The extracellular matrix protein, laminin, supports platelet adhesion through binding to integrin α6β1 In the present study, we demonstrate that human laminin, purified from placenta, also stimulates formation of filopodia and lamellipodia in human and mouse platelets through a pathway that is dependent on α6β1 and the collagen receptor GPVI. The integrin α6β1 is essential for adhesion to laminin, as demonstrated using an α6-blocking antibody, whereas GPVI is dispensable for this response, as shown using “knockout” mouse platelets. On the other hand, lamellipodia formation on laminin is completely inhibited in the absence of GPVI, although filopodia formation remains and is presumably mediated via α6β1 Lamellipodia and filopodia formation are inhibited in Syk-deficient platelets, demonstrating a key role for the kinase in signaling downstream of GPVI and integrin α6β1 GPVI was confirmed as a receptor for laminin using surface plasmon resonance spectroscopy and by demonstration of lamellipodia formation on laminin in the presence of collagenase. These results identify GPVI as a novel receptor for laminin and support a model in which integrin α6β1 brings laminin to GPVI, which in turn mediates lamellipodia formation. We speculate that laminin contributes to platelet spreading in vivo through a direct interaction with GPVI.
Introduction
Platelets play a critical role in the hemostatic process through a combination of adhesion, activation, and aggregation events that lead to formation of a platelet plug and occlusion of the site of damage. Our current understanding of this process highlights a critical role for the interaction of von Willebrand factor (VWF) with the platelet surface receptor, GPIb-IX-V, in mediating platelet tethering at arterial rates of flow.1,2 This is followed by platelet activation mediated by binding of collagen to the immunoglobulin receptor, GPVI, leading to inside-out stimulation of the integrins, α2β1 and αIIbβ3.3 The 2 integrins bind to their respective ligands, collagen and VWF, mediating stable adhesion. Platelet adhesion is further strengthened by integrin-dependent platelet spreading.4-7 Platelet aggregation is mediated through binding of fibrinogen to integrin αIIbβ3 in a process dependent on release of the secondary mediators, ADP and thromboxanes.8-10
The contribution of other extracellular matrix proteins to the hemostatic process is unclear. Recently, however, Nieswandt's group11 has highlighted a possible role for the 2 β1-integrins, α5β1 and α6β1, which are receptors for fibronectin and laminin, respectively, in supporting adhesion in vivo in concert with integrins α2β1 and αIIbβ3. This was achieved by comparing platelet adhesion and aggregate formation in vivo using fluorescent intravital microscopy in mice deficient in α2 and β1 integrin subunits and in the presence of αIIbβ3 blockade.11 This study therefore illustrates that laminin, fibronectin, and other ligands for α5β1 and α6β1 may contribute to the events that underlie platelet adhesion and platelet activation at sites of damage to the vasculature, leading the authors to conclude that “shear-resistant platelet adhesion on the injured vessel wall in vivo is a highly integrated process involving integrin-ligand interaction, none of which by itself is essential.”11
Laminin is highly expressed in the basement membrane and is therefore one of the first extracellular matrix proteins to which platelets are exposed following mild damage to the vasculature. The present results demonstrate that, in addition to supporting adhesion, laminin is able to stimulate platelet spreading through binding to the collagen receptor GPVI but in a manner that is dependent on prior adhesion to integrin α6β1 Thus, these observations demonstrate that laminin is able to support both platelet adhesion and platelet spreading at sites of damage to the vasculature, thereby contributing to the hemostatic process.
Materials and methods
Materials
Anti-SLP-76 polyclonal antibody (pAb) was kindly donated by Dr G. Koretzky (University of Pennsylvania). Anti-Btk pAb was a gift from Dr M. Tomlinson (University of Birmingham, United Kingdom). Syk-deficient radiation chimeric mice were obtained as previously described.12 FcR γ chain–deficient mice13 and PLCγ2-deficient mice14 were bred on a B6 background. GPVI-deficient mice15 were bred on a C57BL/6J background. FcR γ chain–, PLCγ2-, and GPVI-deficient mice were genotyped by polymerase chain reaction (PCR) and wild-type littermates used as a control. The monomeric form of soluble GPVI (GPVIex) and its dimeric form (GPVI-Fc2) were generated as described before.16 Laminin from human placenta, anti–integrin α6 monoclonal antibody (mAb) (GoH3), collagenase, and N-Tris(hydroxymethyl)-methyl-2-amino-ethanesulfonic acid (TES) were purchased from Sigma-Aldrich (St Louis, MO). The 8% to 16% gradient gel (sodium dodecyl sulfate–polyacrylamide gel electrophoresis [SDS-PAGE]) was from TEFCO (Tokyo, Japan). All other reagents were from previously named sources.7,17,18
Platelet preparation
Venous blood from healthy drug-free volunteers was collected into 10% sodium citrate (3.8% sodium citrate, wt/vol). Platelet-rich plasma (PRP) was obtained after centrifugation at 150g for 11 minutes. PRP was centrifuged at 400g for 10 minutes in the presence of 15% acid-citrate-dextrose (ACD; 2.5% sodium citrate, 2% glucose, and 1.5% citric acid, wt/vol) and EGTA (2 mM). The platelet pellet was resuspended in modified Tyrode buffer (CFT; 137 mM NaCl, 11.9 mM NaHCO3, 0.4 mM Na2HPO4, 2.7 mM KCl, 1.1 mM MgCl2, and 5.6 mM glucose, pH 7.3) at a density of 2 × 107/mL. The platelet suspension was incubated for 30 minutes at room temperature and, where necessary, incubated with 1 mM Gly-Arg-Gly-Asp-Ser peptide (GRGDS) for 10 minutes before experimentation. Murine blood was drawn from methoxyfluorane-anesthetized mice by retro-orbital puncture using microcapillary tubes in accordance with the local law. Approximately 500 μL of blood was collected into 100 μL of ACD and a 50 μL CFT-containing Eppendorf tube (Eppendorf, Hamburg, Germany). Blood was centrifuged at 85g for 10 minutes in a microfuge. After removal of PRP plus some erythrocytes into a new Eppendorf tube, PRP was separated by centrifugation at 100g for 10 minutes in a bucket rotor centrifuge. PRP was removed to a new tube and centrifuged with 2 mM EGTA at 400g for 10 minutes. Platelet pellet was isolated and resuspended into CFT at a cell density of 0.3 × 108/mL to 4.0 × 108/mL, the platelet suspension was rested for 30 minutes and, then, where necessary, incubated with 1 mM GRGDS for 10 minutes before experimentation unless otherwise stated.
Platelet spreading assay
For the spreading experiments, coverslips were coated with 50 μg/mL laminin, 50 μg/mL collagen, or 200 μg/mL fibrinogen overnight at 4°C After twice washing with phosphate-buffered saline (PBS), the coverslips were blocked with 1% fatty acid–free purified BSA in PBS for 2 hours at room temperature and then rinsed by CFT. BSA-coated coverslips were prepared for negative control. Where necessary, laminin- or collagen-coated surfaces were treated with 0.1 mg/mL collagenase in TES-calcium buffer (50 mM TES, 0.36 mM CaCl2, pH 7.4) for 2 to 3 hours at 35°C before BSA blocking.
Washed murine platelets (3 × 107/mL) or washed human platelets (2 × 107/mL) were seeded on the coverslips for the indicated time at room temperature in the presence or absence of 20 μg/mL anti–integrin α6β1 antibody, 20 μg/mL rat control IgG, 100 μg/mL GPVIex, or 100 μg/mL GPVI-Fc2. After removal of unbound platelets, coverslips were washed with calcium-free modified Tyrode buffer (CFT), and then adherent platelets were fixed by 3% paraformaldehyde for 30 minutes at room temperature, permeabilized by 0.3% Triton X-100 for 5 minutes, and stained by TRITC-conjugated phalloidin for 2 hours as described elsewhere.17 Platelets were visualized by an inverted fluorescent microscope IX71 (Olympus, Tokyo, Japan) equipped with a 100×/1.30 objective lens, a monochromatic light source, and a DP-70 digital camera (Olympus, Figures 1A, B, 3A, 4A, 6A, and 7A). Up to 8 images where chosen at random per experiment and analyzed by 2 individuals, 1 of whom performed the analysis under blind conditions. The analysis involved both counting of adhered platelets (0.006 mm2 per image) and the analysis of platelet surface area using NIH image (National Institutes of Health [NIH], Bethesda, MD) for Macintosh. Statistical significance was evaluated by the Student t test. In each case, P values of less than .05 were taken as the minimum to indicate statistical significance. All the adhesion assays were performed as described earlier in this paragraph unless otherwise stated later in this paragraph. For differential interference contrast (DIC) imaging, adherent platelets on laminin-coated glass coverslips were fixed by 3% paraformaldehyde for 30 minutes at room temperature and then visualized using differential interference contrast optics Uplan Apo (Olympus; Figure 2A). Real-time imaging of platelet spreading on laminin-coated glass coverslips was also performed using DP controller software for Windows OS (Olympus; Figure 1C).
Protein precipitation studies
Flat-bottomed dishes for cell culture were coated with 50 μg/mL laminin or 50 μg/mL collagen overnight at 4°C. The dishes were washed and BSA-blocked, as described in “Platelet spreading assay.” Only BSA-coated dishes were used as a control. A total of 300 μL washed murine platelets (4.0 × 108/mL) were incubated 1 mM GRGDS and seeded on dishes for 15 to 30 minutes at room temperature. After incubation, 300 μL 2 × ice-cold lysis buffer (2% vol/vol Nonidet P-40, 20 mM Tris, 300 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 2 mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin A, pH 7.3) was added to each dish. Ten microliters of each sample was mixed with the same volume of Laemmli sample buffer, and protein were separated by 1-dimensional SDS-PAGE (8% to 16% gradient gel). Protein tyrosine phosphorylation was measured by Western blotting with 4G10. Protein loading was measured by Western blotting with anti-PLCγ2 pAb. Immunoprecipitation studies were performed as previously described.19 In brief, whole-cell lysates were prepared as described earlier in this paragraph. After removing debris and preclearing with 25 μL protein A–Sepharose beads for 1 hour at 4°C, supernatants were incubated with 2 μL FcR γ chain mAb, 1.5 μL anti-Syk pAb, 2 μL anti–SLP-76 pAb, 4 μL anti-LAT pAb, 2 μL anti-Btk pAb, or 10 μL anti-PLCγ2 pAb with 30 μL protein A–Sepharose beads overnight at 4°C. After centrifugation, pellets were washed with 1 × lysis buffer once and twice with TBS-T and then solubilized by addition of 2 × Laemmli sample buffer, and proteins were separated by SDS-PAGE on 8% or 15% gels. Protein tyrosine phosphorylation was detected by Western blotting with 4G10. Gels were reprobed by FcR γ chain mAb, anti-Syk pAb, anti–SLP-76 pAb, anti-LAT pAb, anti-Btk pAb, or anti-PLCγ2 pAb. Tyrosine phosphorylation of Syk and PLCγ2 were quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA) for Macintosh. Optical density measurements were standardized by the recruitments of these proteins.
Surface plasmon resonance spectroscopy
Specific interactions between GPVI-Fc2 and laminin or collagen were analyzed using BIAcore X system (BIAcore, Uppsala, Sweden) at 25°C. Laminin or collagen was covalently coupled to CM5 chip (BIAcore) using an Amine Coupling Kit (BIAcore) according to the manufacturer's instructions. Regeneration of the protein-coated surfaces was achieved by running 15 μL of 10 mM HCL thorough the flow cell at 30 μL/min 2 times. A control surface was reacted with the amine coupling reagent in the absence of ligand and then blocked with ethanolamine. GPVI-Fc2 in PBS of several concentrations was perfused over the control surface and immobilized laminin or collagen surfaces at a flow rate of 10 μL/min, and the resonance changes were recorded. The sensorgram of the immobilized laminin or collagen surfaces was subtracted from that of the control surfaces and the dissociation constants (Kd) determined using BIAevaluation software (BIAcore).
Results
Human and murine platelets adhere to and spread on human laminin
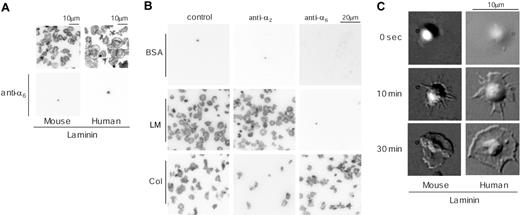
We initially investigated the ability of a laminin monolayer to support spreading of human and murine platelets. Washed platelets were allowed to adhere to a laminin surface for up to 30 minutes in the presence of the αIIbβ3 inhibitor, GRGDS, and then visualized either by staining for F-actin using rhodamine-phalloidin or by DIC microscopy. Most (more than 95%) of the adhered human and mouse platelets had undergone extensive spreading on the laminin surface by 30 minutes, characterized by formation of large, sheetlike lamellipodia (Figure 1A). In comparison, there was no adhesion in the absence of laminin as illustrated for mouse platelets (Figure 1B). Adhesion of both human and mouse platelets was completely blocked in the presence of blocking antibodies to integrin α6 (Figure 1A) but was maintained in the presence of blocking antibodies to α2 (Figure 1B and not shown). In marked contrast, adhesion and spreading of human and mouse platelets on collagen was maintained in the presence of blocking antibodies to α6 but markedly inhibited in the presence of blocking antibodies to α2 (Figure 1B and data not shown). These findings demonstrate that platelet adhesion to laminin is critically dependent on integrin α6β1 but does not require integrins α2β1 or αIIbβ3.
Time-lapse videomicroscopy was used to study platelet spreading on the laminin surface. Adhesion of platelets to laminin was followed by a series of characteristic changes in platelet morphology, including rounding, formation of filopodia, and extensive formation of lamellipodia (Figure 1C). The pattern of spreading of human platelets on laminin is very similar to that of spreading of human platelets on fibrinogen. In contrast, mouse platelets are able to form extensive lamellipodia on laminin (Figure 1C) but not on fibrinogen.5,6 Thus, these observations suggest either that integrin α6β1 generates a stronger profile of intracellular signals in mouse platelets in comparison with integrin αIIbβ3 or that laminin binds to a second receptor, in an α6β1-dependent manner, which is able to drive formation of lamellipodia. The second of these 2 hypotheses is favored on the grounds that integrin αIIbβ3 is expressed at approximately a 50 times greater level than integrin α6β1 and yet is unable to support formation of lamellipodia.
Adhesion and spreading of human and mouse platelets on laminin. (A) Washed murine platelets (3 × 107/mL; left column) or washed human platelets (2 × 107/mL; right column) were pretreated with 1 mM GRGDS peptide in the presence (bottom row) or absence (top row) of 20 μg/mL anti–integrin α6β1 antibody. Coverslips were coated with 50 μg/mL laminin from human placenta overnight at 4°C and then blocked with 1% fatty acid–free BSA for 2 hours at 4°C. After rinsing with Tyrode buffer, platelets were seeded on the coverslips and incubated at room temperature. After 30 minutes of incubation, unbound platelets were removed and adherent platelets were fixed by 3% paraformaldehyde, permeabilized with 0.3% Triton X-100, and F-actin stained using TRITC-labeled phalloidin. After F-actin staining, platelets were visualized using confocal fluorescent microscopy. (B) Murine platelets, washed as described in “Materials and methods” (3 × 107/mL), were pretreated with or without 20 μg/mL anti–integrin α2-blocking antibody (middle column) or anti–integrin α6 antibody (right column) for 10 minutes and then seeded on the BSA-(top row), 50 μg/mL laminin-(middle row), or 50 μg/mL collagen-coated (bottom row) surfaces. Adherent platelets were fixed and visualized as described in “Materials and methods.” (C) Changes in morphology of adherent washed murine platelets (3 × 107/mL; left column) or washed human platelets (2 × 107/mL; right column) were visualized by time-lapse real-time imaging using videomicroscopy with DIC optics. The results are representative of 3 to 12 experiments. LM indicates laminin; col, collagen.
Adhesion and spreading of human and mouse platelets on laminin. (A) Washed murine platelets (3 × 107/mL; left column) or washed human platelets (2 × 107/mL; right column) were pretreated with 1 mM GRGDS peptide in the presence (bottom row) or absence (top row) of 20 μg/mL anti–integrin α6β1 antibody. Coverslips were coated with 50 μg/mL laminin from human placenta overnight at 4°C and then blocked with 1% fatty acid–free BSA for 2 hours at 4°C. After rinsing with Tyrode buffer, platelets were seeded on the coverslips and incubated at room temperature. After 30 minutes of incubation, unbound platelets were removed and adherent platelets were fixed by 3% paraformaldehyde, permeabilized with 0.3% Triton X-100, and F-actin stained using TRITC-labeled phalloidin. After F-actin staining, platelets were visualized using confocal fluorescent microscopy. (B) Murine platelets, washed as described in “Materials and methods” (3 × 107/mL), were pretreated with or without 20 μg/mL anti–integrin α2-blocking antibody (middle column) or anti–integrin α6 antibody (right column) for 10 minutes and then seeded on the BSA-(top row), 50 μg/mL laminin-(middle row), or 50 μg/mL collagen-coated (bottom row) surfaces. Adherent platelets were fixed and visualized as described in “Materials and methods.” (C) Changes in morphology of adherent washed murine platelets (3 × 107/mL; left column) or washed human platelets (2 × 107/mL; right column) were visualized by time-lapse real-time imaging using videomicroscopy with DIC optics. The results are representative of 3 to 12 experiments. LM indicates laminin; col, collagen.
Dissection of signaling events in platelet spreading on laminin
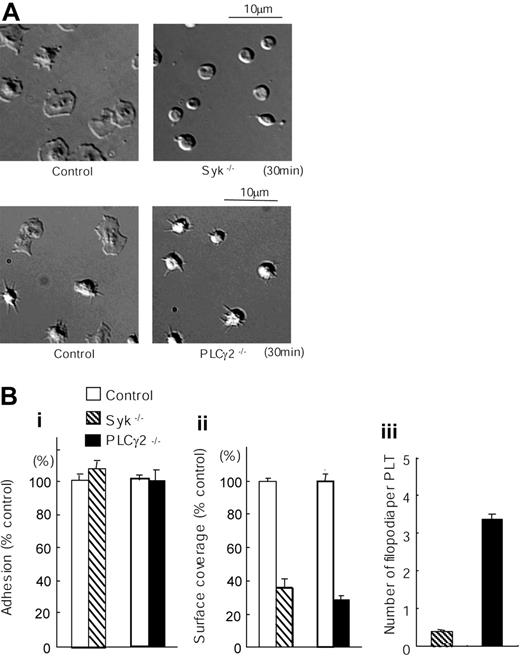
We sought to further investigate the mechanism of spreading on the laminin surface using mice deficient in key signaling proteins. The tyrosine kinase Syk and the effector enzyme PLCγ2 play critical roles in platelet spreading downstream of integrins αIIbβ3 and α2β1.5-7,20,21 As shown in Figure 2, analysis by DIC microscopy revealed that formation of lamellipodia in mouse platelets on laminin was almost completely inhibited in the absence of Syk or PLCγ2, although there was no effect on the level of adhesion. Interestingly, the degree of inhibition of spreading was more marked in the absence of Syk relative to the absence of PLCγ2. In the absence of Syk, the platelets remained as discs, whereas the PLCγ2-deficient platelets undergo rounding and generate a limited number of filopodia. This difference may reflect the presence of a very low level of PLCγ1 in murine platelets,22 in contrast to the absolute requirement of Syk for regulation of both homologs of PLC.
The critical role of Syk and PLCγ2 in mediating spreading on laminin led us to investigate the dependency of this response on the FcR γ chain, which has been shown to play a critical role in mediating spreading in response to activation of the collagen ITAM receptor, GPVI, but not by the integrins α2β1 and αIIbβ36,7,18 To our surprise, formation of lamellipodia on laminin was markedly inhibited in the absence of FcR γ chain, although a residual degree of formation of filopodia was seen (Figure 3). On the other hand, the FcR γ chain was not required for adhesion to laminin. Thus, these results reveal an unexpected role for the FcR γ chain in mediating spreading on laminin, thereby distinguishing the signaling pathway used by the extracellular matrix protein to that regulated by ligands of α2β1 and αIIbβ3.
Spreading but not adhesion of mouse platelets on laminin is dependent on Syk and PLCγ2. (A) Washed murine platelets (3 × 107/mL) from control, Syk-deficient (Syk-/-), or PLCγ2-deficient (PLCγ2-/-) mice were pretreated with the αIIbβ3 blocker, lotrafiban (10 μM), before seeding on laminin-coated coverslips as described in Figure 1. Real-time imaging was performed using videomicroscopy with DIC optics. (B) (i) The number of adherent platelets was counted. The graph illustrates the mean number of percent wild-type adhesion ± SEM per image from at least 4 different images from 2 separate experiments. (ii) Total surface coverage of adherent platelets on laminin was measured using NIH image software. The graph illustrates the percent wild-type surface coverage ± SEM per image from at least 4 different images from 2 separate experiments. (iii) Number of filopodia-like projections in adherent platelets was counted. The graph illustrates the mean number of filopodia-like projection ± SEM per adherent platelet of at least 240 platelets from 2 separate experiments.
Spreading but not adhesion of mouse platelets on laminin is dependent on Syk and PLCγ2. (A) Washed murine platelets (3 × 107/mL) from control, Syk-deficient (Syk-/-), or PLCγ2-deficient (PLCγ2-/-) mice were pretreated with the αIIbβ3 blocker, lotrafiban (10 μM), before seeding on laminin-coated coverslips as described in Figure 1. Real-time imaging was performed using videomicroscopy with DIC optics. (B) (i) The number of adherent platelets was counted. The graph illustrates the mean number of percent wild-type adhesion ± SEM per image from at least 4 different images from 2 separate experiments. (ii) Total surface coverage of adherent platelets on laminin was measured using NIH image software. The graph illustrates the percent wild-type surface coverage ± SEM per image from at least 4 different images from 2 separate experiments. (iii) Number of filopodia-like projections in adherent platelets was counted. The graph illustrates the mean number of filopodia-like projection ± SEM per adherent platelet of at least 240 platelets from 2 separate experiments.
The FcR γ chain is constitutively associated with the collagen receptor GPVI on the platelet surface, and abolition of expression of FcR γ chain also leads to a loss of expression of GPVI. In contrast, the FcR γ chain is retained in platelets deficient in GPVI.15 We therefore investigated whether laminin is able to induce spreading in the absence of GPVI using mice deficient in GPVI. To our further surprise, spreading on a laminin surface was also markedly inhibited in the absence of the collagen receptor as measured by staining for actin (Figure 4) or by DIC microscopy (not shown), although a limited degree of formation of filopodia could be seen, as was the case for the FcR γ chain-/- platelets. This limited formation of filopidia is most likely mediated through stimulation of integrin α6β1, bearing in mind that a similar result is seen in platelets that have undergone adhesion to fibronectin via integrin α5β1, which is expressed at a similar level to α6β1.23 Further, adhesion of the GPVI-deficient platelets to laminin was unchanged but was completely inhibited in the presence of an α6-blocking antibody (Figure 4). These results demonstrate that laminin induces spreading through a GPVI-dependent pathway, which also requires α6β1-mediated adhesion.
To further investigate the molecular basis of platelet adhesion and spreading on laminin, we compared the pattern of protein tyrosine phosphorylation using whole-cell lysates and specific proteins of adherent platelet on laminin in control and GPVI-deficient platelets. Adhesion and spreading of platelets on laminin was associated with a marked increase in tyrosine phosphorylation in the whole-cell lysate through a pathway that is dependent on the GPVI-FcR γ chain complex as demonstrated using both GPVI (Figure 5A) and FcR γ chain knockout platelets (not shown). Immunoprecipitation studies revealed that proteins that undergo an increase in tyrosine phosphorylation upon adhesion to laminin are present in the GPVI signaling cascade, including FcR γ chain, the tyrosine kinases Syk and Btk, the adapter proteins LAT and SLP-76, and PLCγ2 (Figure 5B). Tyrosine phosphorylation of this group of proteins was not detectable in the GPVI knockout platelets, with the exceptions of Syk and PLCγ2, which underwent a markedly reduced increase in phosphorylation of 1.2- and 3-fold, respectively (Figure 5B). These results thereby demonstrate a critical role for GPVI in mediating tyrosine phosphorylation of this group of proteins upon adhesion to laminin. The small increases in tyrosine phosphorylation of Syk and PLCγ2 that are seen in the absence of GPVI may reflect regulation downstream of integrin α6β1 by laminin, consistent with the observation that both proteins are regulated by integrins α2β1 and αIIbβ3 in platelets.6,7,20
Spreading but not adhesion of mouse platelets on laminin is dependent on FcR γ chain. (A) Washed murine platelets (3 × 107/mL) from control or FcR γ chain–deficient mice (FcR γ chain-/-) were pretreated with or without 1 mM GRGDS peptide. Coverslips were coated with 50 μg/mL laminin and blocked with fatty acid–free BSA as described in Figure 1. BSA-coated coverslips were used for negative control. GRGDS-treated platelets were seeded on BSA- or laminin-coated coverslips for 30 minutes at room temperature. After unbound platelets were removed, platelets were fixed, stained, and photographed as described in Figure 1. (B) (i) The number of adherent platelets was counted. The graph illustrates the mean number of adherent platelets ± SEM per 0.006 mm2 from at least 8 different images from 3 separate experiments. (ii) Total surface coverage of adherent platelets on laminin-coated surfaces was measured. The graph illustrates the percent wild-type surface coverage ± SEM per 0.006 mm2 from at least 8 different images from 3 separate experiments. **P < .01.
Spreading but not adhesion of mouse platelets on laminin is dependent on FcR γ chain. (A) Washed murine platelets (3 × 107/mL) from control or FcR γ chain–deficient mice (FcR γ chain-/-) were pretreated with or without 1 mM GRGDS peptide. Coverslips were coated with 50 μg/mL laminin and blocked with fatty acid–free BSA as described in Figure 1. BSA-coated coverslips were used for negative control. GRGDS-treated platelets were seeded on BSA- or laminin-coated coverslips for 30 minutes at room temperature. After unbound platelets were removed, platelets were fixed, stained, and photographed as described in Figure 1. (B) (i) The number of adherent platelets was counted. The graph illustrates the mean number of adherent platelets ± SEM per 0.006 mm2 from at least 8 different images from 3 separate experiments. (ii) Total surface coverage of adherent platelets on laminin-coated surfaces was measured. The graph illustrates the percent wild-type surface coverage ± SEM per 0.006 mm2 from at least 8 different images from 3 separate experiments. **P < .01.
Spreading but not adhesion of mouse platelets on laminin is dependent on GPVI. (A) Washed murine platelets (3 × 107/mL) from control or GPVI-deficient mice (GPVI-/-) were pretreated with or without 1 mM GRGDS peptide in the presence or absence of 20 μg/mL anti–integrin α6 antibody. Laminin-coated coverslips were prepared as described in Figure 1. Platelets were seeded on laminin-coated coverslips for 30 minutes at room temperature. Adherent platelets were fixed, permeabilized, stained, and photographed using fluorescent microscopy as described in Figure 1. (B) The number of adherent platelets (i) and total surface coverage of adherent platelets on laminin (ii) were from at least 10 different images from 2 separate experiments.
Spreading but not adhesion of mouse platelets on laminin is dependent on GPVI. (A) Washed murine platelets (3 × 107/mL) from control or GPVI-deficient mice (GPVI-/-) were pretreated with or without 1 mM GRGDS peptide in the presence or absence of 20 μg/mL anti–integrin α6 antibody. Laminin-coated coverslips were prepared as described in Figure 1. Platelets were seeded on laminin-coated coverslips for 30 minutes at room temperature. Adherent platelets were fixed, permeabilized, stained, and photographed using fluorescent microscopy as described in Figure 1. (B) The number of adherent platelets (i) and total surface coverage of adherent platelets on laminin (ii) were from at least 10 different images from 2 separate experiments.
These results reveal that spreading on laminin is dependent on engagement of 2 receptors, the integrin α6β1 and the GPVI-FcR γ chain complex, whereas adhesion is primarily dependent on the former. Activation of the GPVI-FcR γ chain complex is the major mechanism underlying the increase in platelet tyrosine phosphorylation that is seen in platelets that have undergone spreading on laminin.
Spreading on laminin is not due to contamination with collagen
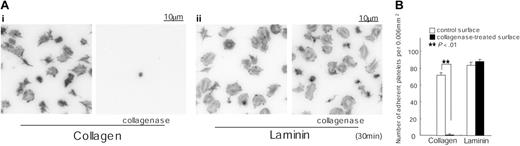
We considered the possibility that the ability of the purified preparation of placental laminin to support spreading through a GPVI-dependent mechanism is due to the presence of a residual amount of collagen in the sample. To address this, we incubated laminin-coated surfaces with 0.1 mg/mL collagenase for 2 hours. A collagen-coated surface was used as a control. Adhesion of murine platelets to the collagen-coated surface was abolished following treatment with collagenase, whereas spreading and adhesion on laminin were not altered (Figure 6). These observations provide strong evidence against a role for contaminating collagen in mediating spreading on laminin and thereby raise the possibility that GPVI is also a laminin receptor. Significantly, the ability of a laminin monolayer to interact stably with GPVI is dependent on engagement of α6β1. This dependency is similar to the role of integrin α2β1 in producing a net increase in the affinity of collagen to the low-affinity receptor, GPVI,3 that enables the adhesion molecule to form a stable interaction with GPVI.
Laminin-induced protein phosphorylation is dependent on GPVI. Washed murine platelets (4 × 108/mL) from control or GPVI-deficient mice (GPVI-/-) were pretreated with 1 mM GRGDS peptide prior to adhesion to laminin as described in Figure 1. BSA-coated dishes were used for negative control; 300 μL of washed platelets were seeded on dishes for the indicated times at room temperature prior to stopping the reaction by addition of 300 μL of 2 × lysis buffer. (A) Proteins were separated by 6% to 20% gradient SDS-PAGE and protein-tyrosine phosphorylation visualized by Western blotting with 4G10. Protein loading was measured by Western blotting with anti-PLCγ2 pAb. (B) Fc receptor γ chain (i), SLP-76 (ii), LAT (iii), Btk (iv), Syk (v), or PLCγ2 (vi) were isolated by immunoprecipitation using specific antibodies before blotting with 4G10. Gels were reprobed with the antibody that was used in the immunoprecipitation studies. Tyrosine phosphorylation of Syk and PLCγ2 were quantified using Quantity One software for Macintosh. Optical density measurements were standardized by the recruitments of these proteins. The optical densities are shown above the corresponding lanes. The results are representative of 2 experiments.
Laminin-induced protein phosphorylation is dependent on GPVI. Washed murine platelets (4 × 108/mL) from control or GPVI-deficient mice (GPVI-/-) were pretreated with 1 mM GRGDS peptide prior to adhesion to laminin as described in Figure 1. BSA-coated dishes were used for negative control; 300 μL of washed platelets were seeded on dishes for the indicated times at room temperature prior to stopping the reaction by addition of 300 μL of 2 × lysis buffer. (A) Proteins were separated by 6% to 20% gradient SDS-PAGE and protein-tyrosine phosphorylation visualized by Western blotting with 4G10. Protein loading was measured by Western blotting with anti-PLCγ2 pAb. (B) Fc receptor γ chain (i), SLP-76 (ii), LAT (iii), Btk (iv), Syk (v), or PLCγ2 (vi) were isolated by immunoprecipitation using specific antibodies before blotting with 4G10. Gels were reprobed with the antibody that was used in the immunoprecipitation studies. Tyrosine phosphorylation of Syk and PLCγ2 were quantified using Quantity One software for Macintosh. Optical density measurements were standardized by the recruitments of these proteins. The optical densities are shown above the corresponding lanes. The results are representative of 2 experiments.
Further studies were designed to confirm that laminin is able to interact directly with GPVI through the use of recombinant extracellular domain of human GPVI expressed either as a monomeric (Myc-His6) tag fusion protein (GPVIex) or as a dimeric human immunoglobin Fc domain fusion protein (GPVI-Fc2).16 These experiments were carried alongside similar studies on collagen, which serve as control.
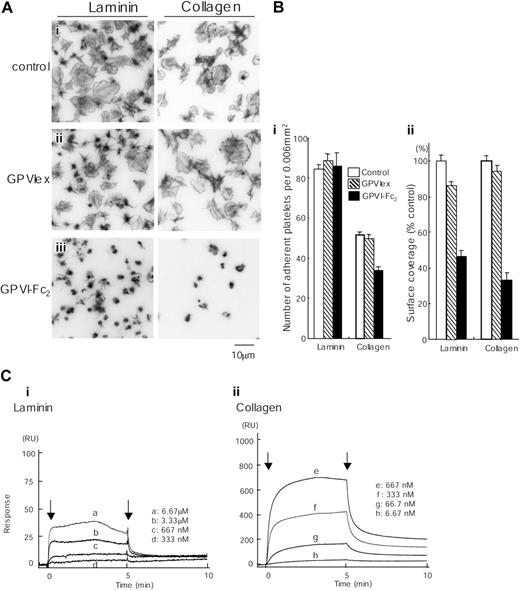
GPVI-Fc2 strongly inhibits collagen-induced platelet aggregation, whereas GPVIex does not, indicating that collagen binds preferentially to the dimeric form of the molecule.16 GPVI-Fc2 (100 μg/mL) but not monomeric GPVIex (100 μg/mL) also markedly inhibited spreading on collagen and partially (approximately 30%) reduced adhesion to collagen, although a limited degree of formation of filopodia remained (Figure 7A-B). The GPVI-independent adhesion and limited formation of filopodia is most likely mediated through the interaction of collagen with integrin α2β1.7 GPVI-Fc2 also markedly inhibited spreading on laminin, reducing the surface area of the adhered platelets by over 50%. In contrast, GPVIex had only a small inhibitory effect on the degree of spreading, reducing the degree of coverage by just over 10%. Neither recombinant GPVI isoform had an inhibitory effect on adhesion to laminin. These results are consistent with observations in GPVI-deficient murine platelets and strongly suggest that laminin induces formation of lamellipodia in both mouse and human platelets through engagement of GPVI, even though binding is dependent on integrin α6β1.
Adhesion to collagen but not laminin is inhibited by prior treatment with collagenase. (A) Coverslips were coated with 50 μg/mL collagen (i) or 50 μg/mL laminin (ii) as described in Figure 1. After rinsing with PBS, coverslips were incubated with 0.1 mg/mL collagenase in TES buffer containing 0.36 mM CaCl2 for 2 hours at 35°C. After washing with PBS, coverslips were blocked with 2% fatty acid–free BSA for 2 hours. Washed murine platelets (3 × 107/mL) were incubated with 2 mM GRGDS peptide and seeded on coverslips for 30 minutes at room temperature. Adherent platelets were fixed, permeabilized, stained, and photographed using fluorescent microscopy as described in Figure 1. (B) The number of adherent platelets was counted as described in Figure 3.
Adhesion to collagen but not laminin is inhibited by prior treatment with collagenase. (A) Coverslips were coated with 50 μg/mL collagen (i) or 50 μg/mL laminin (ii) as described in Figure 1. After rinsing with PBS, coverslips were incubated with 0.1 mg/mL collagenase in TES buffer containing 0.36 mM CaCl2 for 2 hours at 35°C. After washing with PBS, coverslips were blocked with 2% fatty acid–free BSA for 2 hours. Washed murine platelets (3 × 107/mL) were incubated with 2 mM GRGDS peptide and seeded on coverslips for 30 minutes at room temperature. Adherent platelets were fixed, permeabilized, stained, and photographed using fluorescent microscopy as described in Figure 1. (B) The number of adherent platelets was counted as described in Figure 3.
Spreading of human platelets on laminin is mediated through GPVI. (A) Coverslips were coated with 50 μg/mL laminin or 50 μg/mL collagen and blocked with 2% BSA. Washed human platelets (2 × 107/mL) were pretreated with PBS (i), 100 μg/mL GPVIex (ii), or 100 μg/mL GPVI-Fc2 (iii) in the presence of 1 mM GRGDS. Platelets were seeded on BSA-, laminin- or collagen-coated coverslips for 30 minutes at room temperature. Unbound platelets were removed, and adhered platelets were fixed, stained, and visualized as described in Figure 1. (B) (i) The number of adherent platelets was counted. The graph illustrates the mean number of adherent platelets ± SEM per 0.006 mm2 from at least 8 different images in 1 experiment, which is representative of 2 separate experiments. (ii) Total surface coverage of adherent platelets on laminin- or collagen-coated surfaces was measured. The graph illustrates the percent control surface coverage ± SEM per 0.006 mm2 from at least 8 different images in 1 experiment, which is representative of 2 experiments. (C) Different concentrations of GPVI-Fc2 were flowed over an immobilized laminin (i), collagen (ii), or a control surface. The arrows indicate the beginning and end of perfusion of GPVI-Fc2. The results are shown from 1 experiment that is representative of 4 others. RU indicates resonance units.
Spreading of human platelets on laminin is mediated through GPVI. (A) Coverslips were coated with 50 μg/mL laminin or 50 μg/mL collagen and blocked with 2% BSA. Washed human platelets (2 × 107/mL) were pretreated with PBS (i), 100 μg/mL GPVIex (ii), or 100 μg/mL GPVI-Fc2 (iii) in the presence of 1 mM GRGDS. Platelets were seeded on BSA-, laminin- or collagen-coated coverslips for 30 minutes at room temperature. Unbound platelets were removed, and adhered platelets were fixed, stained, and visualized as described in Figure 1. (B) (i) The number of adherent platelets was counted. The graph illustrates the mean number of adherent platelets ± SEM per 0.006 mm2 from at least 8 different images in 1 experiment, which is representative of 2 separate experiments. (ii) Total surface coverage of adherent platelets on laminin- or collagen-coated surfaces was measured. The graph illustrates the percent control surface coverage ± SEM per 0.006 mm2 from at least 8 different images in 1 experiment, which is representative of 2 experiments. (C) Different concentrations of GPVI-Fc2 were flowed over an immobilized laminin (i), collagen (ii), or a control surface. The arrows indicate the beginning and end of perfusion of GPVI-Fc2. The results are shown from 1 experiment that is representative of 4 others. RU indicates resonance units.
We further investigated the interaction of laminin with GPVI-Fc2 using BIAcore. GPVI-Fc2 was perfused over the laminin-coated sensor tip as described in “Materials and methods.” The sensorgrams at different analyte concentrations were obtained and normalized by subtracting background signals from the laminin surface (Table 1). The arrows indicate the beginning and end of perfusion of GPVI-Fc2. The Kd for the interaction of laminin with GPVI-Fc2 was calculated as 6.4 μM, which is more than 10-fold lower than that between GPVI-Fc2 and collagen (Figure 7C). This is likely to account for the inability of the laminin surface to support adhesion in the presence of a blocking antibody to α6β1.
Dissociation constants of GPVI-FC2
Ligand . | Kd . |
|---|---|
| Laminin | 6.36 × 10-6 ± 1.44 × 10-6 |
| Collagen | 5.67 × 10-7 ± 0.73 × 10-7 |
Ligand . | Kd . |
|---|---|
| Laminin | 6.36 × 10-6 ± 1.44 × 10-6 |
| Collagen | 5.67 × 10-7 ± 0.73 × 10-7 |
Kd plus or minus SEM of GPVI-FC2 and each ligand determined as described in “Materials and methods.”
Discussion
Although laminin is richly expressed in the basement membrane of the vasculature, its overall significance in hemostasis and thrombosis remains unclear. Recently, the major platelet receptor for laminin, α6β1, together with the receptor for fibronectin, α5β1, was demonstrated to mediate platelet adhesion under arterial rates of flow in the absence of functional α2β1 and αIIbβ311 Thus, the laminin receptor, α6β1, plays an important role in combination with other platelet integrins in supporting shear-resistant adhesion at arterial rates of flow. In the present study, we demonstrate that laminin is also able to promote platelet spreading through the GPVI-FcR γ chain complex via a pathway that is dependent on binding to integrin α6β1 Thus, laminin is able to support both platelet adhesion and platelet activation, thereby further emphasizing its role alongside that of collagen in supporting platelet activation at sites of damage to the vasculature.
The present observations confirm and extend a much earlier study by Hindriks et al who reported that platelets spread when perfused over laminin at a shear rate of 300/s.24 On the other hand, a number of other groups have reported that laminin is unable to promote platelet spreading.25,26 These discrepant reports may reflect differences in the type of laminin, because many of the previous studies used murine laminin that had been isolated from an Englebreth-Holm-Swarm (EHS) tumor cell.24,25,27 EHS-laminin has been reported to have aberrant glycosylation, which could interfere with receptor-ligand interactions.28,29 In the present study, we used human laminin that had been purified from human placenta.
Laminin stimulates platelet spreading through binding to GPVI via a pathway that is dependent on integrin α6β1 This mechanism of platelet adhesion and activation is similar to the interaction of platelets with collagen. In the latter case, adhesion of platelets to collagen is initiated through inside-out activation of α2β1 by GPVI and through direct binding to α2β1 independent of activation.7,30 Significantly, binding of collagen to α2β1 facilitates the interaction with GPVI.7,.31-33 In contrast, we have shown that adhesion of platelets to laminin is not altered in the absence of GPVI, demonstrating that under static conditions inside-out signals are not required to promote the interaction of laminin with integrin α6β1. The binding of laminin to α6β1, however, is essential for a stable interaction with GPVI, because an anti–α6-blocking antibody completely inhibited platelet adhesion to laminin. This therefore indicates that the binding affinity of GPVI for laminin is too weak to support stable adhesion, even under static conditions.
The α6β1-dependent interaction of laminin with GPVI leads to tyrosine phosphorylation of the same set of signaling molecules as those regulated by collagen, including FcR γ chain, Syk, LAT, SLP-76, Btk, and PLCγ2. Phosphorylation of all of these proteins is abolished or markedly reduced in the absence of the GPVI-FcR γ chain complex, demonstrating that phosphorylation is primarily regulated by the immunoglobulin receptor. The residual phosphorylation of Syk and PLCγ2 that is seen in the absence of GPVI is presumably mediated through clustering of α6β1, because other platelet integrins—namely, α2β1 and αIIbβ3—have been shown to regulate PLCγ2 downstream of Syk.6,7 Engagement of GPVI by laminin, however, induces a much greater level of tyrosine phosphorylation of all proteins, including Syk and PLCγ2, to that induced by integrin α6β1, consistent with the observation that lamellipodia formation is also dependent on engagement of the immunoglobulin receptor.
Despite the ability of laminin to stimulate spreading via GPVI, it is noteworthy that laminin is unable to induce aggregation or tyrosine phosphorylation in suspensions of human or mouse platelets (data not shown). In contrast, collagen promotes robust aggregation and marked tyrosine phosphorylation in platelet suspensions through a pathway that is critically dependent on GPVI. The inability of laminin to promote activation of platelets, despite binding to GPVI, is similar to a previous set of observations made using the anti-GPVI mAb, JAQ1, or a non–cross-linked form of a collagen-related peptide, which contains a repeat sequence of the GPVI-binding motif, glycine-proline-hydroxyproline (GPO).34-36 Thus, it would seem that the interaction of these 2 ligands and also that of laminin with GPVI is insufficient to generate a sufficient strength of signal to promote aggregation in platelet suspensions. In the case of laminin, this is likely to be accounted for by the 10 times lower affinity of laminin for GPVI relative to collagen and also by a lower number of GPVI binding sites in laminin relative to collagen, as indicated by the 30-fold lower magnitude of response determined by BIAcore. This is further supported by the observation that platelets can adhere to a collagen monolayer through GPVI in the absence of integrin α2β1,33 whereas platelets are unable to bind to a monolayer of laminin in the absence of α6β1.
The interaction of GPVI with collagen is mediated through a GPO motif molecule.35 Significantly, there is no GPO triplet in laminin, thereby demonstrating that a distinct motif mediates the interaction with the immunoglobulin receptor. This is similar to the situation with the snake venom toxin, convulxin, which also lacks a GPO motif but is a powerful GPVI receptor agonist. Identification of the binding site in laminin supports interaction with GPVI is of considerable interest, and is presently under investigation.
The present results raise the possibility of a physiologic role for laminin in supporting platelet spreading in vivo may have been overlooked because of the more powerful interaction between collagen and GPVI. In particular, we predict that the interaction with laminin may have particular relevance upon mild damage of the vascular endothelial lining, because this leads to exposure of the laminin-rich basement membrane. The significance of this is emphasized by the observation that the major form of collagen in the basement membrane—namely, type IV—is able to only induce weak activation of platelets.37
The present study identifies laminin as a novel stimulatory component in the subendothelial matrix that, in addition to mediating platelet adhesion through binding to integrin α6β1, stimulates platelet spreading via GPVI. This observation adds to the growing recognition that thrombus formation is regulated through a network of overlapping and redundant interactions with a number of proteins in the subendothelial matrix and further emphasize the importance of GPVI in this process.
Prepublished online as Blood First Edition Paper, October 11, 2005; DOI 10.1182/blood-2005-06-2406.
Supported by the grants from the British Heart Foundation, the National Institutes of Health, and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (no. 17924029). S.P.W. holds a British Heart Foundation (BHF) Chair.
O.I. and K.S.-I. designed research, performed research, analyzed data, and wrote the paper; O.J.T.M. performed research; M.M. contributed vital reagents; Z.M.R. and T.J.K. contributed genetically modified mice; Y.O. designed research; and S.P.W. designed research, analyzed data, and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Jerry Ware, University of Arkansas for Medical Sciences, Little Rock, for the supply of GPVI-deficient mice, to Dr Yoshiki Miura for donating GPVIex and GPVI-Fc2, to Mr Yann Cheli for his support at the Scripps Research Institute, and to Edina Schweighoffer and Victor Tybulewicz for the supply of Syk-deficient mice. Gratitude is expressed to Mrs Chiaki Komatsu and Mrs Yumi Sakamoto for their excellent technical assistance with this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal