmune cytopenias that are sometimes severe or even lethal. There was a trend for a higher incidence of AIHA in general in the fludarabine arm that might have been related to the lower dose of fludarabine within the FC regimen or to some protective effects of cyclophosphamide. Similar results have been reported by the United Kingdom CLL study group.23
In summary, the FC combination yields significantly higher response rates and longer progression-free survival and treatment-free survival than therapy with fludarabine alone. With regard to progression-free survival and treatment-free survival, this phase 3 study shows the largest difference between 2 treatment regimens that has ever been reported in CLL. FC therapy can be given with acceptable adverse effects. The GCLLSG will, therefore, use FC as standard first-line therapy in CLL patients who are physically fit. It remains to be seen whether this regimen allows prolonged overall survival time of patients with advanced CLL.
Appendix
Investigators of the German CLL Study Group are as follows (cities, institutions, and names of participating physicians appear in alphabetical order): Ansbach: S. Müller. Aschaffenburg: M. Weslau. Arnstadt: C. Müller. Augsburg: Zentralklinikum: J. Dani, P. Seufert. Aurich: Kreiskrankenhaus: W. Langer. Bad Hersfeld: Kreiskrankenhaus, Majunke. Bad Mergentheim: Caritas Krankenhaus: E. Hartung. Bad Saarow-Pieskow: Humaine-Klinikum: P. Frenzel, H. Fuss. Bad Salzuflen: R. Weinert. Bayreuth: A. Hübner. Bergisch Gladbach: H. Culmann. Berlin: Clinic of the Humboldt University Charité Mitte: J. Eucker, O. Sezer; Evangelisches Waldkrankenhaus Spandau: J. Potenberg; Robert-Rössle-Klinik: B. Dörken; University Hospital Benjamin Franklin: A. Krackhardt; University Hopsital Charité, Campus Virchow-Clinic: S. Srock; A. Dietzmann; A. Kirsch; A. Koschuth; H. Seibt-Jung; F. Strohbach, K. Zuchold. Bietigheim-Bissingen: Krankenhaus: G. Dietrich. Bremen: Evangelische Diakonissenanstalt: C. Diekmann. Chemnitz: M. Grundeis. Cottbus: U. von Grünhagen. Dresden: S. Dörfel. Düsseldorf: Krankenhaus Düsseldorf-Benrath: B. Günther. Eisenach: St Georg Klinikum: B. Ismer. Emden: Hans-Susemihl-Krankenhaus: H. Becker. Erfurt: H. Franke; U. Hauch; J. Weniger; Helios-Klinikum: K. Härtwig, M. Herold. Erlangen: M. Eckart. Eschweiler: St Antonius Hospital: S. Schäfer. Essen: Evangelisches Krankenhaus Essen-Werden: W. Heit; University Hospital: U. Dührsen, J. Dürig, H. Müller-Beisenhirtz; R. Rudolph. Esslingen: Städtische Kliniken: R. Eckert. Forchheim: R. Thiemann. Frankfurt Oder: Klinikum: W. Stein. Freiburg: Reiber. Garmisch-Partenkirchen: Kreiskrankenhaus: H. Lambertz. Gerlingen: H.-R. Schmitt. Germering: J. Mittermüller. Giessen: G. Schliesser. Göppingen: Klinik A. M. Eichert: B. Maier-Bay. Göttingen: University Hospital: C. Binder. Greifswald: Ernst-Moritz-Arndt University Hospital: G. Dölken, B. Kallinich, M. Wimmer; B. Meyer. Güstrow: H. Eschenburg. Hagen: Katholisches Krankenhaus: W. Lindemann. Halle: H.-J. Hurtz, A. Oppenhorst, R. Rohrberg, M. Schmidt; C. Spohn. Hamburg: Allgemeines Krankenhaus: B. Seyfarth; University Hospital Eppendorf: M. Brügmann, M. de Wit, G. Schuch. Hamm: Evangelisches Krankenhaus: Fouth, E. Lange, C. Voss; St Marienhospital: H. Dürck. Hannover: B. Gaede. Heilbronn: Städtisches Klinikum: K.-H. Koniczek; P. Porowski. Herrsching: H. Dietzfelbinger. Hildesheim: W. Freier. Hövelhof: M. Schneider. Holzminden: Evangelisches Krankenhaus: F. Burghardt. Homburg Saar: University Hospital Saarland: J. Marell, N. Murawski, H. Schmitt. Idar-Oberstein: Städtisches Krankenhaus: A. Fauser, D. Linck. Ingolstadt: P. Maubach. Jena: S. Hahnfeld. Kaiserslautern: Westpfalzklinikum: J. Kirsch, H. Link; R. Hansen. Karlsruhe: Städtisches Klinikum: M. Haag, M. Schmier; St Vicentius Krankenhaus: G. Göckel, J. Metzger. Kassel: U. Söling. Kempten: Klinikum Kepmpten Oberallgäu: O. Prümmer. Kiel: University Hospital: M. Kneba, S. Matutat, M. Ritgen. Köln: University Hospital: V. Diehl, T. Elter, G. Fingerle-Rowson, R. Schnell, H. Schulz; T. Kim. Kronach: M. Stauch. Landshut: U. Vehling-Kaiser. Leer: Kreiskrankenhaus: G. Köchling; L. Müller. Lemgo: Klinikum Lippe-Lemgo: H. Middeke. Ludwigsburg: Ulshöfer. Magdeburg: Städtisches Klinikum Krankenhaus Altstadt: E. Kettner; Otto-von-Guericke University Hospital: A. Franke, C. Maas, S. Mewes, K. Wieker, M. Wiermann. Mannheim: University Hospital: O. Maywald, M. Schatz. Mönchengladbach: U. Grabenhorst. München: Krankenhaus München-Schwabing: D. Adorf; University Hospital Grosshadern: R. Forstpointner, A. Golf, E. Hiller, H.-J. Kolb, N. Lang; University Hospital Rechts der Isar: G. Fischer, I. Ringshausen, F. Schneller; W. Abenhardt; M. Kimmich. Münster: University Hospital: M. Kropff. Muhr A. M. See: B. Göttler. Neunkirchen: P. Schmidt. Norderstedt: R. Hoffmann. Nordhausen: Südharz Krankenhaus: K. Dachselt. Nürnberg: Klinikum Nord: C. Falge, A. Lechner, K. Schäfer-Eckart, G. Schmidt. Offenbach: H. Balló. Oldenburg: B. Ottembra, D. Reschke. Ostfildern: Paracelsus-Krankenhaus: U. Abele. Pasewalk: Asklepios Klinik: T. Ehlert. Plauen: Vogtlandklinikum: V. Schirmer. Potsdam: Klinikum Ernst von Bergmann: K. Akrivakis, A. Gerhardt, F. Rothmann; A. Sauer. Regensburg: Krankenhaus Barmherzige Brüder: E.-D. Kreuser. Rosenheim: Klinikum: C. Hempfling. Rostock: University Hospital: B. Krammer-Steiner, A. Steffen, S. Wilhelm; V. Lackner. Saarbrücken: Caritas-Klinik St. Theresia-Rastpfuhl: A. Rotter; G. Jacobs, J. Schimke. Schwerin: Klinikum: D. Hähling. Siegen: St Marien-Krankenhaus: T. Gaska. Stuttgard: Diakonissenkrankenhaus: K. Kaesberger; Katharinenhospital: S. Krauss; Marienhospital: B. Schmid; Robert-Bosch-Krankenhaus: W. Aulitzky; H.Fiechtner; E. Höring, M. Respondek. Trier: Krankenanstalt Mutterhaus der Borromäerinnen: B. Meuter, C. Peter; B. Rendenbach. Tübingen: University Hospital: O. Aichele, D. Benz; M. Haen. Ulm: University Hospital: J. Greiner, A. Kröber, E. Leupolt. Vechta: St. Marienhospital: J. Diers. Weilheim: Perker. Wendlingen: T. Kamp. Worms: O. Burkhard, B. Reimann. Würzburg: University Hospital: M.-E. Goebler, R. Grossmann, H. Rückle-Lanz; R. Schlag. Wuppertal: Klinikum St Antonius: M. Sandmann. Zittau: M. Schulze.
Prepublished online as Blood First Edition Paper, October 11, 2005; DOI 10.1182/blood-2005-06-2395.
A complete list of the members of the German CLL Study Group (GCLLSG) appears in the “Appendix.”
Supported by the Deutsche Krebshilfe (grant 70-2353), Bonn; the Fresenius Foundation (grant CLL4VH), Bad Homburg; the Sander Foundation (grant 2003.086.1), Munich; and Medac Schering Onkologie, Munich, Germany.
B.F.E. supervised and monitored the trial, conducted the analysis, and wrote the report. R.B. conducted the analysis of the trial. G.H., R.P., M. Hensel, C. Steinbrecher, S. Siehl, U.J., M.B., and C.W. were responsible for patient accrual and for monitoring and managing the clinical data at their referring center. S. Stilgenbauer and H.D. were responsible for the genetic analysis. C. Schweighofer monitored the study. G.B. and B.E. contributed to the design of the study. M. Hallek designed and supervised the trial and wrote the report. All authors critically contributed to the final preparation of the article.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank A. Westermann, K. Posse, K. Leiwig, and D. Glogger of the study office, and we thank K. Klein and C. Ilgen for statistical collection and analyses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal