The causal relationship between HIV-specific CD4+ T-cell responses and viral control and the effect of these responses on the natural history of HIV infection is unclear. In a detailed longitudinal study, functional HIV-1 Gag-specific CD4+ T cells were analyzed in long-term asymptomatic individuals (LTA; n = 6) and progressors to AIDS (n = 7) with a median follow-up of, respectively, 118 and 57 months. Next, HIV-specific CD4+ T-helper cell responses were measured in a prospective cohort study among 96 HIV seroconverters and were related to clinical endpoints using Cox proportional hazard analyses. In the detailed study, no difference for HIV-specific helper-cell responses between LTAs and progressors was observed early in infection, but Gag-specific CD4+ T cells producing IL-2 or IFNγ were lost in progressors late in infection. Multivariate proportional hazard analyses in the prospective cohort study showed that HIV-specific IL-2+, IFNγ+, or IL-2+IFNγ+ CD4+ T cells early after seroconversion had no prognostic value for the rate of progression to AIDS. Our results are compatible with viral load determining the nature and magnitude of HIV-specific CD4+ T-cell responses, rather than HIV-specific CD4+ T-cell responses controlling HIV plasma viral load.
Introduction
HIV-specific CD4+ T-cell proliferative responses have been shown to be inversely related to viral load and were initially only observed in long-term nonprogressors (LTNPs) and in individuals who were treated during acute HIV-1 infection.1,2 More recently developed techniques, like intracellular cytokine staining (ICS) after antigen-induced proliferation showed that Gag-specific IFNγ-producing CD4+ T cells were detectable in almost all individuals infected with HIV-1, regardless of disease progression.3-5 Differences in frequencies of Gag-specific IFNγ+ CD4+ T cells were initially observed between LTNPs and individuals progressing to AIDS,6 but this observation could not be confirmed by others.3,4,7,8 Several reports have indicated that single IL-2-producing CD4+ T cells3,9 and HIV-specific CD4+ T cells producing both IFNγ and IL-210,11 on antigen activation in vitro were associated with immunologic memory and protective immunity from HIV disease progression. This is compatible with studies in mice in which memory CD4+ T cells are associated with long-term immunity to Leishmania.12
In acute13 and chronic HIV-1 infection,9 it has been clearly demonstrated that HIV-specific CD4+ T cells in viremic individuals are of the effector memory phenotype (Tem; CD27-CCR7-CD45RO+), whereas in aviremic individuals both central memory (Tcm; CD27+CCR7-CD45RO+) and effector memory cells were observed. On the basis of this observation, it has been hypothesized that functional HIV-specific CD4+ T cells of the central memory phenotype which produce IL-2 and have a high capacity to proliferate continuously arise but because of persistent viremia rapidly differentiate into effector memory CD4+ T cells, only capable of producing IFNγ and without self-renewal capacity.13,14
So far, in HIV infection only cross-sectional studies have been performed, in which the presence of memory CD4+ T-cell responses was correlated with cytotoxic T lymphocyte (CTL) function and/or HIV disease progression. Here, we used the unique material of the Amsterdam Cohort Studies (ACS) on HIV-1 infection and AIDS, which is a prospective cohort study with well-defined clinical endpoints, to investigate HIV-specific CD4+ T-cell function during the natural course of HIV-1 infection in selected long-term asymptomatics (LTAs) and progressors to AIDS. We found that progression to AIDS could not be prevented by the presence of strong HIV-specific CD4+ T-cell responses early after seroconversion, and this observation was confirmed by a large prospective study, including 96 patients of the same cohort.
Patients, materials, and methods
Subjects and samples
Thirteen HIV-1 seropositive individuals of the Amsterdam Cohort on HIV-1 infection and AIDS with a known date of seroconversion were selected based on HLA type.15 Peripheral-blood mononuclear cells (PBMCs) were cryopreserved according to a standard protocol in a computerized freezing device. During follow-up, all individuals remained treatment naive. Within the Amsterdam cohort, long-term asymptomatic individuals (LTAs) were classified based on a nested case-cohort study by at least 9 years of AIDS-free follow-up and a mean CD4+ T-cell count of more than 400 cells/μL during the 8th to 9th year following seroconversion. Progressors were classified by the occurrence of AIDS-defining illnesses within 2 to 7 years after seroconversion.16 We selected 6 LTAs and 7 individuals who developed AIDS (progressors), as was defined by the occurrence of AIDS-defining illnesses (Table 1). None of them were diagnosed with CMV-related diseases. One of the LTAs remained symptom free but had a CD4 count of fewer than 400 cells/μL during the 9th year of follow-up. Based on the lack of symptoms, this individual was included in the LTA group. For characteristics of the study participants see Table 1. Individuals were analyzed at an early time point (12 months after seroconversion) and a late time point (for LTAs at a median follow-up of 118 months; range, 82-148 months), for progressors at a median follow-up of 57 months (range, 25-65 months) after HIV seroconversion. In addition, 10 HIV negative healthy blood bank donors were included as control subjects.
Characteristics of the study participants
. | . | . | CD4 counts, 106 cells/mL . | . | CD8 counts, 106 cells/mL . | . | Viral load, copies/mL . | . | % Gag-specific CFSElow CD3+CD4+ T cells . | . | % CMV-specific CFSElow CD3+CD4+ T cells . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group and subject ID . | Follow-up*time after sc, mo . | AIDS-defining illness . | Early . | Late . | Early . | Late . | Early . | Late . | Early . | Late . | Early . | Late . | |||||
| CTA | |||||||||||||||||
| 57 | 122 | NA | 0.68 | 0.47 | 1.40 | 4.40 | 1 550 | 1 000 | 4.3 | 4.9 | 3.5 | 59.3 | |||||
| 658 | 124 | NA | 1.21 | 0.51 | 0.80 | 1.16 | 450 000 | 24 000 | 2.4 | 4.2 | 1.5 | 3.4 | |||||
| 1160 | 111 | NA | 0.67 | 0.28 | 0.90 | 1.25 | 12 000 | 11 550 | 1.9 | 0.4 | 2.0 | 4.3 | |||||
| 126 | 148 | NA | 0.55 | 0.46 | 1.00 | 1.31 | 1 000 | 110 | 1.0 | 1.0 | 10.8 | 18.8 | |||||
| 44 | 114 | NA | 0.47 | 0.37 | 0.80 | 1.02 | 1 000 | 1 000 | 1.2 | 1.4 | 2.4 | 4.3 | |||||
| 1171 | 82 | NA | 0.77 | 0.44 | 1.70 | 0.86 | 5 000 | 1 000 | 1.7 | 15.4 | 9.2 | 53.3 | |||||
| Median | 118 | NA | 0.68 | 0.47 | 0.95 | 1.21 | 3 275 | 1 000 | 1.8 | 2.8 | 2.9 | 11.6 | |||||
| Progressors | |||||||||||||||||
| 159 | 65 | Pneumocystis carinii | 0.59 | 0.21 | 1.40 | 2.00 | 180 000 | 160 000 | 0.8 | ND | 3.8 | 2.1 | |||||
| 748 | 63 | Microsporidiosis | 0.45 | 0.22 | 0.70 | 0.92 | 160 000 | 100 000 | 1.1 | 1.1 | 30.8 | 20.2 | |||||
| 656 | 60 | Cryptosporidiasis | 0.24 | 0.12 | 0.30 | 0.71 | 52 000 | 25 000 | 8.5 | 0.9 | ND | 14.4 | |||||
| 1211 | 25 | Cryptococcal meningitis | 0.7 | 0.18 | 1.00 | 0.50 | 590 000 | 76 000 | 5.1 | 1.3 | 14.1 | 12.0 | |||||
| 547 | 32 | P carinii | 0.87 | 0.3 | 0.60 | 0.60 | 5 500 | 13 000 | 1.7 | 0.2 | 39.1 | 47.0 | |||||
| 208 | 48 | P carinii | 0.79 | 0.04 | 0.80 | 1.30 | 20 000 | 640 000 | 0.8 | 1.2 | 1.0 | 0.9 | |||||
| 1234 | 57 | P carinii | 0.86 | 0.39 | 1.60 | 1.96 | 33 000 | 360 000 | 1.2 | 0.3 | 12.6 | 8.2 | |||||
| Median | 57 | NA | 0.70 | 0.21 | 0.80 | 0.92 | 52 000 | 76 000 | 1.2 | 1.0 | 13.3 | 12.1 | |||||
. | . | . | CD4 counts, 106 cells/mL . | . | CD8 counts, 106 cells/mL . | . | Viral load, copies/mL . | . | % Gag-specific CFSElow CD3+CD4+ T cells . | . | % CMV-specific CFSElow CD3+CD4+ T cells . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group and subject ID . | Follow-up*time after sc, mo . | AIDS-defining illness . | Early . | Late . | Early . | Late . | Early . | Late . | Early . | Late . | Early . | Late . | |||||
| CTA | |||||||||||||||||
| 57 | 122 | NA | 0.68 | 0.47 | 1.40 | 4.40 | 1 550 | 1 000 | 4.3 | 4.9 | 3.5 | 59.3 | |||||
| 658 | 124 | NA | 1.21 | 0.51 | 0.80 | 1.16 | 450 000 | 24 000 | 2.4 | 4.2 | 1.5 | 3.4 | |||||
| 1160 | 111 | NA | 0.67 | 0.28 | 0.90 | 1.25 | 12 000 | 11 550 | 1.9 | 0.4 | 2.0 | 4.3 | |||||
| 126 | 148 | NA | 0.55 | 0.46 | 1.00 | 1.31 | 1 000 | 110 | 1.0 | 1.0 | 10.8 | 18.8 | |||||
| 44 | 114 | NA | 0.47 | 0.37 | 0.80 | 1.02 | 1 000 | 1 000 | 1.2 | 1.4 | 2.4 | 4.3 | |||||
| 1171 | 82 | NA | 0.77 | 0.44 | 1.70 | 0.86 | 5 000 | 1 000 | 1.7 | 15.4 | 9.2 | 53.3 | |||||
| Median | 118 | NA | 0.68 | 0.47 | 0.95 | 1.21 | 3 275 | 1 000 | 1.8 | 2.8 | 2.9 | 11.6 | |||||
| Progressors | |||||||||||||||||
| 159 | 65 | Pneumocystis carinii | 0.59 | 0.21 | 1.40 | 2.00 | 180 000 | 160 000 | 0.8 | ND | 3.8 | 2.1 | |||||
| 748 | 63 | Microsporidiosis | 0.45 | 0.22 | 0.70 | 0.92 | 160 000 | 100 000 | 1.1 | 1.1 | 30.8 | 20.2 | |||||
| 656 | 60 | Cryptosporidiasis | 0.24 | 0.12 | 0.30 | 0.71 | 52 000 | 25 000 | 8.5 | 0.9 | ND | 14.4 | |||||
| 1211 | 25 | Cryptococcal meningitis | 0.7 | 0.18 | 1.00 | 0.50 | 590 000 | 76 000 | 5.1 | 1.3 | 14.1 | 12.0 | |||||
| 547 | 32 | P carinii | 0.87 | 0.3 | 0.60 | 0.60 | 5 500 | 13 000 | 1.7 | 0.2 | 39.1 | 47.0 | |||||
| 208 | 48 | P carinii | 0.79 | 0.04 | 0.80 | 1.30 | 20 000 | 640 000 | 0.8 | 1.2 | 1.0 | 0.9 | |||||
| 1234 | 57 | P carinii | 0.86 | 0.39 | 1.60 | 1.96 | 33 000 | 360 000 | 1.2 | 0.3 | 12.6 | 8.2 | |||||
| Median | 57 | NA | 0.70 | 0.21 | 0.80 | 0.92 | 52 000 | 76 000 | 1.2 | 1.0 | 13.3 | 12.1 | |||||
For early CD4 counts, P > .999; for late CD4 counts, P = .008; for early CD8 counts, P = .37; for late CD8 counts, P = .46; for early viral load, P = .05; for late viral load, P = .002; for early Gag-specific CFSElow CD3+CD4+ T cells, P = .63; for late Gag-specific CFSElow CD3+CD4+ T cells, P = .13; for early CMV-specific CFSElow CD3+CD4+ T cells, P = .13; and for late CMV-specific CFSElow CD3+CD4+ T cells, P = .63.
ND indicates not done; and NA, not applicable.
Follow-up in months after seroconversion
P values indicate the differences between the 2 groups at each time point (Mann-Whitney)
To analyze a possible protective role of functional HIV-specific CD4+ T cells in progression to AIDS, a prospective study was performed in 96 participants of the ACS with a documented date of seroconversion. This seroconverter cohort has been described earlier17,18 (www.amsterdamcohortstudies.org). Follow-up time was censored at July 1, 1997, to prevent the inclusion of subjects receiving highly active antiretroviral therapy (HAART). Median follow-up to AIDS diagnosis or censoring was 82.7 months (range, 14.9-153.9 months) from seroconversion and 70.1 months (range, 11.9-136.7 months) from 1 year after seroconversion. Forty of the 96 participants progressed to AIDS during follow-up. Informed written consent was obtained from all participants, and this study was approved by the Medical Ethical Committee of the Academic Medical Center.
Intracellular cytokine staining after antigenic stimulation
Intracellular cytokine staining was performed as previously described.19 Briefly, cryopreserved PBMCs were thawed, divided into aliquots at 2 × 106 cells/mL, and stimulated with a Gag-peptide pool (15 mers with 11 overlap, final concentration of the individual peptides was 2 μg/mL, HXB2; National Institutes of Health [NIH] AIDS Research and Reagent program, Bethesda, MD) in the presence of costimulation (2 μg/mL αCD28; Sanquin Reagents, Amsterdam, The Netherlands) and 2 μg/mL αCD49d (Pharmingen, San Jose, CA) for 6 hours at 37°C, 5% CO2. After 1 hour, Brefeldin A (Becton Dickinson [BD], San Jose, CA) was added at a final concentration of 1 μg/mL. Next, cells were stained with CD27-biotin (Sanquin Reagents) followed by streptavidin-coupled APC-Cy7 (BD) and CD45RO-APC (Pharmingen, San Diego, CA). After fixation and permeabilization (permeabilization reagents; BD) cells were stained with CD3-PerCP, CD4-APC-Cy7, IL-2-PE, and IFNγ-FITC monoclonal antibodies (BD) for 20 minutes at 4 °C. Cells were then fixed in Cellfix (BD), and 6-color flow cytometry was performed. At least 300 000 events were acquired using an LSRII flow cytometer (BD). Lymphocytes were gated by forward and sideward scatter. Data were analyzed using the software program CELL Quest (BD). Frequencies of cytokine-producing cells were reported after subtraction of the frequencies in medium controls. Cytokine production was above the background levels observed in individuals without HIV (median, 0.02% of CD4+ T cells; data not shown). Absolute numbers per volume blood were calculated by multiplication of the fraction of cytokine-positive CD4+ T cells with the absolute T-cell count per microliter of blood.
Antigen-specific proliferation
In vitro T-cell proliferation to HIV peptide pools was measured as previously described19 using the fluorescent, cell-permeable dye CFSE (5,6-carboxyfluorescein diacetate succinimidyl ester; Molecular Probes, Leiden, The Netherlands) according to the manufacturer's protocol. Briefly, PBMCs were thawed, and cells were incubated with CFSE for 8 minutes, after which labeling was stopped using Human Pool Serum (HPS). Cells were washed, and 2 × 106 cells in 0.5 mL RPMI-1640 medium were divided into aliquots in round-bottom tubes (polystyrene; Falcon, BD). Cells were then stimulated with either a Gag-derived peptide pool (concentration of individual peptides in pool 2 μg/mL) or a 1/100 dilution of CMV lysate (Microbix Biosystems, Toronto, ON, Canada). As a positive control, a combination of 0.2 μg/μL αCD3 (Sanquin Reagents) and 2 μg/μL αCD28 (Sanquin Reagents) was used. After 6 days of incubation at 37°C, 5% CO2, cells were stained using CD4-APC and CD3-PerCP monoclonal antibodies (BD) and analyzed using a fluorescence-activated cell sorting (FACS) calibur. Percentages of proliferation greater than 0.7% were considered positive, based on a median percentage HIV-1 Gag-specific CFSElow CD4+ T cells of 0.7% in PBMCs from HIV seronegative individuals stimulated with the Gag-peptide pool.
Statistical analyses
Nonparametric statistical tests were used because the assumption of normally distributed data was not met. Differences between the patient groups were analyzed using Mann-Whitney tests. Wilcoxon tests were performed to determine differences in HIV-specific CD4+ T-cell percentages and numbers between early and late time points. Correlations were tested using the nonparametric Spearman correlation test. The predictive value of HIV-specific cytokine-producing CD4+ T cells for progression to AIDS was studied using Cox proportional hazards analyses and Kaplan-Meier survival curves. HIV-specific CD4+ T cells producing IFNγ, IL-2, or IL-2 and IFNγ were analyzed. Each individual marker was first tested univariately. In multivariate analyses, each marker was adjusted for baseline log plasma HIV-1 RNA (as a continuous variable) and square root CD4+ T-cell counts (as a continuous variable)20 and in addition for expression of Ki67 on CD4+ T cells (2 categories), expression of CD38 on CD4+ T cells (2 categories), or coexpression of CD38 and HLA-DR on CD8+ T cells (2 categories). Expression of Ki67 and CD38 on CD4+ T cells and coexpression of CD38 and HLA-DR on CD8+ T cells have all been previously shown to be markers of T-cell activation predictive for progression to AIDS.18 A P value of .05 was considered statistically significant. All statistical analyses were performed using the software program SPSS 11.5 (SPSS, Chicago, IL).
Results
Comparable numbers of Gag-specific cytokine-producing CD4+ T cells in LTAs and progressors early in infection
First, a detailed study was performed in 13 patients infected with HIV of whom 7 rapidly progressed to AIDS (progressors) and 6 remained asymptomatic for at least 9 years. One year after seroconversion, LTAs and progressors showed no difference in peripheral-blood CD4+ T-cell and CD8+ T-cell numbers, but in progressors a higher viral load was found (Table 1). Late in infection, progressors had lower CD4+ T-cell numbers and a higher viral load compared with LTAs (Table 1).
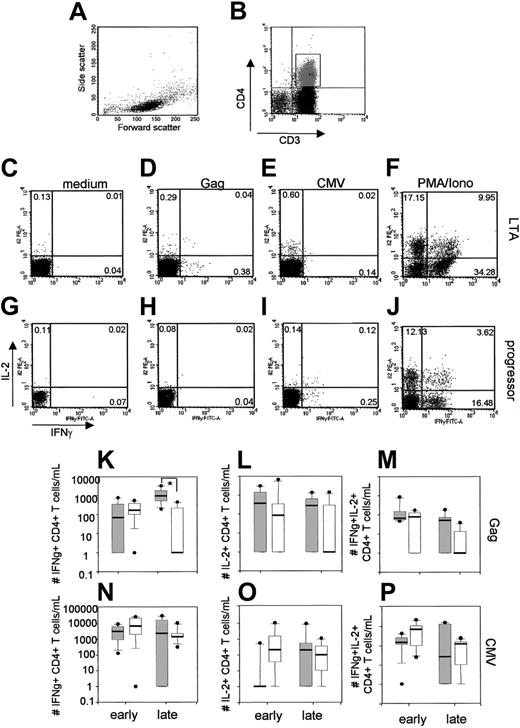
Gag-specific single IFNγ+, single IL-2+, or IL-2+IFNγ+ CD4+ T cells were analyzed (for a representative example see Figure 1D,H). We calculated the absolute numbers of cytokine-producing CD4+ T cells, because this may be a more informative read-out than percentages as we have shown previously.19 One year after seroconversion, HIV-specific cytokine-producing CD4+ T cells were detected in all progressors (median number of IFNγ+CD4+ T cells, 174 cells/mL; IL-2+CD4+ T cells, 87 cells/mL; and IL-2+IFNγ+CD4+ T cells, 79 cells/mL; Figure 1K-M) and were not significantly different from the number observed in LTAs. In LTAs, the median number of HIV-specific single IFNγ+CD4+ T cells tended to increase during HIV-1 infection (from 68 to 950 cells/mL, P = .23; Figure 1K), whereas median numbers of single IL-2+CD4+ T cells decreased (from 379 cells/mL to 283 cells/mL, P = .04; Figure 1L) and of IL-2+IFNγ+ CD4+ T cells did not change (from 68 to 52 cells/mL; P = .69; Figure 1M). Although LTAs and progressors initially had equal numbers of Gag-specific single IL-2+ and IL-2+IFNγ+ CD4+ T cells, these numbers decreased in individuals who progressed to AIDS. Late in infection, single IFNγ+CD4+ T cells were only detected in 4 of 7 progressors, resulting in a significant difference between LTAs and progressors (P < .01, Mann-Whitney), whereas single IL-2+ and IL-2+IFNγ+ CD4+ T cells were only found in 3 of 7 progressors. In contrast, CMV-specific CD4+ T-cell cytokine production did not decrease in progressors during HIV-infection (Figure 1N-P).
Loss of HIV-specific CD4+ T cells and progression to AIDS despite high levels of HIV-specific cytokine-producing CD4+ T cells early in infection. Representative FACS plots showing the lymphocyte gate (A), gating of the CD3+CD4+ T cells (B), production of IFNγ and IL-2 of CD3+CD4+ T cells in the absence of stimulus (C,G), after stimulation with a Gag-peptide pool (D,H), CMV lysate (E,I), and PMA/ionomycin as a positive control (F,J). Percentages of cytokine-producing CD3+CD4+ T cells are indicated. Results of HIV-specific single IFNγ+ CD4+ T cells, single IL-2+ CD4+ T cells, and IL-2+ and IFNγ+ CD4+ T cells are summarized in box plots and depicted in panels K to M. CMV-specific single IFNγ+ CD4+ T cells, single IL-2+ CD4+ T cells, and IL-2+ and IFNγ+ CD4+ T cells are summarized in panels N to P. Each box summarizes results of 6 LTAs (gray) or 7 progressors (white). The horizontal bar represents the median, the box length represents the interquartile range, and the error bars show the outliers.
Loss of HIV-specific CD4+ T cells and progression to AIDS despite high levels of HIV-specific cytokine-producing CD4+ T cells early in infection. Representative FACS plots showing the lymphocyte gate (A), gating of the CD3+CD4+ T cells (B), production of IFNγ and IL-2 of CD3+CD4+ T cells in the absence of stimulus (C,G), after stimulation with a Gag-peptide pool (D,H), CMV lysate (E,I), and PMA/ionomycin as a positive control (F,J). Percentages of cytokine-producing CD3+CD4+ T cells are indicated. Results of HIV-specific single IFNγ+ CD4+ T cells, single IL-2+ CD4+ T cells, and IL-2+ and IFNγ+ CD4+ T cells are summarized in box plots and depicted in panels K to M. CMV-specific single IFNγ+ CD4+ T cells, single IL-2+ CD4+ T cells, and IL-2+ and IFNγ+ CD4+ T cells are summarized in panels N to P. Each box summarizes results of 6 LTAs (gray) or 7 progressors (white). The horizontal bar represents the median, the box length represents the interquartile range, and the error bars show the outliers.
HIV-specific CD4+ T-cell proliferative responses were readily detected early in infection, in LTAs (median percentage CFSElow HIV-specific CD4+ T cells, 1.8%; range, 1.0%-2.4%) and progressors (median percentage CFSElow HIV-specific CD4+ T cells, 1.2%; range, 0.8%-8.5%). Late in HIV infection, proliferative responses had increased only in the LTA group, although this increase was not significant (median percentage CFSElow HIV-specific CD4+ T cells, 2.8%; range 0.4%-15.4%; P = .17, Wilcoxon signed rank) (Table 1). Analyzing the proliferation data by calculating stimulation indices (divide the percentage of proliferation of CD3+CD4+ T cells after stimulation by the percentage of proliferation in the unstimulated sample) gave similar results (data not shown).
Phenotypical analysis of virus-specific CD4+ T cells in the course of HIV-1 infection
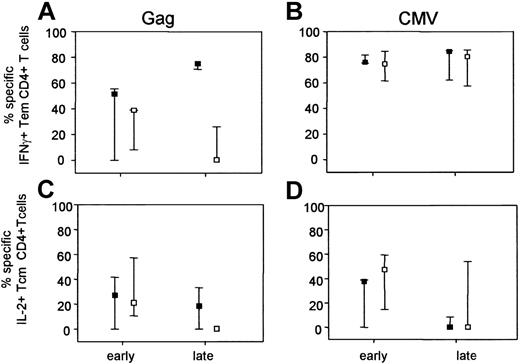
Phenotypes of functional subsets of cytokine-producing CD4+ T cells were compared between LTAs and progressors, with the use of the differentiation markers CD27 and CD45RO. With these markers, one can distinguish 4 populations, designated naive (CD27+CD45RO-), central memory (Cm; CD27+CD45RO+), effector memory (Em: CD27-CD45RO+), and terminally differentiated (CD27-CD45RO-). The contribution of each subpopulation to the total production of IFNγ and IL-2 was calculated. As only very low levels of cytokine production were observed in the naive population, and because the population of terminally differentiated cells was too small to be reliably phenotyped, only the results for the IL-2+ Cm and IFNγ+ Em CD4+ T cells are shown in Figure 3. Early in infection, no differences in phenotype of HIV-specific cytokine-producing CD4+ T cells were observed between LTAs and progressors (Figure 3A,C). Interestingly, late in infection in LTAs a shift was observed from the population of HIV-specific IL-2+ Cm CD4+ T cells toward the further differentiated IFNγ Em CD4+ T-cell population (Figure 3A-C). In progressors, however, hardly any HIV-specific cytokine-positive CD4+ T cells were found late in infection. No differences in phenotype of CMV-specific CD4+ T cells between LTAs and progressors were found (Figure 3B,D).
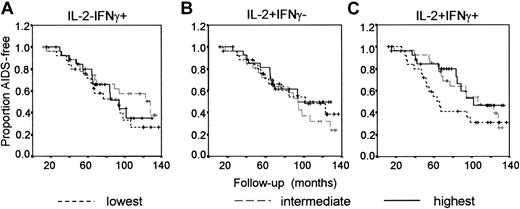
No association between the presence of HIV-specific CD4+ T cells 1 year after seroconversion and AIDS-free survival. HIV-specific CD4+ T cells producing IFNγ, IL-2, or IL-2 and IFNγ were analyzed at a median of 12.3 months after seroconversion (range, 0.3-20.4 months). Numbers of HIV-specific cytokine-producing CD4+ T cells were categorized into 3 groups, and the effect of the presence of functional HIV-specific CD4+ T cells on AIDS-free survival was analyzed using Kaplan-Meier survival curves. (A) Numbers of IFNγ+ CD4+ T cells: lowest (broken line), intermediate (gray line), highest (black line). (B) Numbers of IL-2+ CD4+ T cells. (C) Numbers of IL-2+IFNγ+ CD4+ T cells.
No association between the presence of HIV-specific CD4+ T cells 1 year after seroconversion and AIDS-free survival. HIV-specific CD4+ T cells producing IFNγ, IL-2, or IL-2 and IFNγ were analyzed at a median of 12.3 months after seroconversion (range, 0.3-20.4 months). Numbers of HIV-specific cytokine-producing CD4+ T cells were categorized into 3 groups, and the effect of the presence of functional HIV-specific CD4+ T cells on AIDS-free survival was analyzed using Kaplan-Meier survival curves. (A) Numbers of IFNγ+ CD4+ T cells: lowest (broken line), intermediate (gray line), highest (black line). (B) Numbers of IL-2+ CD4+ T cells. (C) Numbers of IL-2+IFNγ+ CD4+ T cells.
Loss of Gag-specific IL-2+ central memory and IFNγ+ effector memory CD4+ T cells during progression to AIDS. Cytokine+ CD4+ T cells were assessed for surface expression of CD27 and CD45RO in 6 LTAs and 7 progressors both early and late in infection. Within the lymphocyte gate, CD3 was plotted against CD4. Next, the CD4+CD3+ T cells were gated. Within the CD4 gate, CD27 was plotted against CD45RO. Then the Cm population (CD27+CD45RO+) and the Em population (CD27-CD45RO+) were selected and within each gate the specific IFNγ+ or IL-2+CD4+ T cells were determined. Gag-specific IFNγ+ Tem cells (A) and Gag-specific IL-2+ Tcm CD4+ T cells (C) are shown, as well as the percentage of CMV-specific IFNγ+ Tem cells (B) and IL-2+ Tcm cells (D). Naive cells and the terminally differentiated cells were excluded from the analyses because only very low levels of cytokine production were observed in the naive population, and the population of Td cells was too small to be reliably phenotyped. Results are depicted as the median fraction together with the 25th to 75th percentile. ▪ indicates LTAs; □, progressors.
Loss of Gag-specific IL-2+ central memory and IFNγ+ effector memory CD4+ T cells during progression to AIDS. Cytokine+ CD4+ T cells were assessed for surface expression of CD27 and CD45RO in 6 LTAs and 7 progressors both early and late in infection. Within the lymphocyte gate, CD3 was plotted against CD4. Next, the CD4+CD3+ T cells were gated. Within the CD4 gate, CD27 was plotted against CD45RO. Then the Cm population (CD27+CD45RO+) and the Em population (CD27-CD45RO+) were selected and within each gate the specific IFNγ+ or IL-2+CD4+ T cells were determined. Gag-specific IFNγ+ Tem cells (A) and Gag-specific IL-2+ Tcm CD4+ T cells (C) are shown, as well as the percentage of CMV-specific IFNγ+ Tem cells (B) and IL-2+ Tcm cells (D). Naive cells and the terminally differentiated cells were excluded from the analyses because only very low levels of cytokine production were observed in the naive population, and the population of Td cells was too small to be reliably phenotyped. Results are depicted as the median fraction together with the 25th to 75th percentile. ▪ indicates LTAs; □, progressors.
Prognostic value of HIV-specific CD4+ T-cell responses
The observation that LTAs and progressors have equal numbers of HIV-specific CD4+ T cells early in infection suggested that a functional HIV-specific CD4+ T-cell response might not provide protection against progression to AIDS. To further investigate the prognostic value of HIV-specific T-helper responses, a large prospective study was performed that included 96 HIV-infected seroconverters. HIV-specific CD4+ T cells producing IFNγ, IL-2, or IL-2 and IFNγ were analyzed at a median of 12.3 months after seroconversion (range, 0.3-20.4 months). Most individuals were analyzed between 8 and 15 months after seroconversion (85 of 97) The few individuals who were analyzed within 6 months after seroconversion and later than 17 months after seroconversion were not different in total CD4+ T-cell numbers, viral load, activation markers, and HIV-specific CD4+ T-cell percentages.
In univariate analyses, no significant predictive value of the number of cytokine-producing HIV-specific CD4+ T cells was found (Table 2; Figure 2). When adjusted for HIV viral load and CD4+ T cells, also no significant predictive effect of the numbers of single IFNγ+ or single IL-2+ CD4+ T cells was observed. Adjusting for expression of the activation markers Ki67 or CD38 on CD4+ T cells, or coexpression of CD38 and HLA-DR on CD8+ T cells (which have been described as dominant predictors of progression to AIDS18 ) did not change the hazard ratio (HR). Interestingly, multivariate analyses showed that the number of IL-2+IFNγ+ cells appeared to be significantly associated with slow progression to AIDS after adjusting for plasma HIV-1 RNA and CD4+ T-cell numbers (P = .05) and also after including the percentage of CD38+HLA-DR+CD8+ T cells in the model (P = .04). However, after adjusting for the percentage of Ki67+CD4+ T cells or the percentage CD38+CD4+ T cells, the number of IL-2+IFNγ+CD4+ T cells was no longer predictive for protection against progression to AIDS. Gag-specific IL-2+IFNγ+ CD4+ T cells fitted into the model as a continuous instead of a categorical variable were also not significantly associated with protection against HIV progression. Similar results were observed when the percentages of HIV-specific CD4+ T cells rather than the numbers of these cells were analyzed (data not shown).
Cox proportional hazard models for progression to AIDS in 96 study participants
. | . | Hazard ratio (95% CI) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. cases* . | Univariate . | Multivariate 1, adjusted† . | Multivariate 2, adjusted‡ . | Multivariate 3, adjusted§ . | Multivariate 4, adjusted∥ . | ||||
| No. Gag IFNγ+ CD4+ T cells/mL | ||||||||||
| Tertiles¶ | ||||||||||
| 1 (Lowest)# | 27 | 1 | 1 | 1 | 1 | 1 | ||||
| 2 (Intermediate) | 28 | 0.60 (0.28-1.27) | 0.56 (0.26-1.19) | 0.56 (0.26-1.92) | 0.59 (0.28-1.27) | 0.48 (0.22-1.05) | ||||
| 3 (Highest) | 27 | 0.82 (0.38-1.76) | 0.65 (0.29-1.43) | 0.60 (0.27-1.35) | 0.55 (0.24-1.26) | 0.57 (0.26-1.27) | ||||
| No. Gag IL-2+ CD4+ T cells/mL | ||||||||||
| Tertiles** | ||||||||||
| 1 (Lowest)# | 27 | 1 | 1 | 1 | 1 | 1 | ||||
| 2 (Intermediate) | 27 | 1.23 (0.58-2.61) | 1.15 (0.54-2.48) | 1.21 (0.22-2.71) | 1.23 (0.56-2.73) | 1.29 (0.59-2.80) | ||||
| 3 (Highest) | 28 | 0.91 (0.41-2.02) | 0.99 (0.43-2.27) | 1.08 (0.46-2.52) | 1.09 (0.47-2.55) | 1.06 (0.46-2.47) | ||||
| No. Gag IL-2+ + IFNγ+ CD4+ T cells/mL | ||||||||||
| Tertiles†† | ||||||||||
| 1 (Lowest)# | 27 | 1 | 1 | 1 | 1 | 1 | ||||
| 2 (Intermediate) | 28 | 0.58 (0.28-1.19) | 0.57 (0.27-1.18) | 0.61 (0.28-1.32) | 0.62 (0.29-1.31) | 0.54 (0.25-1.14) | ||||
| 3 (Highest) | 27 | 0.50 (0.23-1.10) | 0.44 (0.20-0.98) | 0.47 (0.21-1.08) | 0.50 (0.22-1.13) | 0.40 (0.17-0.94) | ||||
. | . | Hazard ratio (95% CI) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. cases* . | Univariate . | Multivariate 1, adjusted† . | Multivariate 2, adjusted‡ . | Multivariate 3, adjusted§ . | Multivariate 4, adjusted∥ . | ||||
| No. Gag IFNγ+ CD4+ T cells/mL | ||||||||||
| Tertiles¶ | ||||||||||
| 1 (Lowest)# | 27 | 1 | 1 | 1 | 1 | 1 | ||||
| 2 (Intermediate) | 28 | 0.60 (0.28-1.27) | 0.56 (0.26-1.19) | 0.56 (0.26-1.92) | 0.59 (0.28-1.27) | 0.48 (0.22-1.05) | ||||
| 3 (Highest) | 27 | 0.82 (0.38-1.76) | 0.65 (0.29-1.43) | 0.60 (0.27-1.35) | 0.55 (0.24-1.26) | 0.57 (0.26-1.27) | ||||
| No. Gag IL-2+ CD4+ T cells/mL | ||||||||||
| Tertiles** | ||||||||||
| 1 (Lowest)# | 27 | 1 | 1 | 1 | 1 | 1 | ||||
| 2 (Intermediate) | 27 | 1.23 (0.58-2.61) | 1.15 (0.54-2.48) | 1.21 (0.22-2.71) | 1.23 (0.56-2.73) | 1.29 (0.59-2.80) | ||||
| 3 (Highest) | 28 | 0.91 (0.41-2.02) | 0.99 (0.43-2.27) | 1.08 (0.46-2.52) | 1.09 (0.47-2.55) | 1.06 (0.46-2.47) | ||||
| No. Gag IL-2+ + IFNγ+ CD4+ T cells/mL | ||||||||||
| Tertiles†† | ||||||||||
| 1 (Lowest)# | 27 | 1 | 1 | 1 | 1 | 1 | ||||
| 2 (Intermediate) | 28 | 0.58 (0.28-1.19) | 0.57 (0.27-1.18) | 0.61 (0.28-1.32) | 0.62 (0.29-1.31) | 0.54 (0.25-1.14) | ||||
| 3 (Highest) | 27 | 0.50 (0.23-1.10) | 0.44 (0.20-0.98) | 0.47 (0.21-1.08) | 0.50 (0.22-1.13) | 0.40 (0.17-0.94) | ||||
CI indicates 95% confidence level.
Because of missing data on the covariates of the multivariate model, it is possible that a subject was excluded from the analyses
Adjusted for baseline log HIV RNA (continuous) and square root CD4+ T-cell count (continuous)
Adjusted for baseline log HIV RNA (continuous), square root CD4+ T-cell numbers (continuous), and percentage of Ki67+CD4+ T cells (2 categories: 1.6%-5.3%; 5.3%-15.5%)
Adjusted for baseline log HIV RNA (continuous), square root CD4+ T-cell numbers (continuous), and percentage of CD38+CD4+ T cells (2 categories: 40.0%-66.1%; 66.1%-91.1%)
Adjusted for baseline log HIV RNA (continuous), square root CD4+ T-cell numbers (continuous), and percentage of CD38+HLA-DR+CD8+ T cells (2 categories: 0.5%-3.6%; 3.6%-71.4%)
Ranges: first tertile, 1-141 cells/mL; second tertile, 141-365 cells/mL, third tertile, 365-5184 cells/mL
Reference category
Ranges: first tertile, 1-54 cells/mL; second tertile, 54-128 cells/mL; third tertile, 128-6794 cells/mL
Ranges: first tertile, 1-151 cells/mL; second tertile, 151-360 cells/mL; third tertile, 360-1316 cells/mL
Discussion
The role of HIV-specific CD4+ T-cell responses in the natural history of HIV infection is still debated. This is partly related to the fact that mainly cross-sectional studies have been performed, where the presence of CD4+ T-cell responses was correlated with CTL function and/or HIV disease progression. At this time prospective studies to test the protective effect of HIV-specific memory CD4+ T-cell responses for disease progression have not been reported.
Here, we present a prospective cohort study analyzing functional properties of HIV-specific CD4+ T cells in relation to clinical outcome. First, a detailed longitudinal analysis was performed in a small number of progressors and LTAs. Early in infection, equal numbers of Gag-specific single IL-2+ and IL-2+IFNγ+ CD4+ T cells were present in LTA progressors, but in the course of HIV infection these numbers declined in progressors. These changes in CD4+ T-cell function were specific for HIV, as CMV-specific CD4+ T-cell responses did not change over time. In our cohort, we readily detect proliferation of HIV-specific CD4+ T-helper responses in LTAs. However, these responses are clearly lower in magnitude than responses in long-term nonprogressors reported in previous studies. In these studies nonprogressors were selected for having extreme low loads.8,21 Thus, the relatively low T-cell proliferation that we describe here can be explained by the fact that the LTAs in our study were not selected for low viral loads. Phenotypic analysis of the antigen-specific cells was performed in parallel using the T-cell differentiation markers CD45RO and CD27. In agreement with earlier studies13,22 loss of IL-2-producing cells coincided with loss of CD45RO+CD27+CD4+ `central memory' cells. In LTAs, this loss of IL-2+ cells was paralleled by a shift toward the IFNγ+ Em population, whereas in progressors both populations of cytokine-producing HIV-specific CD4+ T cells were lost late in infection. Thus, progression to AIDS was characterized by a quantitative and qualitative loss of HIV-specific CD4+ T cells, despite the fact that HIV-specific CD4+ T-cell responses early in infection were comparable in LTAs and progressors.
These data suggest that the presence of functional HIV-specific CD4+ T cells does not protect against progression to AIDS. We confirmed this finding by analyzing the predictive value of the number of Gag-specific cytokine-producing CD4+ T cells for the development of AIDS in a prospective cohort of 96 individuals infected with HIV-1 with known seroconversion dates. Univarately, none of the markers was significantly associated with progression to AIDS. In multivariate models, only the presence of high numbers of IL-2+IFNγ+ CD4+ T cells protected against progression to AIDS. However, including viral load, total CD4+ T-cell numbers and the percentage of Ki67+CD4+ T cells or the percentage of CD38+CD4+ T cells into the model resulted in a loss of statistical significance.
Taken together, in a detailed longitudinal study and in a prospective cohort study we found that, despite the early presence of functional HIV-specific CD4+ T cells, HIV-infected individuals do progress to AIDS, exhibiting increasing viral load, decreasing CD4+ T-cell numbers, and decreasing numbers of HIV-specific cytokine-producing CD4+ T cells.
In the assays used, HIV-specific CD4+ T cells are detected that have been primed in vivo. Therefore, possible differences in antigen presentation because of antigen-presenting cell (APC) dysfunction in vivo might be visible in our ex vivo assays. One thus cannot exclude the possibility that the loss of HIV-specific CD4+ T-cell responses observed late in infection is related to loss of APC function late in infection.23 For CD8+ T-cell responses it is known that the use of peptides based on a consensus sequence may result in an underestimation of the specific response,24 although others showed that this might be a moderate effect.25 As we used peptides based on the consensus sequence rather than autologous peptides, the responses we measure may be an underestimation. To our knowledge, no studies on ex vivo HIV-specific CD4+ T-cell responses elicited using autologous viruses have been reported.
As the amount of patient material was limited, we analyzed only Gag-specific CD4+ T-cell responses. It has been shown that multiple regions of Gag and Nef are targeted by HIV-specific CD4+ T cells and that other regions of the virus are targeted only infrequently or are not targeted at all.26 In addition, HIV-1 Gag and Nef were shown to have the highest epitope density and to elicit the strongest responses26 and in our hands Nef-specific CD4+ T-cell responses showed a similar trend compared with Gag-specific responses, although of lower magnitude.19 Therefore, we believe that the analysis of Gag-specific responses may give an adequate representation of the HIV-specific CD4+ T-cell response.
Earlier studies have shown that HIV-specific CD4+ T cells of the central memory phenotype, which produce IL-2 and have a high capacity to proliferate, are relatively abundant in patients with low viral load. However, with persistent viremia these cells rapidly differentiate into “effector” memory CD4+ T cells, only capable of producing IFNγ and without self-renewal capacity.13,14 Here in a prospective study we demonstrate that such “memory type” CD4+ T cells apparently do not prevent HIV disease progression but are indeed lost during disease progression. These data support the idea that HIV-viral load level determines the magnitude and characteristics of the CD4+ T-cell response, rather than CD4+ T-helper responses controlling HIV viral load.8,9
It has been well established that the viral load set point characteristic for patients infected with HIV is an important prognostic parameter.20 It has been shown that viral replication in an independent manner induces systemic hyperactivation of the immune system which is a major predictive factor for CD4+ T-cell loss and progression to AIDS.18,27,28 The viral load set point is strongly associated with HLA allele expression which is believed to influence viral fitness and the viral load set point through adaptation to host CTL pressure.29,30 This would suggest that, although we show that CD4+ T-cell responses are not protective once the viral set point is established, early preservation of HIV-specific CD4 responses would have a strong and long-lasting protective effect. This idea indeed has lead to administration of HAART immediately after HIV transmission.2 Recent studies however have shown that even under these conditions HIV-specific CD4+ T-helper responses did not provide long-lasting protection from HIV disease progression.19,31
In conclusion, our data indicate that the early presence of CD4+ T-helper responses with functional and phenotypic properties generally associated with at least partial control of viral replication may not provide protection to progression to disease. Similar observations have been made for HIV-specific CD8+ T-cell responses.32 As it is currently not clear what kind of HIV-specific T cells are involved in protective immunity, careful studies on the possible protective effect of vaccine-induced HIV-specific CD4+ and CD8+ T cells are warranted,33 although recently some doubt has been cast in this regard.34,35
Prepublished online as Blood First Edition Paper, October 18, 2005; DOI 10.1182/blood-2005-07-2907.
Supported by the AIDS Fonds Netherlands (grant 5005).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the participants of the Amsterdam Cohort on HIV-1 infection and AIDS, which is a collaboration of the Municipal Health Service, The Academic Medical Centre and Sanquin Research at CLB. The Gag-derived overlapping peptide pool (15-mers with 11 overlap, HXB2) was obtained through the NIH AIDS Research and Reference Reagent program, Division of AIDS, NIAID, NIH. We thank Daphne van der A (National Institute for Public Health and the Environment, Bilthoven, The Netherlands), Ronald Geskus and Maria Prins (Department of HIV and STI, Cluster of Infectious Diseases, Municipal Health Service, Amsterdam, The Netherlands) for expert statistical assistance, and Mette Hazenberg for critically reading the manuscript. Processing of patient samples was performed by Linda Dekker and coworkers (Department of Clinical Viro-Immunology, Sanquin, Amsterdam, The Netherlands), Suzanne Jurriaans and coworkers (Department of Human Retrovirology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands) provided viral load data for all individuals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal