Natural killer (NK) cells are generally reported as innate effector cells for killing virally infected and transformed cells. It is unclear how NK cells evoke adaptive immunity to eradicate tumors. We now demonstrate that the TNF superfamily member, LIGHT, known as TNFSF14 and a T-cell costimulatory molecule, is a critical ligand for the activation of NK cells. Herpesvirus entry mediator (HVEM) is expressed on NK cells, and its engagement with LIGHT mediates NK-cell activation. The expression of LIGHT inside tumors leads to rapid rejection in a NK-dependent manner. Both NK and CD8+ cells are essential but not sufficient for the rejection of tumors because mice lacking either population fail to reject the tumor. Interestingly, activated NK cells do not kill tumors directly but can facilitate the priming of tumor-specific CD8+ T cells in an IFN-γ–dependent manner. Conversely, intratumor depletion of either NK cells or IFN-γ during tumor progression disrupts CD8+ cell–mediated tumor rejection, suggesting that the tumor is the essential site for the crosstalk between NK and CD8+ cells. Furthermore, IFNG-deficient NK cells fail to effectively activate CD8+ T cells, suggesting IFN-γ plays an important role in NK-mediated activation of cytotoxic T lymphocytes (CTLs). Our findings establish a direct role for LIGHT in NK activation/expansion and a critical helper role of activated NK cells in priming CD8+ T cells and breaking T-cell tolerance at the tumor site.
Introduction
Natural killer (NK) cells are commonly defined as the effectors of the innate immune system, acting as the first line of defense against viral infection and tumors. Direct killing and cytokine production are the 2 major effector responses of NK cells. Target recognition of NK cells is guided by 2 types of NK-cell receptors, inhibitory and activating receptors.1-3 NK cells express inhibitory receptors specific for MHC class I molecules on target cells. As a result of direct ligand interaction, these inhibitory receptors prevent NK cells from killing, thus providing the molecular basis for the “missing-self” hypothesis. In mice, the inhibitory receptors include Ly49 family receptors, Ly49A and CD94/NKG2A.4-6 NK cells also express activating receptors that recognize target-cell ligands and elicit perforin-dependent killing. Many of these receptors, such as Ly49D and Ly49H, are structurally related to the inhibitory receptors, but their signaling activities are limited by the inhibitory receptors.7 Stimulation of NK cells through the activating receptors can result in secretion of cytokines such as IFN-γ, GM-CSF, TNF-α, and chemokines as CCL4, CCL5, and CCL23.8,9 Recent studies suggest that NK cells may play roles in regulating adaptive immunity because of their ability to produce such cytokines and chemokines. However, the relative contribution and significance of these 2 functions, killing targets or immune regulation, to the overall immune response against viral infection and cancers has been difficult to evaluate. Recent reports from NK-depletion experiments showed that NK cells can be critical for tumor immunity.10-12 They suggest that NK cells modulate adaptive immune responses by their interaction with dendritic cells (DCs), which leads to cellular activation, maturation, and even death.8,13,14 DCs can directly trigger NK-cell activation to kill viruses, intracellular bacteria, and tumors.15,16 Previous studies have shown MHC class I–low tumor cells can rapidly trigger NK-cell activation in vivo. These activated NK cells evoke DCs to produce IL-12 and to induce protective CD8+ T-cell responses against tumors.17 Although NK cells are capable of trafficking to draining lymph nodes (DLNs) to prime Th-1 responses,18 their exact role in the activation of CD8+ cells in the inflammatory site or tumor is unclear. Moreover, where and how the interaction between the NK and CD8+ T cells occurs in inflamed or tumor tissues have not been well demonstrated.
LIGHT is a TNF superfamily ligand, known as TNFSF14, which modulates T-cell immune responses by signaling through herpes virus entry mediator (HVEM) and lymphotoxin β receptor (LTβR).19,20 It is predominantly expressed on lymphoid tissues and on the surface of immature DCs and activated T cells.21,22 Interaction of LIGHT with LTβR on stromal cells results in the up-regulation of lymphoid chemokines and adhesion molecules, leading to the increased presence of lymphocytes in peripheral tissues. Up-regulation of LIGHT can cause severe inflammation in nonlymphoid tissues or forced expression of LIGHT inside tumor tissues promote lymphocyte infiltrates and the eradication of highly established tumors in mice.23-26 Interestingly, a recent study showed that LIGHT is constitutively expressed in some human melanoma cells and tumor-derived microvesicles, and tumors that expressed LIGHT were associated with increased lymphocytic infiltration.27 However, the role of NK cells in these models has not been defined. Here, we demonstrate that LIGHT can also serve as a critical ligand for the activation of NK cells. Forced expression of LIGHT inside tumor tissues can lead to rapid rejection of tumor in a NK-dependent fashion. The activated NK cells can directly elicit tumor-specific cytotoxic T lymphocyte (CTL) proliferation and maturation in situ for the rejection of a well-established tumor. Conversely, intratumor depletion of NK cells abrogates the protective CTL response in killing their targets. These findings raise the importance of intratumorally activated NK cells which may effectively cause CTLs to break tolerance for the eradication of the tumor and may provide new guidance for cancer immunotherapy via the employment of NK helper function in the induction of adaptive immunity and delivery of LIGHT to change tumor environment for better immune responses.
Materials and methods
Mice and cell lines
Female C3B6F1 mice, age 5 to 8 weeks, were purchased from the Frederick Cancer Research Facility of the US National Cancer Institute (Frederick, MD). C57BL/6-Rag1-/- (Rag1-/-) mice and Ifng-/- B6 mice were from Jackson Laboratory (Bar Harbor, ME). Hvem-/- B6 mice were generated as described.28 2C T-cell receptor (TCR)–transgenic mice on Rag1-/--B6 background (2C mice) were a gift from J. Chen (MIT, Cambridge, MA). Animal care and experiments were performed in accordance with the institution and NIH guidelines and approved by the animal use committee at the University of Chicago. Ag104, Ag104 Ld, Ag104LIGHT, and Ag104 Ld LIGHT cell lines were established as described.26 YAC-1 cell line was from V. Kumar at the University of Chicago.
Antibodies and reagents
Purified antibodies that reacted with mouse CD16/32 (2.4G2), mouse NK1.1 (pk136), mouse IFNγ (XMG1.2),29 mouse CD8,2.34 mouse HVEM were all from hybridoma. Control rat-IgG, APC–anti-CD8, PE–anti-CD8, PE–anti-NK1.1, APC–anti-NK1.1, biotinylated DX5, anti–mouse Ly49G2, anti–mouse NKG2D, enzyme-linked immunosorbent assay (ELISA) assay kit for IFNγ and GM-CSF were all from BD PharMingen (San Diego, CA). Anti–mouse perforin antibody (Kamiya Biomedical, Seattle, WA), rabbit asialo-GM1 (Wako Chemicals, Richmond, VA) were purchased. Human IL-2 was from NIH; rIL-15 and rIFNγ were from R&D Systems (Minneapolis, MN). NK and CD8+ cell-isolation kits were from Miltenyi Biotec (Auburn, CA). [3H] thymidine (3H-TdR) and 51Cr were from Amersham (Aylesbury, United Kingdom).
Single-cell isolation from tumor tissues
Tumor tissues were collected from tumor-bearing mice, washed in PBS, and cut into pieces and suspended in 5% FCS DMEM with 1.5% collagenase D for 30-minute incubation at 37°C. Cell suspension was harvested after 30 minutes; the remaining tissue clumps were digested for another 30 minutes with collagenase D solution until all of the tumor tissues had resolved into single-cell suspension.
NK and CD8+ cell isolation
For NK-cell isolation, splenocytes or lymph nodes from Rag1-/- or Hvem-/- mice were negatively selected for NK cells with NK isolation kit via the manufacturer's instruction. Greater than 95% of the enriched NK cells expressed pan NK marker DX5 analyzed by fluorescence-activated cell sorting (FACS). CD8+ cells were negatively separated through CD8+ isolation kit according to the manufacturer's instructions. As to isolation from tumor tissues, CD8+ T cells were further separated to get rid of adherent tumor cells via overnight incubation at 37°C. The purity of CD8+ cells is greater than 90% by FACS analysis.
Assay for proliferation and cytokine
Flat-bottom 96-well plates were coated with 1 μg/mL anti-NK1.1 mAb or different doses of rLIGHT overnight at 4°C. NK cells (1 × 105) were added to each well supplemented with 20 IU/mL rIL-2. Alternatively, NK cells were cocultured with 4 × 104 irradiated Ag104 Ld LIGHT tumor cells (3500 Gy) for stimulation. Supernatants were collected after 48 hours, and IFNγ or GM-CSF was detected by ELISA following the manufacturer's instructions. NK-cell proliferation was assessed by the addition of 1 μCi (0.037 MBq) 3H-TdR per well for the last 15 hours of the 3-day culture. 3H-TdR incorporation was measured with Topcount microplate scintillation counter (Packard–Perkin Elmer, Boston, MA).
Direct interaction assay between NK and CD8+ T cells
NK cells were preactivated with rLIGHT, IL-15, or anti-NK1.1 mAb; washed; and added to the culture of 2C T and irradiated Ag104Ld tumor cells (3500 Gy) at days 0, 3, and 5 for 7-day incubation. 2C T-cell proliferation was assayed by adding 1 μCi (0.037 MBq) 3H-TdR per well during the last 15 hours of the 7-day culture as described for NK cells. The cytotoxicity was determined in a standard 4-hour 51Cr-release assay.
Cytotoxicity assay
Cytolytic activity of NK cells and CTLs was assessed against 51Cr-labeled target cells in a standard 4-hour 51Cr-release assay.12 NK cells were cocultured with 51Cr-labeled YAC-1 cells in a 96-well V-bottom plate for 4 hours at various effector-target (E/T) ratios. CTLs were coincubated with 51Cr -labeled Ag104Ld cells with different E/T ratios. Spontaneous release of 51Cr was analyzed by incubating the target cells with medium alone. Maximum release was determined by adding 1% NP-40 to a final concentration of 0.5%. The percentage of specific lysis was calculated as follows: 100 × [(experimental release - spontaneous release)/(maximum release - spontaneous release)]. Each experiment was done at least twice with triplicate samples.
Flow cytometry
Tumor cell lines Ag104 LdLIGHT and Ag104 Ld were confirmed by FACS analysis of their expression molecules as described.26 For detection of HVEM expression, isolated NK cells from Rag1-/- or Hvem-/- mice were stained with PE–anti-NK1.1 mAb and biotinylated anti-HVEM mAb followed by APC-streptavidin. NK and CD8+ cells from DLNs, splenocytes or single tumor cells were stained with PE–anti-NK1.1 and APC–anti-CD8 mAbs. For intracellular perforin staining, surface marker CD8 was first stained, then fixed with 2% paraformaldehyde and permeabilized with 0.5% saponin. Perforin was stained with rat anti–mouse perforin antibody followed by biotinylated goat anti–rat IgG and PE-streptavidin. NKG2D staining was as for CD8 and perforin, but without fixation and permeabilization. Samples were examined on FACScan, and data were analyzed with FlowJo software (Treestar, Ashland, OR).
Depletion of NK or CD8+ cells and blockade of LIGHT or IFNγ in vivo
Mice were depleted of lymphocyte subsets as described.12 Mice were treated with antibody on days -3, 0, and 7 or on days 14, 18, and 22 (Day 0 is the day of primary or secondary tumor injection). For intratumor depletion of NK cells or blockade of IFNγ, antibody was injected inside tumor at days 9, 12, and 15 after tumor inoculation. The following doses of antibodies were used: anti-asialo GM1 antibody (intraperitoneally, 50 μg/time; intratumor injection, 20 μg/time), anti-CD8 mAb (intraperitoneally, 100 μg/time), anti-IFNγ mAb (intraperitoneally, 200 μg/time; intratumor injection, 50 μg/time). These protocols effectively depleted NK or CD8+ cell subsets examined with FACS. LIGHT was blocked with LTβR-Ig through intraperitoneal injection at days 0, 3, and 7 during tumor inoculation (100 μg per injection).
Histology and immunohistochemical staining
Tumor tissues and spleens for histology examination were collected at the indicated times, embedded in OCT compound, and frozen in -70°C. Frozen sections were fixed and stained with anti–mouse Ly49G2, anti–mouse CD8, or together. Biotin-conjugated secondary antibody was used for development by DAB system.26
Results
NK cells are required for LIGHT-mediated tumor rejection
The essential role of NK cells against tumors is difficult to demonstrate because most tumors grow progressively. Therefore, regressive tumors are required to study whether and how NK cells reject tumors. A very low dose (1 × 104) of Ag104 fibrosarcoma, even with strong antigen Ld (Ag104 Ld), can grow aggressively in C3H or C3B6F1 mice.26 However, introducing LIGHT into tumors allows massive recruitment of various immune cells, resulting in tumor rejection, which provides us with an interesting model to study whether and how NK or T cells play a role in tumor rejection. To test whether NK cells play a role in LIGHT-mediated tumor rejection, C3B6F1 mice were treated with anti-asialo GM1 antibody on days -3, 0, and 7 of tumor implantation to systemically deplete NK cells. NK cell–depletion led to 100% outgrowth of Ag104 Ld LIGHT tumors similarly to when CD8+ T cells were depleted (Figure 1A; Table 1). Similar results were observed when mice were treated with anti-NK1.1 mAb (pk136) (data not shown). These data suggest that NK cells, like CD8+ cells, are essential for LIGHT-mediated tumor rejection.
NK-cell depletion abolishes LIGHT-mediated tumor rejection
Primary challenge, mice, secondary challenge, and treatment* . | Tumor incidence, no./no. mice total(%)† . |
|---|---|
| 5 × 106 Ag104LdLIGHT | |
| C3B6F1 mice | |
| No secondary challenge | |
| None | 0/15 (0) |
| NK depletion (early)‡ | 6/6 (100) |
| CD8 depletion (early) | 6/6 (100) |
| LTβR-Ig (early) | 5/5 (100) |
| Anti-IFNγ (early) | 6/6 (100) |
| Control Ig (early) | 0/21 (0) |
| NK depletion (late)§ | 0/5 (0) |
| Anti-IFNγ (late) | 0/6 (0) |
| Control Ig (late) | 0/6 (0) |
| NK depletion (intratumor) | 6/6 (100) |
| Anti-IFNγ (intratumor) | 5/5 (100) |
| Control Ig (intratumor) | 0/5 (0) |
| Secondary challenge, 1 × 107 Ag104Ld | |
| NK depletion | 5/5 (100) |
| CD8 depletion | 5/5 (100) |
| Anti-IFNγ | 6/6 (100) |
| Control Ig | 0/6 (0) |
| 1 × 106 Ag104LdLIGHT | |
| Rag1 KO mice, no secondary challenge or treatment | 9/9 (100) |
| C3B6F1 mice, no secondary challenge or treatment | 0/9 (0) |
Primary challenge, mice, secondary challenge, and treatment* . | Tumor incidence, no./no. mice total(%)† . |
|---|---|
| 5 × 106 Ag104LdLIGHT | |
| C3B6F1 mice | |
| No secondary challenge | |
| None | 0/15 (0) |
| NK depletion (early)‡ | 6/6 (100) |
| CD8 depletion (early) | 6/6 (100) |
| LTβR-Ig (early) | 5/5 (100) |
| Anti-IFNγ (early) | 6/6 (100) |
| Control Ig (early) | 0/21 (0) |
| NK depletion (late)§ | 0/5 (0) |
| Anti-IFNγ (late) | 0/6 (0) |
| Control Ig (late) | 0/6 (0) |
| NK depletion (intratumor) | 6/6 (100) |
| Anti-IFNγ (intratumor) | 5/5 (100) |
| Control Ig (intratumor) | 0/5 (0) |
| Secondary challenge, 1 × 107 Ag104Ld | |
| NK depletion | 5/5 (100) |
| CD8 depletion | 5/5 (100) |
| Anti-IFNγ | 6/6 (100) |
| Control Ig | 0/6 (0) |
| 1 × 106 Ag104LdLIGHT | |
| Rag1 KO mice, no secondary challenge or treatment | 9/9 (100) |
| C3B6F1 mice, no secondary challenge or treatment | 0/9 (0) |
Number of tumor cells as indicated were injected subcutaneously into mice
The results were pooled from independent experiments
Treatment was done by administration of reagents intraperitoneally on days –3, 0, and 7 (day 0 is the day of primary or secondary tumor injection)
Treatment was done by administration of reagents intraperitoneally on days 14, 18, and 22
We next tried to determine whether NK cells alone are sufficient for mediating tumor rejection. Rag1-/- mice deficient of T, B, and NKT cells were subcutaneously inoculated with 1 × 106 Ag104Ld LIGHT tumor cells. Tumors grew readily in Rag1-/- mice, whereas all tumors were rejected in the wild-type (WT) mice after 22 days (Table 1). These findings suggest that NK cells alone are not sufficient to eradicate tumors, and their effect may be dependent on the presence of CD8+ cells. To determine at which stage NK cells play a major role in LIGHT-mediated tumor rejection, we systemically depleted NK cells on days 14, 18, and 22 after tumor inoculation. We found that Ag104 Ld LIGHT tumors were rejected after 22 days in the mice treated with control Ig, whereas tumors in the mice depleted of NK cells at the earlier time points grew aggressively (Table 1). In contrast to early NK-cell depletion which permits tumor growth, late depletion of NK cells fails to affect the tumor rejection. This raises the possibility that the NK cells play an early role to initiate CTL-mediated responses, but this role is diminished after activation of CD8+ cells.
Whether NK cells are required during memory responses has not been demonstrated. To determine the necessity of NK cells in protective immunity, C3B6F1 mice that previously rejected Ag104 Ld LIGHT cells were rechallenged with Ag104 Ld in the opposite flanks and then depleted of either NK or CD8+ cells. In all of the mice that were lacking either NK or CD8+ cells, tumors grew progressively (Figure 1B). In contrast, mice treated with control Ig were tumor free. These findings suggest that NK cells, the innate immune cells, are required not only for primary responses but also for enhancing memory immunity against rechallenge. Therefore, this tumor model allows us to address where, when, and how NK cells play an essential role in tumor rejection and whether there is a link between NK and CD8-mediated tumor rejection.
LIGHT-mediated tumor rejection is NK-cell dependent. (A) NK-cell depletion before tumor formation leads to Ag104Ld LIGHT tumor growth. C3B6F1 mice were subcutaneously inoculated with Ag104Ld LIGHT tumor cells through intraperitoneal treatment by anti-asialo GM1 antibody at days -3, 0, and 7 for NK-cell depletion. CD8+ cells were depleted with anti-CD8 antibody. Tumor growth was monitored every other day, and data are the mean ± SD of 5 mice for each group. (B) NK-cell depletion results in outgrowth of Ag104 Ld tumor at the secondary tumor challenge. The C3B6F1 mice were subcutaneously inoculated with 1 × 107 Ag104 Ld tumor cells after 40 days of primary Ag104 Ld LIGHT tumor rejection, followed by NK-cell depletion. One of 3 independent experiments and a total of 15 mice per group are presented.
LIGHT-mediated tumor rejection is NK-cell dependent. (A) NK-cell depletion before tumor formation leads to Ag104Ld LIGHT tumor growth. C3B6F1 mice were subcutaneously inoculated with Ag104Ld LIGHT tumor cells through intraperitoneal treatment by anti-asialo GM1 antibody at days -3, 0, and 7 for NK-cell depletion. CD8+ cells were depleted with anti-CD8 antibody. Tumor growth was monitored every other day, and data are the mean ± SD of 5 mice for each group. (B) NK-cell depletion results in outgrowth of Ag104 Ld tumor at the secondary tumor challenge. The C3B6F1 mice were subcutaneously inoculated with 1 × 107 Ag104 Ld tumor cells after 40 days of primary Ag104 Ld LIGHT tumor rejection, followed by NK-cell depletion. One of 3 independent experiments and a total of 15 mice per group are presented.
NK cells dramatically increase in LIGHT-mediated tumor microenvironment
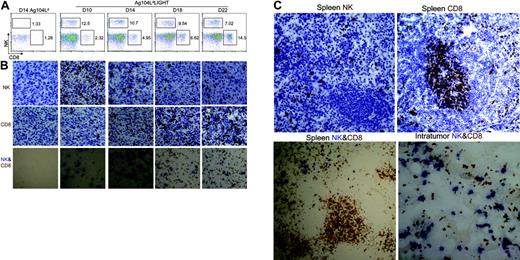
A recent report showed that an increase of NK cells in the draining LNs after injection of mature DCs can enhance priming of CD4+ T cells.18 We sought to determine whether NK-cell accumulation can also be observed in lymphoid organs during tumor growth and regression. NK cells were detected by FACS analysis at days 10, 14, 18, and 22 in the DLNs, non-DLNs, spleen, and tumor tissues from Ag104 Ld LIGHT or Ag104 Ld tumor-bearing mice. The number of NK cells in the DLNs, non-DLNs, or spleens was low and not significantly different at all time points in the mice bearing either Ag104 Ld LIGHT or Ag104 Ld tumors (data not shown). Interestingly, we found nearly a 10-fold increase in NK-cell (but not NKT cells) accumulation inside the Ag104 Ld LIGHT tumor tissue at the early stage of tumor formation compared with those in the LIGHT-negative Ag104 Ld tumor (Figure 2A). During tumor regression, the percentage of infiltrating NK cells peaked at 10 days after inoculation and then decreased, followed by a subsequent increase of CD8+ T cells in the tumor microenvironment. The kinetic ratios of CD8+ T cells over NK cells during LIGHT-mediated tumor regression were increased from 0.18 to 2.1 over 12 days(day 10, 0.18; day 14, 0.46; day 18, 0.69; and day 22, 2.1). In the absence of LIGHT, both NK and CD8+ T cells inside Ag104 Ld tumors were barely detectable at all time points during the course of tumor growth (Figure 2A). To observe the localization of intratumor NK cells, tumor histology was examined. Within the tumor, the dynamic changes of NK and CD8+ T cells during the process of tumor rejection in the presence or absence of LIGHT were similar to the FACS results shown above (Figure 2B). However, our histologic analysis revealed the NK cells were distributed in close proximity with the CD8+ T cells inside Ag104 LdLIGHT tumor tissues, whereas these 2 types of cells were segregated into separate zones in the spleen (Figure 2C). The NK cells in these tumor-bearing mice were predominately located in the splenic red pulps, whereas the CD8+ T cells were in the T-cell zones (Figure C). This was comparable to the ones bearing Ag104 Ld tumor or naive mice (data not shown). These findings suggest that an increase of NK cells inside the LIGHT-positive tumors during early stages of tumor progression might promote the activation of tumor-specific CD8+ T cells inside the tumors.
NK cells dramatically increase inside Ag104 Ld LIGHT tumor. (A) Intratumor NK cells dramatically increased at an early stage of tumor growth, CD8+ T cells increased at a late stage. Tumor tissues from Ag104 Ld- or Ag104Ld LIGHT-bearing mice were collected at days 10, 14, 18, and 22. Single cells were obtained by collagenase D digestion and stained with anti–NK1.1-conjugated PE and anti–CD8-conjugated APC. The lymphocyte area was gated for analysis. Data are representative of at least 3 independent experiments. (B) Dynamic change of increased infiltration of NK and CD8+ cells inside the tumor by immunohistochemical staining. Frozen sections of the indicated tumor tissues were stained with NK-specific receptor marker anti-Ly49G2, anti-CD8, or together. Results shown represent 1 of 3 independent experiments. (C) NK cells distribute closely to CD8+ T cells inside Ag104Ld LIGHT tumor tissues. NK and CD8+ T cells separate at different zones in spleens of Ag104Ld LIGHT tumor. Data shown represent 1 of 3 independent experiments. Images were acquired through an Olympus BX41 microscope equipped with a 20 ×/0.50 objective lens and a 10 ×/0.22 eyepiece (Olympus America, Melville, NY), for a total original magnification of × 200. An Axiocam camera and Axiovision 3.0 software (Carl Zeiss Microimaging, Thornwood, NY) were used to capture and process images.
NK cells dramatically increase inside Ag104 Ld LIGHT tumor. (A) Intratumor NK cells dramatically increased at an early stage of tumor growth, CD8+ T cells increased at a late stage. Tumor tissues from Ag104 Ld- or Ag104Ld LIGHT-bearing mice were collected at days 10, 14, 18, and 22. Single cells were obtained by collagenase D digestion and stained with anti–NK1.1-conjugated PE and anti–CD8-conjugated APC. The lymphocyte area was gated for analysis. Data are representative of at least 3 independent experiments. (B) Dynamic change of increased infiltration of NK and CD8+ cells inside the tumor by immunohistochemical staining. Frozen sections of the indicated tumor tissues were stained with NK-specific receptor marker anti-Ly49G2, anti-CD8, or together. Results shown represent 1 of 3 independent experiments. (C) NK cells distribute closely to CD8+ T cells inside Ag104Ld LIGHT tumor tissues. NK and CD8+ T cells separate at different zones in spleens of Ag104Ld LIGHT tumor. Data shown represent 1 of 3 independent experiments. Images were acquired through an Olympus BX41 microscope equipped with a 20 ×/0.50 objective lens and a 10 ×/0.22 eyepiece (Olympus America, Melville, NY), for a total original magnification of × 200. An Axiocam camera and Axiovision 3.0 software (Carl Zeiss Microimaging, Thornwood, NY) were used to capture and process images.
LIGHT stimulates proliferation and activation of NK cells
LIGHT has not been reported to directly stimulate NK cells. To examine a potential direct role of LIGHT expressed in Ag104 Ld tumor on NK cells, we cocultured NK cells with irradiated Ag104 Ld LIGHT tumor cells for 3 days. NK cells showed significantly increased proliferation and IFN-γ secretion in the presence of LIGHT-expressing tumor cells compared with NK cells alone or tumor cells alone (Figure 3A). LTβR-Ig is a chimeric protein comprising the LTβR extracellular domain that binds to LIGHT plus the Fc portion of human IgG1. LTβR-Ig can block LIGHT-mediated NK-cell activation and IFN-γ production, whereas the control LFA-1–Ig had no effect (Figure 3B). These results suggest that NK-cell activation by Ag104 Ld LIGHT tumor cells is specifically induced by LIGHT. To confirm the direct effect of LIGHT on NK-cell activation, plate-bound recombinant LIGHT (rLIGHT) was used as stimulators. Freshly isolated NK cells from Rag1-/- mice were used as responders. After 72 hours of incubation, rLIGHT induced NK-cell proliferation to an extent similar to that observed on crosslinking NK1.1 with anti-NK1.1 mAb, a known NK cell–activating receptor (Figure 3C). NK cells stimulated by rLIGHT produced high levels of IFN-γ and GM-CSF (Figure 3D). LTβR-Ig (100 μg) was injected into mice on the day of tumor challenge, leading to outgrowth of Ag104LdLIGHT tumor (Table 1). It suggests that rejection of Ag104LdLIGHT tumor is LIGHT dependent. Although there is an apparent difference in NK-cell receptors between mice and humans, intracellular signaling pathways that activate NK cells may also be conserved.30,31 We observed that recombinant human LIGHT can also activate human NK cells, indicating that LIGHT-HVEM signaling for NK cells is conserved between mice and humans (data not shown). Our study confirms for the first time that LIGHT can directly stimulate both murine and human NK cells.
LIGHT stimulates NK-cell proliferation and activation. (A) Ag104Ld LIGHT tumor cells profoundly induce NK-cell proliferation and IFNγ secretion. Purified NK cells from Rag1-/- mice were cocultured with irradiated Ag104Ld LIGHT cells for 3 days. 3H-TdR incorporation and IFN-γ detection were described as above. Tumor or NK cells alone do not show much proliferation and cytokine production. Tumor cells with LIGHT can significantly stimulate NK cells (P < .001). The data are presented from 3 independent experiments. (B) Blocking LIGHT disrupts Ag104 Ld LIGHT-mediated NK-cell proliferation and activation. The low dose (5 μg/mL) of LTβR-Ig or control Ig was added to the reaction of NK and Ag104Ld LIGHT-cell culture for 3-day incubation. Significant inhibition was found with LTβR-Ig group over control (P < .001). (C) rLIGHT induces NK-cell proliferation. Purified NK cells were isolated through NK purification kit from Rag1-/- mice and incubated with rLIGHT-coated plates for 3 days. 3H-TdR was added 15 hours before incorporation assay. rLIGHT can stimulate NK cells (P < .001). (D) rLIGHT enhances IFN-γ and GM-CSF secretion. IFN-γ and GM-CSF were detected with ELISA from the supernatants as treated above. Data analysis was performed by using the Student t test. Averaged results are expressed as mean plus or minus SD.
LIGHT stimulates NK-cell proliferation and activation. (A) Ag104Ld LIGHT tumor cells profoundly induce NK-cell proliferation and IFNγ secretion. Purified NK cells from Rag1-/- mice were cocultured with irradiated Ag104Ld LIGHT cells for 3 days. 3H-TdR incorporation and IFN-γ detection were described as above. Tumor or NK cells alone do not show much proliferation and cytokine production. Tumor cells with LIGHT can significantly stimulate NK cells (P < .001). The data are presented from 3 independent experiments. (B) Blocking LIGHT disrupts Ag104 Ld LIGHT-mediated NK-cell proliferation and activation. The low dose (5 μg/mL) of LTβR-Ig or control Ig was added to the reaction of NK and Ag104Ld LIGHT-cell culture for 3-day incubation. Significant inhibition was found with LTβR-Ig group over control (P < .001). (C) rLIGHT induces NK-cell proliferation. Purified NK cells were isolated through NK purification kit from Rag1-/- mice and incubated with rLIGHT-coated plates for 3 days. 3H-TdR was added 15 hours before incorporation assay. rLIGHT can stimulate NK cells (P < .001). (D) rLIGHT enhances IFN-γ and GM-CSF secretion. IFN-γ and GM-CSF were detected with ELISA from the supernatants as treated above. Data analysis was performed by using the Student t test. Averaged results are expressed as mean plus or minus SD.
HVEM signaling mediates LIGHT-induced NK-cell activation
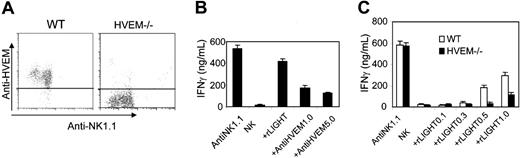
LIGHT is known to bind to 2 distinct members of the TNFR family, including HVEM and LTβR.19,32 We next attempted to determine which receptor mediates NK-cell activation through LIGHT. Because LTβR was not detected on NK cells, we generated an anti-HVEM mAb to assess whether HVEM is expressed on NK cells. We found that HVEM was highly expressed on the surface of purified NK cells, whereas NK cells deficient of HVEM (HVEM-/-) showed no increased binding to anti-HVEM mAb over the control antibody (Figure 4A). To study whether HVEM is an important receptor for LIGHT-mediated NK-cell activity, a low dose of anti-HVEM mAb was added into culture in the presence of rLIGHT. The data showed that addition of anti-HVEM antibody blocked rLIGHT-induced NK-cell proliferation and IFN-γ secretion in a dose-dependent fashion (Figure 4B and not shown). To further test whether HVEM on NK cells is required for LIGHT-mediated NK activity, NK cells from Hvem-/- mice were stimulated with rLIGHT, which showed impaired rLIGHT-induced NK-cell proliferation and IFN-γ production (Figure 4C and not shown). These findings indicate that LIGHT engagement with HVEM can sufficiently elicit proliferation and activation of NK cells.
Activated NK cells trigger CTL immunity
Although NK cells are thought to play a role in antitumor activity, their actual contribution to adaptive immunity is unclear. Both NK cells and CTLs are essential but not sufficient for some tumors, including LIGHT-mediated tumor eradication. But whether NK cells can directly promote activation and expansion of CD8+ T cells is unknown. We observed that the percentage of CTLs, which were accompanied by NK cells in the close vicinity inside the tumor, continued to expand 10 days after implantation followed by tumor regression. We wondered whether NK cells can directly activate antigen-specific CD8+ T cells in the presence of tumor. To examine their direct interaction, we used 2C T cells which recognize the model tumor antigen H-2 Ld expressed on the Ag104 Ld tumor cell line to define the role of NK cells in H-2 Ld-driven T-cell activation in vitro. Isolated NK cells from Rag1-/- mice were preactivated with rLIGHT and then transferred to the culture containing naive 2C T cells that were primed with irradiated Ag104 Ld tumor cells. NK cells were added into the culture on day 0, 3, or 5 during a 7-day incubation of 2C T cells with Ag104 Ld tumor cells. 2C T cells responded poorly to Ag104 Ld without activated NK cells but showed the highest level of proliferation and cytolytic activity with the addition of NK cells to the culture on day 0. The costimulatory effect of NK cells dramatically diminished when NK cells were added to the culture on day 5 (Figure 5A-B). NK alone with irradiated Ag104 Ld tumor cells barely induced proliferation and cytotoxicity. 2C T cells stimulated with NK cells that were preactivated by IL-15 or anti-NK1.1 mAb exhibited a similar response, suggesting that NK cells activated with various other stimuli can directly activate T cells (data not shown). 2C T cells activated by NK cells fully expressed the activation molecule NKG2D and the mature granule molecule perforin (Figure 5C). Perforin is a pore-forming protein and delivers granzymes into target cells to trigger granule-mediated apoptosis.33,34 Transfection experiments and mice deficient in perforin, granzyme A, or granzyme B demonstrated that perforin or either granzyme is sufficient for granule-mediated tumor lysis by CTLs and NK cells.33,35 To determine the maturation and effector capacity of the CD8+ T cells, which received help from LIGHT-activated NK cells, we analyzed perforin expression in the tumor-infiltrating CD8+ T cells during the process of tumor regression. CD8+ perforin-positive (not NK) cells dynamically increased inside the Ag104 Ld LIGHT tumor, followed by rapid tumor regression (Figure 5D and not shown). In contrast, perforin-positive CD8+ T cells inside the Ag104 Ld tumor were rarely detectable at all time points as representatively shown at day 14 (Figure 5D). Despite their own killing ability, it seems to be CTLs, not NK cells, from the tumor tissues or the DLNs of Ag104 Ld LIGHT tumor-bearing mice which show profound cytotoxicity (Figure 5E and not shown). Together, these data suggest that activated NK cells can enhance the maturation of CTLs to kill tumor cells.
HVEM signaling mediates LIGHT-induced NK-cell activation. (A) HVEM is expressed on the surface of NK cells. NK cells from Rag1-/- or Hvem-/- mice were enriched by NK purification kits and then stained with anti–NK1.1-conjugated PE and biotin-labeled anti-HVEM followed by streptavidin-APC. Data are representative of at least 3 independent experiments. (B) Anti-HVEM mAb blocks LIGHT-mediated NK-cell IFN-γ production and proliferation (data not shown). Different doses of anti-HVEM antibody were added to rLIGHT-treated NK-cell culture for 3 days. 3H-TdR incorporation and IFN-γ assays were described previously. Anti-HVEM antibody can significantly inhibit rLIGHT-mediated stimulation (P < .001). (C) Hvem-deficient mice impair LIGHT-mediated IFN-γ production by NK cells. NK cells from Rag1-/- or Hvem-/- mice were stimulated with different amounts of rLIGHT with assessment as above. Hvem-/- mice showed reduced response to rLIGHT (P < .001). The results are representative of 6 independent experiments. Data analysis was performed by using the Student t test. Averaged results are expressed as mean plus or minus SD.
HVEM signaling mediates LIGHT-induced NK-cell activation. (A) HVEM is expressed on the surface of NK cells. NK cells from Rag1-/- or Hvem-/- mice were enriched by NK purification kits and then stained with anti–NK1.1-conjugated PE and biotin-labeled anti-HVEM followed by streptavidin-APC. Data are representative of at least 3 independent experiments. (B) Anti-HVEM mAb blocks LIGHT-mediated NK-cell IFN-γ production and proliferation (data not shown). Different doses of anti-HVEM antibody were added to rLIGHT-treated NK-cell culture for 3 days. 3H-TdR incorporation and IFN-γ assays were described previously. Anti-HVEM antibody can significantly inhibit rLIGHT-mediated stimulation (P < .001). (C) Hvem-deficient mice impair LIGHT-mediated IFN-γ production by NK cells. NK cells from Rag1-/- or Hvem-/- mice were stimulated with different amounts of rLIGHT with assessment as above. Hvem-/- mice showed reduced response to rLIGHT (P < .001). The results are representative of 6 independent experiments. Data analysis was performed by using the Student t test. Averaged results are expressed as mean plus or minus SD.
Activated NK cells promote CD8+ cell responses. (A) NK cells stimulate CD8+ T-cell proliferation. Purified NK cells from Rag1-/- mice were prestimulated with 0.5 μg/mL rLIGHT for 2 days and added to the culture of 2C T and irradiated Ag104 Ld cells at the indicated days for 7-day incubation. 3H-TdR incorporation and IFNγ assays were described previously. Significant stimulation of NK + 2C T cells to tumor can be detected by all 3 groups. (B) NK cells promote CD8+ cytolytic activity. 2C T cells with above treatment were cocultured with 51Cr-labeled Ag104 Ld at 50 of E/T ratio for specific lysis assay. (C) NK cells induce CD8+ T-cell maturation. Activated 2C T cells as above were stained with anti-CD8+ and anti-NKG2D (top) or anti–perforin through intracellular staining by permeabilization with 0.5% saponin (bottom). Data shown represent 1 of at least 3 independent experiments. Number in each quadrant indicates percentage of gated lymphocytes. (D) Perforin-positive CD8+ cells dynamically increased inside Ag104 Ld LIGHT tumor during the process of rejection. Single-cell suspension of tumor tissues from different times was stained with anti-CD8 and anti–perforin antibodies. Data shown represent 1 of at least 3 independent experiments. (E) Freshly isolated CD8+ T cells, not NK cells, from intratumor show marked killing activity. NK and CD8+ cells were isolated by NK or CD8+ purification kit from tumor tissues of day 22 Ag104 Ld LIGHT or Ag104 Ld tumor, followed by coculturing with 51Cr-labeled Ag104 Ld cells at the indicated E/T ratios for specific lysis assay. The results are representative of at least 3 independent experiments. Data analysis was performed by using the Student t test. Averaged results are expressed as mean plus or minus SD.
Activated NK cells promote CD8+ cell responses. (A) NK cells stimulate CD8+ T-cell proliferation. Purified NK cells from Rag1-/- mice were prestimulated with 0.5 μg/mL rLIGHT for 2 days and added to the culture of 2C T and irradiated Ag104 Ld cells at the indicated days for 7-day incubation. 3H-TdR incorporation and IFNγ assays were described previously. Significant stimulation of NK + 2C T cells to tumor can be detected by all 3 groups. (B) NK cells promote CD8+ cytolytic activity. 2C T cells with above treatment were cocultured with 51Cr-labeled Ag104 Ld at 50 of E/T ratio for specific lysis assay. (C) NK cells induce CD8+ T-cell maturation. Activated 2C T cells as above were stained with anti-CD8+ and anti-NKG2D (top) or anti–perforin through intracellular staining by permeabilization with 0.5% saponin (bottom). Data shown represent 1 of at least 3 independent experiments. Number in each quadrant indicates percentage of gated lymphocytes. (D) Perforin-positive CD8+ cells dynamically increased inside Ag104 Ld LIGHT tumor during the process of rejection. Single-cell suspension of tumor tissues from different times was stained with anti-CD8 and anti–perforin antibodies. Data shown represent 1 of at least 3 independent experiments. (E) Freshly isolated CD8+ T cells, not NK cells, from intratumor show marked killing activity. NK and CD8+ cells were isolated by NK or CD8+ purification kit from tumor tissues of day 22 Ag104 Ld LIGHT or Ag104 Ld tumor, followed by coculturing with 51Cr-labeled Ag104 Ld cells at the indicated E/T ratios for specific lysis assay. The results are representative of at least 3 independent experiments. Data analysis was performed by using the Student t test. Averaged results are expressed as mean plus or minus SD.
IFN-γ plays an essential role in NK-induced CTL immunity
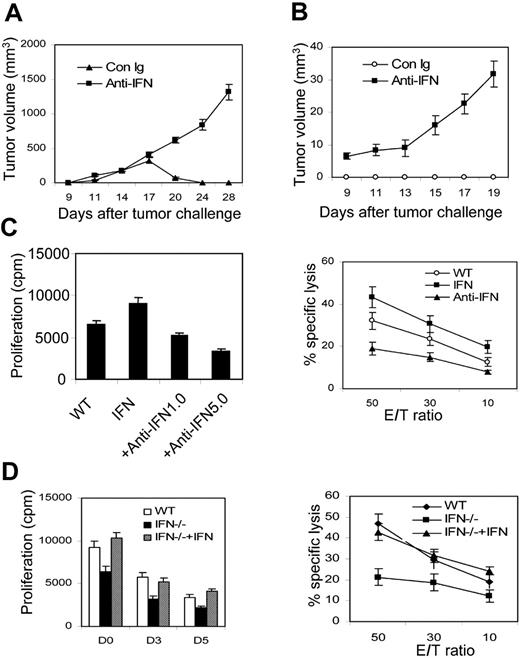
IFN-γ is mainly produced by NK cells at early stages of many immune responses.18,36 Some studies showed that IFN-γ may exert a negative (proapoptotic) effect on CTLs, and mice lacking Ifng generate a greater number of Listeria-specific CTLs.37 But others showed that IFN-γ acts directly on CD8+ T cells to increase their abundance during viral infection.38 To define whether NK-cell–derived IFN-γ can activate CTLs, we subcutaneously inoculated Ag104 LdLIGHT tumor cells into C3B6F1 mice, then treated the mice with anti–IFN-γ mAb or control Ig at the early phases of tumor inoculation. Tumors grew aggressively with a tumor incidence of 100% in anti–IFN-γ mAb-treated mice, whereas control Ig-treated mice rejected the tumors (Figure 6A; Table 1). To further test the role of IFN-γ in the memory responses, the C3B6F1 mice were rechallenged with 1 × 107 Ag104 Ld tumor cells 40 days after the primary Ag104Ld LIGHT tumor regression and simultaneously treated with anti–IFN-γ mAb for 2 weeks. Blockade of IFN-γ led to tumor outgrowth. In contrast, control Ig-treated mice were resistant to the rechallenge of parental Ag104 Ld tumors (Figure 6B). To assess the direct action of IFN-γ for NK cell–induced CTL responses, we preactivated NK cells by rLIGHT and then added the NK cells into the culture of 2C T cells and irradiated Ag104 Ld tumor cells with either the addition of anti–IFN-γ mAb (1 μg/mL) or 100 ng/mL rIFN-γ and incubated for 7 days. At a low dose, anti–IFN-γ mAb markedly blocked proliferation and cytolytic activity of 2C T cells. However, the addition of rIFN-γ further enhanced 2C T-cell proliferation and cytotoxicity induced by LIGHT-activated NK cells (Figure 6C). To confirm the direct role of NK cell–derived IFN-γ in the proliferation and maturation of CTLs, isolated NK cells from Ifng-deficient mice were activated and incubated with 2C T cells in the presence of irradiated Ag104 Ld tumor cells. NK cells deficient of Ifng showed impaired stimulatory ability for CD8+ T-cell proliferation. Even more obvious was a deficit in the ability of Ifng-deficient NK cells to stimulate the cytolytic activity of 2C T cells. Interestingly, rIFN-γ alone can restore the Ifng-/- NK-cell deficiency in triggering CTL responses (Figure 6D). These data suggest that IFN-γ secreted by NK cells plays a critical role for the priming of CTL responses for tumor rejection.
IFN-γ plays an essential role in NK-induced CD8+ responses. (A) In vivo IFN-γ blockade leads to outgrowth of Ag104Ld LIGHT tumor at the primary tumor challenge. C3B6F1 mice were subcutaneously inoculated with Ag104Ld LIGHT tumor cells and treated twice weekly with anti–IFN-γ antibody or control anti–rat Ig for 2 weeks. (B) Blocking IFN-γ causes tumor growth at the secondary tumor challenge. The C3B6F1 mice were subcutaneously inoculated with 1 × 107 Ag104Ld tumor cells after 40 days of primary Ag104Ld LIGHT tumor rejection and treated with anti–IFN-γ antibody or control anti–rat Ig as previously described. (C) IFN-γ blockade impairs NK cell–induced CD8+ cell proliferation. rLIGHT-preactivated NK cells from Rag-1-/- mice were incubated with the culture of 2C T and irradiated Ag104Ld cells with different doses of anti–IFN-γ antibody or 100 ng/mL rIFN-γ for 7 days. 3H-TdR incorporation and specific lysis assays were performed as previously described. (D) IFN-γ–deficient mice impair NK cell–induced CD8+ proliferation and lytic activity. rLIGHT-preactivated NK cells from Rag1-/- or Ifng-/- mice were added to the culture of 2C T and irradiated Ag104Ld cells at days 0, 3, and 5 for 7 days. NK cells from Ifng-/- mice showed reduced responses (P < .01). rIFN-γ was added to the culture of Ifng-/- NK cells at the indicated times. Data analysis was performed by using the Student t test. Averaged results are expressed as mean plus or minus SD.
IFN-γ plays an essential role in NK-induced CD8+ responses. (A) In vivo IFN-γ blockade leads to outgrowth of Ag104Ld LIGHT tumor at the primary tumor challenge. C3B6F1 mice were subcutaneously inoculated with Ag104Ld LIGHT tumor cells and treated twice weekly with anti–IFN-γ antibody or control anti–rat Ig for 2 weeks. (B) Blocking IFN-γ causes tumor growth at the secondary tumor challenge. The C3B6F1 mice were subcutaneously inoculated with 1 × 107 Ag104Ld tumor cells after 40 days of primary Ag104Ld LIGHT tumor rejection and treated with anti–IFN-γ antibody or control anti–rat Ig as previously described. (C) IFN-γ blockade impairs NK cell–induced CD8+ cell proliferation. rLIGHT-preactivated NK cells from Rag-1-/- mice were incubated with the culture of 2C T and irradiated Ag104Ld cells with different doses of anti–IFN-γ antibody or 100 ng/mL rIFN-γ for 7 days. 3H-TdR incorporation and specific lysis assays were performed as previously described. (D) IFN-γ–deficient mice impair NK cell–induced CD8+ proliferation and lytic activity. rLIGHT-preactivated NK cells from Rag1-/- or Ifng-/- mice were added to the culture of 2C T and irradiated Ag104Ld cells at days 0, 3, and 5 for 7 days. NK cells from Ifng-/- mice showed reduced responses (P < .01). rIFN-γ was added to the culture of Ifng-/- NK cells at the indicated times. Data analysis was performed by using the Student t test. Averaged results are expressed as mean plus or minus SD.
NK-cell depletion inside tumor abolishes CD8+ T-cell–mediated tumor rejection
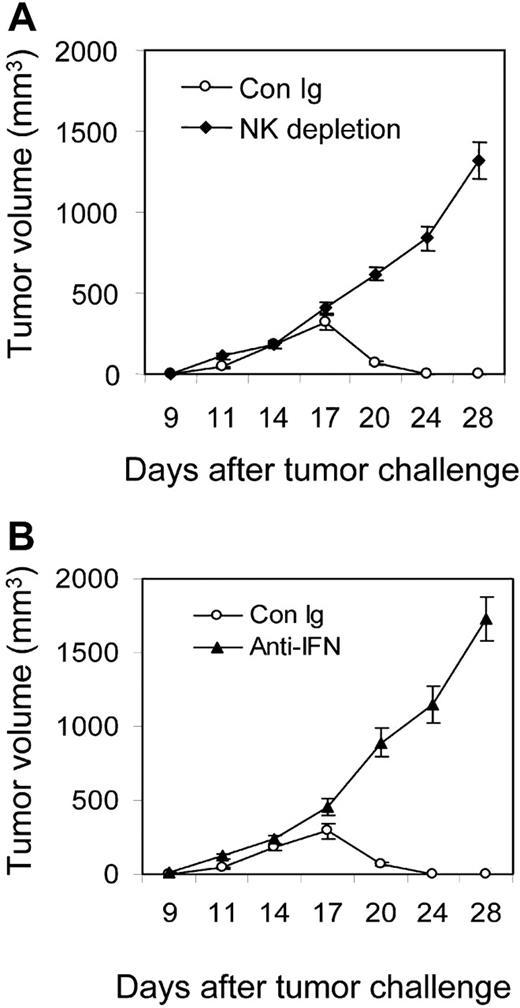
The primary site for activation of CTLs by NK cells has not been demonstrated. We found an accumulation of NK cells, followed by CTLs inside the tumor, suggesting that NK cells may initially interact with CD8+ cells inside the tumor. To further confirm an essential function of NK cells inside the tumor for tumor rejection, C3B6F1 mice were inoculated with Ag104Ld LIGHT tumor cells followed by intratumor NK-cell depletion using an anti-asialo GM1 antibody on days 9, 12, and 15. NK-cell depletion inside the tumor was sufficient for a 100% incidence of tumor growth (Figure 7A; Table 1). To test whether IFN-γ inside tumor is also essential for tumor rejection, blockade of IFN-γ via anti–IFN-γ mAb was done on days 9, 12, and 15. All treated mice with Ag104Ld LIGHT tumor showed an outgrowth of tumor, whereas control Ig-treated mice rejected the tumors (Figure 7B; Table 1). These results clearly suggest that intratumor NK cells and local IFN-γ are required for priming CTLs inside tumors.
Intratumor depletion of NK cells or blockade of IFN-γ causes tumor outgrowth. C3B6F1 mice were subcutaneously inoculated with Ag104LdLIGHT tumor cells with intratumor NK depletion by anti-asialo GM1 antibody (A) or blockade of IFN-γ via anti–IFN-γ mAb (B) at days 9, 12, and 15. Tumor growth was monitored every other day and data are the mean ± SD of 5 mice for each group.
Intratumor depletion of NK cells or blockade of IFN-γ causes tumor outgrowth. C3B6F1 mice were subcutaneously inoculated with Ag104LdLIGHT tumor cells with intratumor NK depletion by anti-asialo GM1 antibody (A) or blockade of IFN-γ via anti–IFN-γ mAb (B) at days 9, 12, and 15. Tumor growth was monitored every other day and data are the mean ± SD of 5 mice for each group.
Discussion
The NK cell was named initially because of its ability to spontaneously kill certain tumor cells in vitro. However, the significance of such killing for the rejection of a solid tumor has not been well established, nor have mechanisms that elicit NK activation inside the tumor. NK cells may be critical for the regression of some tumors shown by NK-depleting experiments, but their direct role for CTL activation and expansion has not been clearly demonstrated.10-12 It is difficult to evaluate an actual role of NK cells in tumor immunity in progressive tumor models. We recently observed that expression of LIGHT on tumor cells results in significant infiltration of immune cells, followed by rapid rejection of the established tumors.26 Therefore, this model allows us to further elucidate which immune cells are essential for eradicating nonimmunogenic and highly progressive tumors. Surprisingly, our study revealed that both NK and CD8+ T cells are essential but not sufficient for tumor rejection because the absence of either cell population during early stages of tumor inoculation fails to induce rejection. We also demonstrated that a critical site of interaction between NK and CD8+ cells for tumor rejection occurs inside tumor tissues. The essential role of NK cells is not merely limited to LIGHT-expressing tumors. We recently observed that the regression of MC57 (fibrosarcoma) and MC38 (colon cancer) is also dependent on NK cells (data not shown). Contrary to the initial belief that the main function of NK cells is to directly kill tumor cells, our data suggest that NK cells may play a critical role in assisting CTL activation, expansion, and maturation to elicit tumor rejection. (1) We did not detect any direct killing of Ag104Ld by activated NK cells. Ag104Ld was not killed by NK cells but obviously by CD8+ cells freshly purified from tumor tissues. (2) Tumor-infiltrating activated NK cells did not express perforin amid tumor growth (data not shown), whereas CD8+ T cells had a gradual increase in the level of perforin expression during tumor regression, suggesting that CTLs play a more active role in killing tumor cells compared with NK cells. (3) The percentage of tumor-infiltrating NK cells dramatically peaked during the priming phase with a subsequent increase in CTLs that correlated with eventual tumor regression. (4) Depletion of NK cells at a early phase rather than a late stage of tumor growth causes tumor growth, suggesting that the NK cells are essential for early activation of CD8+ cells but not the direct killing of tumor cells by NK themselves. (5) The tumor growth pattern in Rag1-/- mice did not change in the presence or absence of LIGHT, suggesting that NK cells alone are not sufficient to induce rejection without the presence of CD8+ T cells. Collectively, these findings support the notion that NK cells are critical in providing a helper function to initiate CD8+ T-cell responses against tumor progression.
Although NK cells are less abundant than the total number of T and B cells, as members of innate immunity, they respond much earlier. Actually, their frequency is much higher than antigen-specific lymphocytes against a given tumor at early immune responses. This raises the possibility that NK cells may be critical for bridging adaptive immune responses by costimulating tumor-responding CD8+ cells. In this study, we observed that NK cells dramatically increase in tumor tissues at the beginning of tumor formation followed by an increase in CTLs. Interestingly, the NK cells remain inside the tumor until the tumor regresses. Furthermore, depletion of NK cells at an early stage, but not at a late stage, of tumor inoculation ablates CD8+-mediated tumor rejection. Therefore, LIGHT-expressing tumors can activate and expand NK cells rapidly in the tumor tissues to effectively trigger tumor antigen-specific CD8+ T cells during the priming phase. Once CTLs are activated and expanded, a helper role of NK cells may become less pivotal.
NK cells, unlike T cells, can be activated in the absence of MHC class I molecules and play a crucial role in the earliest stage of immune responses.12,39 NK-cell activity depends on the balance of their inhibitory and activating molecules.1 Most studies focused on NK-cell activation through a series of family members, such as Ly49D and NKG2D.40,41 A recent proteomic analysis of unactivated and activated human NK-cell membrane-enriched fractions demonstrated that activated NK cells can efficiently stimulate T cells.42 Some studies showed that activated human NK cells are able to promote TCR-dependent proliferation of resting autologous peripheral-blood CD4+ T cells through costimulatory molecules of the immunoglobulin (Ig) and tumor necrosis factor (TNF) superfamilies. Several other costimulatory receptors were found to activate both T cells and NK cells.11,12,43-45 Recent studies demonstrated that tumors expressing NKG2D receptor ligand (NKG2DRL) can also activate both NKG2DR-expressing NK cells and CD8+ T cells to trigger protective CTL immunity.46,47 A recent report showed activated NK cells can enhance CTL responses against tumors via a DC-dependent pathway.17 In our study, we revealed that NK cells can be activated by LIGHT via HVEM, one of the receptors for LIGHT. Activated NK cells can in turn trigger a potent CTL response, independent of DCs. It is possible that costimulatory molecules like LIGHT provide additional signals for activation and expansion of NK cells which further activate incoming T cells. In the event that the tumor antigen is not sufficient to fully activate infiltrating T cells in some tumor models, it is very likely that activated NK cells inside the tumor may be more critical to prevent the tolerance of T cells inside the tumor. Being consistent with our hypothesis, one recent study of patients with melanoma observed LIGHT constitutively expressed by the cancer cells can be associated with enhanced infiltration of lymphocytes into the tumor tissues.27 In addition, activated T cells inside the tumor may also up-regulate LIGHT which may be associated with increase of infiltration in some tumors. How tumor cells avoid LIGHT-mediated activation of NK cells and CTLs remains to be determined.
An exact interaction site for the maturation of tumor-specific CD8+ cells by NK cells has not been demonstrated, and most studies focused on draining LNs. Our study indicates that tumor tissues can be the major site. (1) NK cells initially accumulate inside tumor tissues, followed by CD8+ T cells; (2) NK cells distribute near CD8+ T cells inside tumor; (3) intratumor depletion of NK cells leads to the reduction of CTLs and Ag104 LdLIGHT tumor regrowth; (4) transfer of activated NK cells can limit the growth of tumor cells (data not shown). Therefore, intratumor NK cells could be a potent activator for incoming CD8+ T cells.
We first showed that LIGHT is a new ligand for NK activation and further established a critical role of NK cells in breaking T-cell tolerance for tumor regression. Intratumor depletion of NK cells or blocking IFN-γ at an early stage of tumor formation abolished protective CD8+ responses against tumors, revealing key kinetics and location of NK-T interaction. IFNG-deficient NK cells fail to activate and maturate CTLs, suggesting the critical role of IFN-γ from NK cells is for the maturation of CTLs. Together, these results suggest this cytokine is one of the key bridging factors to initiate adaptive immunity by NK cells. It is likely that IFN-γ from both NK cells and CD8+ T cells are important and can form a positive loop to counter a suppressive environment inside the tumor. Some studies showed IFN-γ may exert a negative effect on T cells in vitro.48 Mice lacking Ifng generate a large number of Listeria-specific CTLs,37 but others showed IFN-γ acts directly on CD8+ T cells to increase their abundance during virus infection.38 Therefore, IFN-γ may play a distinct role in regulating CTLs depending on the phase of the immune response. Our study also suggests that an IFN-γ–independent pathway may also parallel with an IFN-γ–dependent pathway in NK-mediated CTL maturation. But the actual pathway has yet to be elucidated.
It is very challenging to break T-cell tolerance or unresponsiveness inside the tumor environment. Tumor-specific T cells have been difficult to isolate or expand because most tumor antigens are not well defined. The systemic transfer of lymphokine activated killer (LAK) cells into cancer hosts had been used to promote tumor immunity for many years, but the efficacy was limited because of high toxicity. However, local transfer of activated NK cells into tumor tissues or local activation of NK cells rather than systemic administration may be more potent to abolish tolerance with very limited side effects. Understanding of the mechanism for activation and expansion of NK cells inside the tumor may open a new avenue to break T-cell tolerance inside tumor tissues. Our recent and current studies have clearly shown that the forced expression of LIGHT in tumor cells in vitro or inside tumor tissues in vivo by plasmid DNA containing LIGHT might help to activate NK cells and CTLs, leading to the rejection of a primary tumor and even at a distal site.22,26 Our preliminary data using adenovirus to deliver LIGHT inside the tumor tissue also promoted early IFN-γ production inside the tumor, followed by the tumor rejection (data not shown). It is possible that activation of NK cells using gene therapy at a primary tumor site may create a better stimulatory environment for infiltrated CTLs. Activated and expanded CTLs in LIGHT-expressing tumors may become the major players to eradicate the distal tumors.26 Therefore, a better understanding of LIGHT-mediated NK and T-cell activation may provide a new strategy for immunotherapy of cancers. Considering the difficulty in generating and activating tumor-specific CTLs inside the tumor, the successful recruitment and activation of NK cells inside tumor tissues by various gene therapies could become a promising alternative strategy to enhance both NK and CTL function for eradicating established tumors. Additionally, direct activation and expansion of CTLs by activated NK cells may play an important role in bridging innate and adaptive immunity for a variety of other nontumor-related immune responses.
Prepublished online as Blood First Edition Paper, October 13, 2005; DOI 10.1182/blood-2005-08-3485.
Supported by the National Institutes of Health (NIH) (grants R01-062026, R01-DK58897, and P01-CA09296-01); NIH training grant (5T32DK07 074) (P.Y.) and Digestive Diseases Research Core Center (DDRCC) seeding grant (P30-DK42086) (P.Y.).
Z.F. designed and performed most experiments as well as wrote the paper; P.Y. designed experiments, generated LIGHT-expressing tumor lines, and made initial key observations; Yang Wang cloned LIGHT; Yugang Wang generated and produced recombinant LIGHT; M.L.F. assisted Z.F., W.L. handled pathology, Y.S. helped Z.F. with some experiments, Y.-X.F. designed, organized, and wrote the paper.
Z.F. and P.Y. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Vinay Kumar and Hans Schreiber for discussion, advice, and reagents.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal