Eighty patients with chronic myeloid leukemia (CML) underwent T cell-depleted stem cell transplantation from an HLA-identical sibling, with add-back of donor T cells on days 30 to 45 and days 60 to 100 in patients in whom grade 2 or greater acute graft-versus-host disease (GVHD) developed. The outcomes for 54 patients with chronic-phase (CP) and 26 with advanced-phase (AP) disease were as follows: overall survival, 85% ± 5% versus 36% ± 10%; transplantation-related mortality (TRM), 13% ± 5% versus 43% ± 11%; and current leukemia-free survival, 76% ± 6% versus 34% ± 9%. The day-30 lymphocyte count (LC30) was strongly associated with outcome. For patients in CP with counts greater than the median of 0.30 × 109/L, survival was 100% versus 70% ± 9% (P = .003); current LFS 100% versus 56% ± 9% (P = .002); and TRM 0% versus 26% ± 8% (P = .006). Higher-than-median LC30 correlated significantly with molecular remission (MR) at 3, 6, and 12 months and with higher CD34 doses. Lymphocyte subset analysis performed in 20 patients available for phenotyping showed that LC30 was highly correlated with absolute CD56+CD3- natural killer cell numbers (NK30), which also predicted for survival and MR. CD34 cell dose, LC30, and NK30, but not day-30 CD3+ cell count, were highly correlated and were significant predictors of transplantation outcome. These results suggest that transplanted CD34 cell doses greater than 5 × 106/kg may improve outcomes by increasing the early recovery of NK cells.
Introduction
Elective stem cell transplantation (SCT) in patients with chronic-phase (CP) chronic myelogenous leukemia (CML) using HLA-identical family donors usually has a favorable outcome because transplant-related mortality (TRM) and relapse rates are low.1 Furthermore, relapse after SCT can be successfully treated with donor lymphocyte infusion (DLI).2 In a study of 346 patients with CML, Radich et al3 showed that between 6 and 12 months after transplantation, persistent BCR-ABL positivity was associated with a high risk for relapse and inferior survival. It is generally agreed that monitoring BCR-ABL mRNA transcripts by reverse transcription-polymerase chain reaction (RT-PCR) is a useful means of predicting cytogenetic and hematologic relapse.4-7 The opportunity to monitor residual disease has encouraged several transplantation groups to perform T cell-depleted SCT followed by elective DLI to treat residual disease if detected on regular monitoring. This approach spares patients who have not had relapses from graft-versus-host disease (GVHD) induced by donor lymphocytes.
Since 1993, our transplantation approach has been to perform T cell-depleted SCT followed by 1 or 2 rounds of DLI between 1 and 3 months after transplantation. To identify and treat persistent disease, patients with CML were monitored regularly by PCR for BCR-ABL. Here we present the results of this treatment approach in 80 patients with CML, along with an analysis of factors predictive for disease control and transplantation outcome. Our findings emphasize an important predictive role of the lymphocyte count 30 days after transplantation and the transplanted CD34 dose and suggest that higher stem cell doses improve outcomes by promoting early natural killer (NK) cell recovery.
Patients, materials, and methods
Study group
Between December 1993 and May 2004, 80 consecutive patients with CML underwent T cell-depleted SCT from an HLA-identical sibling in 6 successive National Heart, Lung and Blood Institute (NHLBI) institutional review board-approved protocols (93-H-0212, 97-H-0099, 99-H-0046, 02-H-0111, 03-H-0192, 04-H-0112). All patients and donors gave written informed consent before enrolling in the transplantation protocol.
Conditioning regimens
Four transplantation regimens were evaluated: (1) 13.5 Gy total body irradiation (TBI), cyclophosphamide (Cy) 120 mg/kg, standard dose (SD) cyclosporine (CSA; target levels, 200-400 μg/L), and bone marrow transplantation (BMT) (n = 25); (2) peripheral blood stem cell transplantation (PBSCT) (n = 16); (3) PBSCT/TBI/Cy and low-dose (target levels, 100-200 μg/L) or no (LD/N) CSA (n = 22); and (4) PBSCT/12.0 Gy TBI/Cy/fludarabine (Flu) 125 mg/m2 and LD/N CSA (n = 17). PBSCT was used in all protocols after December 1996 (Table 1).
Characteristics of transplantation regimens used for CML patients from 1993 to 2004
Protocol . | Study period . | No. patients . | Stem cell source . | TBI dose, cGy . | CD3 dose, × 105/kg . | Mean CD34 dose, × 106/kg . | CSA dose . |
|---|---|---|---|---|---|---|---|
| 1 | 1993-1996 | 25 | BM | 1360 | 2 | 2 | STD |
| 2 | 1996-1999 | 16 | PB | 1360 | 1 | 4 | STD |
| 3 | 1999-2001 | 22 | PB | 1200 | 0.5 | 8 | N/LD |
| 4 | 2001-2004 | 17 | PB | 1200 | 0.2 | 6 | N/LD |
Protocol . | Study period . | No. patients . | Stem cell source . | TBI dose, cGy . | CD3 dose, × 105/kg . | Mean CD34 dose, × 106/kg . | CSA dose . |
|---|---|---|---|---|---|---|---|
| 1 | 1993-1996 | 25 | BM | 1360 | 2 | 2 | STD |
| 2 | 1996-1999 | 16 | PB | 1360 | 1 | 4 | STD |
| 3 | 1999-2001 | 22 | PB | 1200 | 0.5 | 8 | N/LD |
| 4 | 2001-2004 | 17 | PB | 1200 | 0.2 | 6 | N/LD |
BM indicates bone marrow; PB, peripheral blood; STD, standard; N, none; LD, low dose.
Transplantation approach
In the first protocol (93-H-0212), stem cells were collected after bone marrow harvesting and were depleted of T cells by elutriation,8 whereas in all subsequent protocols the donor underwent granulocyte-colony-stimulating factor (G-CSF)-mobilized peripheral blood apheresis followed by stem cell collection. In protocol 97-H-0099, patients underwent T cell-depleted G-CSF-mobilized PBSCT prepared by using the CellPro TCD system, consisting of CD34+ selection on the Ceprate SC immunoabsorption column (CellPro, Bothell, WA), followed by negative selection of T cells using anti-CD2 and a second, smaller column (CellPro). More recent protocols used the Isolex 300i immunomagnetic cell separation system, version 2.5 (Nexell Therapeutics, Irvine, CA), for positive selection of CD34+ cells, followed by negative selection of T cells using an antibody cocktail of anti-CD2, anti-CD6, and anti-CD7, as previously described.9 In the absence of GVHD, or unless molecular remission was documented in CML, T cells were added back on days 30 to 45 and day 100 (n = 79) or day 60 (n = 1). Cyclosporine dose varied according to protocol: 41 patients received standard-dose (SD) CSA (target plasma level, 200-400 μg/mL); 5 received low-dose (LD) CSA (target plasma level, 100-200 μg/mL), starting on day -4 until an oral dose was tolerated; and 34 received no (N) CSA during the first 6 weeks after transplantation. Patients started CSA the day before the first DLI. CSA was continued for 6 months and then tapered if chronic GVHD did not develop. Standard prophylaxis against infection included fluconazole, Bactrim for at least 6 months after transplantation, and weekly surveillance for CMV antigenemia as described previously.9,10
Day-30 lymphocyte monitoring (LC30)
All patients survived to day 30 after transplantation for day-30 lymphocyte evaluation based on routine blood count. Twenty randomly cryopreserved day-30 blood samples were available for lymphocyte subset analysis by flow cytometry. The samples were derived from patients who underwent BMT and PBSCT and were collected between 1993 and 2004.
Flow cytometry
Sample staining was performed using 1 × 106 peripheral mononuclear cells (PBMCs) in 50 μL 1% FCS/PBS. Cells were stained with a panel of directly conjugated antibodies to CD3, CD4, CD8, CD56, and CD16 (Becton Dickinson, San Jose, CA). Fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), peridin chlorophyll protein (PerCP), and APC-Cy7 were used as fluorophores. The lymphocytes were then washed in 1% FCS/PBS and resuspended in 1% paraformaldehyde in PBS. Data acquisition was performed with LSR II (Becton Dickinson). A minimum of 50 000 gated cells were acquired. Data were analyzed using FACS Diva (Becton Dickinson) software.
Molecular analysis
BCR-ABL expression was monitored serially in patient leukocytes using RT-PCR. Until 2003, each sample was processed as described previously11 using double-nested PCR to amplify BCR-ABL and a single round of PCR to amplify ABL housekeeper cDNA. Product bands were detected using ethidium bromide and agarose gel electrophoresis. After July 2003, the method was changed to make use of real-time PCR: leukocyte RNA was purified using RNeasy (Qiagen, Valencia, CA) and transcribed using random hexamer primers and the GeneAmp transcription kit (Applied Biosystems, Branchburg, NJ). Duplicate samples of the resultant cDNA were amplified 50 cycles in a LightCycler (Roche, Mannheim, Germany) using the primers and fluorescence-labeled TaqMan probe described by Mensink et al.12 To assess RNA integrity, the housekeeper gene G6PDH was measured using a commercial real-time PCR kit (Roche, Indianapolis, IN). In all cases, serial 10-fold dilutions of RNA prepared from the BCR-ABL-positive cell line K562 were used as a positive control, and RNA from the BCR-ABL-negative cell line HMy was used as a negative control to detect sample contamination. Both assays could detect b2a2 and b3a2 translocations but not translocations involving the minor breakpoint cluster region.
For the purposes of this analysis, a patient sample was considered positive for BCR-ABL if a detectable band was noted in a double-nested assay or if either of the duplicate samples generated a measurable crossing point in the real-time PCR assay. Samples were considered negative if no BCR-ABL band was detected and the housekeeper gene expression assay confirmed RNA integrity. The double-nested assay and the quantitative real-time BCR-ABL assays were comparable in sensitivity. Each could detect BCR-ABL transcripts in K562 RNA diluted to 1:100 000 to 1 000 000 in control RNA.
Definitions
Chronic phase (CP) was distinguished from advanced phase (AP) (accelerated and blastic phase) using the International Bone Marrow Transplantation Registry (IBMTR) criteria.13 Overall survival (OS) was defined as the time from transplantation until death from any cause. Current leukemia-free survival (LFS) was defined as the survival without evidence of leukemia (molecular remission) at the time of most recent assessment.14 Transplant-related mortality (TRM) was defined as the time from transplantation until death from infectious cause, graft failure, or graft-versus-host disease (GVHD)-related causes. Molecular remission in CML was defined as absence of BCR-ABL mRNA transcripts by RT-PCR.
Statistical methods
Summary statistics, such as proportions, means, standard deviations, 95% confidence intervals, medians, and ranges, were used to describe the patient characteristics, pretransplantation variables, and posttransplantation outcomes. Standard techniques in survival analysis, including Kaplan-Meier estimates and the Cox proportional hazard models, were used to estimate the time-to-event distributions of overall survival (OS), current LFS, relapse, and TRM. In particular, Kaplan-Meier curves were used to display the distributions of survival and mortality among subgroups of patients, and the Cox proportional hazard models with univariate or multivariate covariates were used to evaluate the effects of covariates on survival times. Statistical associations between pretransplantation variables were investigated using correlation analysis, including Pearson correlation coefficients and Spearman rank correlation coefficients, and multiple regression analysis. Statistical tests were based on t tests, and χ2 tests and F-tests were used to evaluate the statistical significance of covariates in multiple regression models or the Cox proportional hazard models. The Wald score and likelihood ratio tests were used to evaluate the fitness of the Cox proportional hazard models. Variables included in multivariate analysis were as follows: age, sex, donor-patient sex match, disease phase (CP vs AP), type of transplantation (BMT vs PBSCT), CD34 dose, CD3 dose, LC30, and CSA dose. Data analysis was performed using SPSS 13 for Windows (SPSS, Chicago, IL) software.
Results
Patient characteristics are described in Table 2. The median follow-up time for the cohort was 43 months (range, 0.5-135 months). Thirty-eight percent of patients have survived more than 5 years after transplantation. Twenty-four patients died. Causes of death are summarized in Table 3.
Patient characteristics and univariate analysis
. | . | OS . | . | Current LFS . | . | TRM . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor . | No. patients . | No. (%) . | P . | No. (%) . | P . | No. (%) . | P . | |||
| Age | .38 | .38 | .29 | |||||||
| Less than 36 y | 38 | 25 (65 ± 7) | 23 (59 ± 8) | 10 (26 ± 7) | ||||||
| At least 36 y | 42 | 31 (73 ± 7) | 28 (66 ± 7) | 7 (17 ± 6) | ||||||
| Sex | .40 | .29 | .76 | |||||||
| Male | 53 | 35 (65 ± 7) | 31 (57 ± 7) | 12 (24 ± 5) | ||||||
| Female | 27 | 21 (78 ± 8) | 20 (74 ± 9) | 5 (19 ± 7) | ||||||
| Disease phase | < .001 | < .001 | .007 | |||||||
| Chronic | 54 | 46 (85 ± 5) | 42 (76 ± 6) | 7 (13 ± 5) | ||||||
| Advanced | 26 | 10 (36 ± 10) | 9 (34 ± 9) | 10 (43 ± 11) | ||||||
| Donor-patient sex match | .88 | .82 | .82 | |||||||
| F to M | 13 | 9 (69 ± 13) | 9 (69 ± 13) | 3 (24 ± 9) | ||||||
| Others | 67 | 47 (69 ± 6) | 42 (61 ± 6) | 14 (22 ± 8) | ||||||
| Transplantation type | .16 | .54 | .08 | |||||||
| BMT | 25 | 15 (60 ± 10) | 15 (60 ± 10) | 8 (33 ± 10) | ||||||
| PBSCT | 55 | 41 (74 ± 6) | 36 (63 ± 7) | 9 (16 ± 5) | ||||||
| CSA dose | .29 | .27 | .48 | |||||||
| SD | 41 | 26 (63 ± 8) | 26 (63 ± 8) | 10 (25 ± 7) | ||||||
| LD | 5 | 5 (100) | 5 (100) | 0 (0) | ||||||
| N | 34 | 25 (74 ± 8) | 20 (54 ± 9) | 7 (21 ± 6) | ||||||
| CD34 dose | .005 | .01 | .05 | |||||||
| Less than 4.5 × 106/kg | 40 | 22 (55 ± 8) | 20 (50 ± 8) | 12 (31 ± 8) | ||||||
| More than 4.5 × 106/kg | 40 | 34 (85 ± 6) | 31 (76 ± 7) | 5 (12 ± 5) | ||||||
| CD3 dose | .43 | .29 | .38 | |||||||
| 1 × 105/kg | 16 | 11 (69 ± 12) | 11 (69 ± 12) | 2 (13 ± 8) | ||||||
| 0.2 × 106/kg | 17 | 12 (68 ± 12) | 9 (55 ± 13) | 3 (19 ± 9) | ||||||
| 2 × 106/kg | 25 | 15 (60 ± 10) | 15 (60 ± 10) | 8 (33 ± 9) | ||||||
| 0.5 × 106/kg | 22 | 18 (81 ± 8) | 16 (73 ± 9) | 4 (18 ± 8) | ||||||
| LC30 | < .001 | < .001 | .01 | |||||||
| Less than 0.30 × 109/L | 40 | 19 (46 ± 8) | 17 (41 ± 8) | 14 (33 ± 3) | ||||||
| More than 0.30 × 109/L | 40 | 37 (93 ± 4) | 34 (85 ± 6) | 3 (8.5 ± 4.7) | ||||||
. | . | OS . | . | Current LFS . | . | TRM . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor . | No. patients . | No. (%) . | P . | No. (%) . | P . | No. (%) . | P . | |||
| Age | .38 | .38 | .29 | |||||||
| Less than 36 y | 38 | 25 (65 ± 7) | 23 (59 ± 8) | 10 (26 ± 7) | ||||||
| At least 36 y | 42 | 31 (73 ± 7) | 28 (66 ± 7) | 7 (17 ± 6) | ||||||
| Sex | .40 | .29 | .76 | |||||||
| Male | 53 | 35 (65 ± 7) | 31 (57 ± 7) | 12 (24 ± 5) | ||||||
| Female | 27 | 21 (78 ± 8) | 20 (74 ± 9) | 5 (19 ± 7) | ||||||
| Disease phase | < .001 | < .001 | .007 | |||||||
| Chronic | 54 | 46 (85 ± 5) | 42 (76 ± 6) | 7 (13 ± 5) | ||||||
| Advanced | 26 | 10 (36 ± 10) | 9 (34 ± 9) | 10 (43 ± 11) | ||||||
| Donor-patient sex match | .88 | .82 | .82 | |||||||
| F to M | 13 | 9 (69 ± 13) | 9 (69 ± 13) | 3 (24 ± 9) | ||||||
| Others | 67 | 47 (69 ± 6) | 42 (61 ± 6) | 14 (22 ± 8) | ||||||
| Transplantation type | .16 | .54 | .08 | |||||||
| BMT | 25 | 15 (60 ± 10) | 15 (60 ± 10) | 8 (33 ± 10) | ||||||
| PBSCT | 55 | 41 (74 ± 6) | 36 (63 ± 7) | 9 (16 ± 5) | ||||||
| CSA dose | .29 | .27 | .48 | |||||||
| SD | 41 | 26 (63 ± 8) | 26 (63 ± 8) | 10 (25 ± 7) | ||||||
| LD | 5 | 5 (100) | 5 (100) | 0 (0) | ||||||
| N | 34 | 25 (74 ± 8) | 20 (54 ± 9) | 7 (21 ± 6) | ||||||
| CD34 dose | .005 | .01 | .05 | |||||||
| Less than 4.5 × 106/kg | 40 | 22 (55 ± 8) | 20 (50 ± 8) | 12 (31 ± 8) | ||||||
| More than 4.5 × 106/kg | 40 | 34 (85 ± 6) | 31 (76 ± 7) | 5 (12 ± 5) | ||||||
| CD3 dose | .43 | .29 | .38 | |||||||
| 1 × 105/kg | 16 | 11 (69 ± 12) | 11 (69 ± 12) | 2 (13 ± 8) | ||||||
| 0.2 × 106/kg | 17 | 12 (68 ± 12) | 9 (55 ± 13) | 3 (19 ± 9) | ||||||
| 2 × 106/kg | 25 | 15 (60 ± 10) | 15 (60 ± 10) | 8 (33 ± 9) | ||||||
| 0.5 × 106/kg | 22 | 18 (81 ± 8) | 16 (73 ± 9) | 4 (18 ± 8) | ||||||
| LC30 | < .001 | < .001 | .01 | |||||||
| Less than 0.30 × 109/L | 40 | 19 (46 ± 8) | 17 (41 ± 8) | 14 (33 ± 3) | ||||||
| More than 0.30 × 109/L | 40 | 37 (93 ± 4) | 34 (85 ± 6) | 3 (8.5 ± 4.7) | ||||||
Median age of patients was 36 years; range was 12 to 58 years. Median CD34 dose was 4.5 × 106/kg. Median LC30 was 0.297 × 109/L.
Causes of death after transplantation
Cause . | No. patients (%) . |
|---|---|
| Relapse | 6 (7.5) |
| TRM | 17 (21.3) |
| Infection, viral | 7 (8.7) |
| IPS, ARDS-2 | 5 (6.3) |
| GVHD | 2 (2.5) |
| Graft failure | 1 (1.25) |
| Intracranial bleeding | 1 (1.25) |
| Transfusion reactions | 1 (1.25) |
| Motor vehicle injury | 1 (1.25) |
| Total | 24 (30) |
Cause . | No. patients (%) . |
|---|---|
| Relapse | 6 (7.5) |
| TRM | 17 (21.3) |
| Infection, viral | 7 (8.7) |
| IPS, ARDS-2 | 5 (6.3) |
| GVHD | 2 (2.5) |
| Graft failure | 1 (1.25) |
| Intracranial bleeding | 1 (1.25) |
| Transfusion reactions | 1 (1.25) |
| Motor vehicle injury | 1 (1.25) |
| Total | 24 (30) |
IPS indicates idiopathic pneumonia syndrome; ARDS, acute respiratory distress syndrome.
Univariate and multivariate analyses of risk factors for transplantation outcome
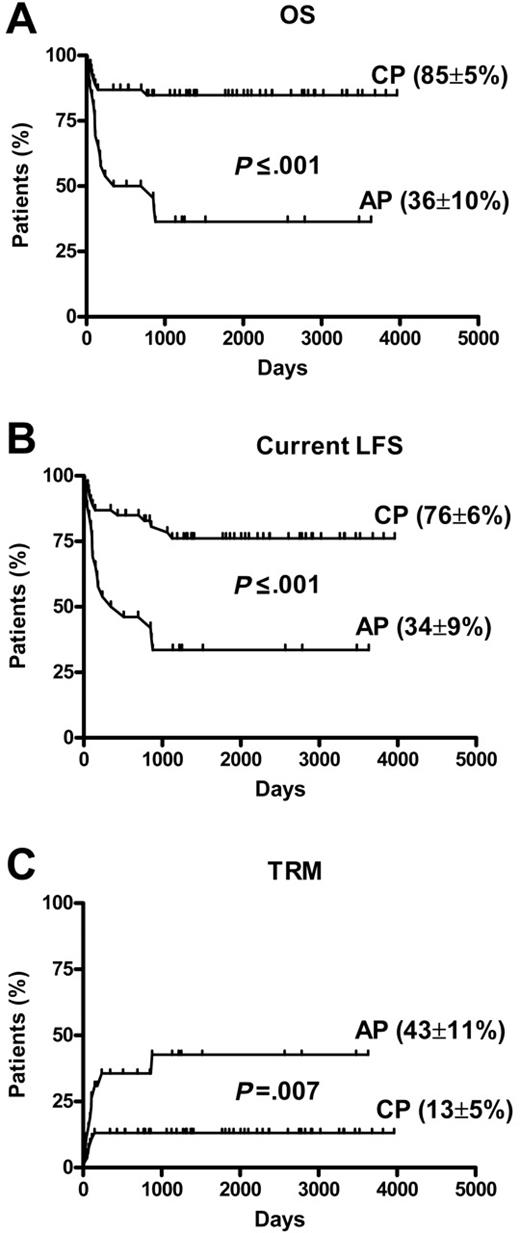
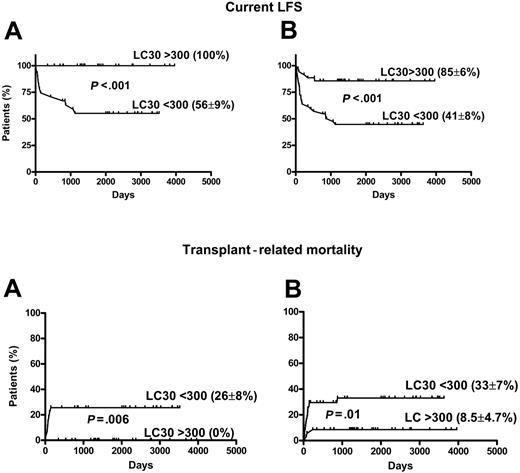
Univariate analysis data are presented in Table 2. Compared with patients with CP disease, those with AP CML had significantly inferior survival (85% ± 5% vs 36% ± 10%; P < .001) current LFS (76% ± 6% vs 34% ± 9%; P < .001) and higher TRM (13% ± 5% vs 43% ± 11%; P = .007) (Figure 1). There was a trend toward improved survival and current LFS and inferior TRM after PBSCT compared with those after BMT (Table 2). In 56 patients with CP disease, there was significantly superior survival and lower TRM after PBSCT compared with rates after BMT (OS, 91% ± 4% vs 71% ± 11%; P = .03) and TRM (5.5% ± 3.8% vs 29.4% ± 11%; P = .01). The current LFS was higher in patients with CP disease in the PBSCT group than in the BMT group, but the difference was not significant (78% ± 7% vs 70% ± 11%; P = .36). The 2 other factors significantly affecting outcome were the CD34 cell dose and LC30 treated as dichotomous variables above or below the median value. There was no impact of age, sex, donor-patient sex match, GVHD prophylaxis (cyclosporine dose), and CD3 dose on transplantation outcome in univariate analysis. No correlation was found between LC30 or CD34 cell dose and development of acute or chronic GVHD. Factors identified as significant in univariate analysis were entered into stepwise multivariate analysis (Table 4). LC30 and disease phase emerged as independent factors strongly associated with outcome. Figure 2 shows the impact of LC30 on current LFS and TRM in CP and in all patients. For patients in CP with lymphocyte counts higher than the median of 0.30 × 109/L, survival was 100% compared with 70% ± 9% for those with counts lower than the median (P = .003); current LFS 100% compared with 56% ± 9% (P = .002); and TRM 0% compared with 26% ± 8% (P = .006). Lower-than-median LC30 was associated with 6.7- and 13.2-fold relative risks for worse OS and current LFS, respectively. Separate analysis of PBSCT data revealed that LC30 remained a significant predictive factor for outcome. Compared with CP disease, AP disease was associated with a 4.6-, 3.4-, and 2.9-fold relative risk for worse OS, current LFS, and TRM (Table 4).
Multivariate analysis: factors affecting transplantation outcome
Group . | Relative ratio (95% CI) . | P . |
|---|---|---|
| All patients | ||
| Overall survival | ||
| Disease phase | 4.6 (1.9-10.9) | .001 |
| LC30 | 6.7 (1.3-34.1) | .02 |
| Current LFS | ||
| Disease phase | 3.4 (1.6-7.5) | .02 |
| LC30 | 13.2 (3.2-55.1) | < .001 |
| TRM | ||
| Disease phase | 2.9 (1.1-7.8) | .03 |
| PBSCT patients | ||
| Overall survival | ||
| Disease phase | 9.7 (2.6-36) | .001 |
| LC30 | 5.8 (1.1-31.6) | .04 |
| Current LFS | ||
| Disease phase | 5.3 (1.9-14.5) | .001 |
| LC30 | 13.7 (3.1-59.7) | <.001 |
| TRM | ||
| Disease phase | 7.8 (1.6-38.8) | .01 |
Group . | Relative ratio (95% CI) . | P . |
|---|---|---|
| All patients | ||
| Overall survival | ||
| Disease phase | 4.6 (1.9-10.9) | .001 |
| LC30 | 6.7 (1.3-34.1) | .02 |
| Current LFS | ||
| Disease phase | 3.4 (1.6-7.5) | .02 |
| LC30 | 13.2 (3.2-55.1) | < .001 |
| TRM | ||
| Disease phase | 2.9 (1.1-7.8) | .03 |
| PBSCT patients | ||
| Overall survival | ||
| Disease phase | 9.7 (2.6-36) | .001 |
| LC30 | 5.8 (1.1-31.6) | .04 |
| Current LFS | ||
| Disease phase | 5.3 (1.9-14.5) | .001 |
| LC30 | 13.7 (3.1-59.7) | <.001 |
| TRM | ||
| Disease phase | 7.8 (1.6-38.8) | .01 |
Comparisons were between chronic versus advanced phase and LC30 greater than or less than 0.30 × 109/L. Chronic phase and LC30 above 0.30 × 109/L were considered favorable factors, while advanced phase and LC30 below 0.30 × 109/L were considered unfavorable.
Transplantation outcomes. (A) OS (n = 80). (B) Current LFS (n = 80). (C) TRM chronic phase (CP) (n = 54) compared with advanced phase (AP) (n = 26).
Transplantation outcomes. (A) OS (n = 80). (B) Current LFS (n = 80). (C) TRM chronic phase (CP) (n = 54) compared with advanced phase (AP) (n = 26).
Effect of LC30 on current LFS and TRM. LC30 is measured in microliters. (A) Chronic phase (CP) (n = 54). (B) All patients (n = 80).
Effect of LC30 on current LFS and TRM. LC30 is measured in microliters. (A) Chronic phase (CP) (n = 54). (B) All patients (n = 80).
Time to molecular remission
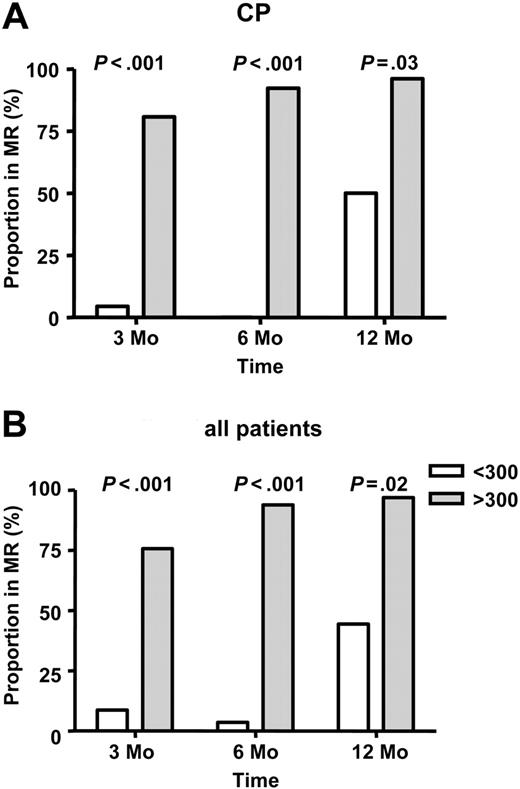
In all surviving patients, the rate of molecular remission (BCR-ABL negative) at 3, 6, and 12 months after transplantation was 28 (41%) of 68 patients, 32 (52%) of 61 patients, and 43 (73%) of 59 patients, respectively. Fifty-one (91%) of 56 patients in complete hematological remission at last follow-up were negative for BCR-ABL (median, 64.5 months; range, 5.5-135 months). In patients with CP disease, 22 of 48 patients were negative for BCR-ABL at 3 months after transplantation; this increased to 35 of 46 patients at 12 months, with 42 of 46 patients currently disease free. Among all patients, those with higher-than-median LC30 were significantly more likely to be in molecular remission at 3 months: 25 of 33 patients compared with 3 of 35 patients (P < .001); at 6 months, 31 of 33 patients compared with 1 of 28 patients (P <. 001); and at 12 months, 31 of 32 patients compared with 12 of 27 patients (P = .02) (Figure 3). Similarly, patients receiving a higher-than-median CD34 dose were more likely to be in molecular remission at 3 months (24 of 36 patients vs 4 of 32 patients; P < .001), 6 months (29 of 35 patients vs 3 of 26 patients; P < .001), and 12 months (29 of 33 patients vs 14 of 26 patients; P = .007). In multivariate analysis, higher LC30 and higher-than-median CD34 dose PBSCT emerged as independent factors associated with more rapid and complete molecular remission (Table 5).
Multivariate analysis: factors affecting molecular remission after transplantation
Group . | Relative ratio (95% CI) . | P . |
|---|---|---|
| 3 mo | ||
| Type of transplantation | 0.075 (0.02-0.028) | < .001 |
| LC30, × 109/L | 9.5 (2.9-30.2) | < .001 |
| 6 mo | ||
| Type of transplantation | 0.41 (0.005-0.34) | .003 |
| LC30, × 109/L | 37.3 (6.4-217.8) | < .001 |
| 12 mo | ||
| Type of transplantation | 0.09 (0.01-0.81) | .03 |
| LC30, × 109/L | 48.4 (4.5-518.6) | .001 |
Group . | Relative ratio (95% CI) . | P . |
|---|---|---|
| 3 mo | ||
| Type of transplantation | 0.075 (0.02-0.028) | < .001 |
| LC30, × 109/L | 9.5 (2.9-30.2) | < .001 |
| 6 mo | ||
| Type of transplantation | 0.41 (0.005-0.34) | .003 |
| LC30, × 109/L | 37.3 (6.4-217.8) | < .001 |
| 12 mo | ||
| Type of transplantation | 0.09 (0.01-0.81) | .03 |
| LC30, × 109/L | 48.4 (4.5-518.6) | .001 |
Comparisons were between PBSCTs and BMTs and LC30 above and below 0.30 × 109/L. Favorable factors were defined as PBSCT and LC30 above 0.30 × 109/L; unfavorable factors, as BMT and LC30 below 0.30 × 109/L.
Interrelationship of day-30 lymphocyte count and other factors
Because cohorts of patients underwent different transplantation regimens with different efficiencies of T-cell depletion and CD34 selection, the cyclosporine dose (standard or low), CD34 dose, and CD3 dose at transplantation were studied to explore relationships between LC30 and possible confounding factors. LC30 and CD34 cell dose were strongly correlated, whether treated as a continuous variable (correlation coefficient, 0.71; P < .001) or as a discontinuous variable. Among patients receiving more than the median of 4.5 × 106/kg CD34 cells dose, 34 (85%) of 40 patients had a higher-than-median LC30, compared with only 6 (15%) of 40 patients receiving less than the median CD34 dose (P < .001). LC30 was inversely correlated with CSA dose and transplantation lymphocyte dose: only 29% of patients who received SD cyclosporine had greater-than-median LC30, compared with 72% receiving no or low-dose CSA (P = .003). CD3 dose was inversely correlated with LC30: only 28.5% of patients receiving a transplantation CD3 dose exceeding 1 × 105/kg had greater-than-median LC30, compared with 60.5% who received less than 0.5 × 105/kg (P = .006). In multivariate analysis, low CD34 dose (less than 4.5 × 10 × 6 cells/kg) and standard-dose CSA were independently correlated with low LC30 (RR, 5.9 [P = .02]; RR, 3.6 [P = .04], respectively) (Table 6).
Factors affecting LC30 in multivariate analysis
Group . | Relative ratio (95% CI) . | P . |
|---|---|---|
| CD34 dose | 5.9 (1.3-27.2) | .02 |
| CSA dose | 3.6 (1.0-12.0) | .04 |
Group . | Relative ratio (95% CI) . | P . |
|---|---|---|
| CD34 dose | 5.9 (1.3-27.2) | .02 |
| CSA dose | 3.6 (1.0-12.0) | .04 |
Comparisons were between CD34 doses of more or less than 4.5 × 106/kg and N/LD or STD CSA doses. Favorable factors were defined as CD34 doses above 4.5 × 106/kg and N/LD CSA doses; CD34 doses below 4.5 × 106/kg and STD CSA doses were defined as unfavorable factors.
LC30 and MR in the first year after transplantation. LC30 is measured in microliters (A) Chronic phase (CP). (B) All patients.
LC30 and MR in the first year after transplantation. LC30 is measured in microliters (A) Chronic phase (CP). (B) All patients.
Lymphocyte subset analysis
Results of lymphocyte subset analysis on 10 patients with values above the median LC30 and 10 patients with values below the median LC30 are shown in Table 7. Predominant populations were donor-derived NK cells. All 10 patients with NK30 (day-30 NK cell) counts greater than 1 × 109/L survive in molecular remission (MR). Of 10 patients with lower NK30 counts, only one achieved MR; 2 patients had relapses, and 7 have died (Table 7). LC30 and NK30 were highly correlated (correlation coefficient, 885; P < .001). Similarly, CD34 dose and absolute NK cell counts were significantly correlated (correlation coefficient, 498; P = .025). In contrast, no significant correlation was seen between day-30 CD3+ cell count and CD34 dose. Higher NK cells counts were associated with rapid molecular remission (all patients with absolute NK cell counts higher than 100/μL achieved MR, compared with only one patient whose absolute NK cell count was less than 100/μL; P = .002).
Lymphocyte subset analyses and outcomes
Group, Patient no. . | LC30, × 109/L . | CD8 CD3+CD8+, % (× 109/L) . | CD4 CD3+CD4+, % (× 109/L) . | NK30, % (× 109/L) . | MR at 3 mo . | Outcome . |
|---|---|---|---|---|---|---|
| Group A | ||||||
| 12 | 0.032 | 18.3 (0.006) | 17.7 (0.006) | 56.7 (0.018) | Yes | Died; GVHD |
| 17 | 0.052 | 17.8 (0.009) | 5.1 (0.003) | 65.7 (0.034) | No | Died; relapse |
| 27 | 0.171 | 23.3 (0.040) | 17.9 (0.031) | 33.9 (0.058) | No | Alive; in MR |
| 36 | 0.030 | 1.54 (0.001) | 2.23 (0.007) | 86.3 (0.026) | NA | Died; viral encephalitis |
| 73 | 0.102 | 20.6 (0.021) | 19.4 (0.020) | 60.2 (0.061) | No | Died; relapse |
| 258 | 0.117 | 13.1 (0.015) | 3 (0.004) | 61.9 (0.072) | No | Died; IP |
| 283 | 0.278 | 48.7 (0.135) | 10.9 (0.030) | 22.7 (0.063) | No | Alive; MRel |
| 285 | 0.172 | 31.5 (0.054) | 17.4 (0.030) | 36.5 (0.063) | NA | Died; IP |
| 286 | 0.199 | 70.3 (0.140) | 6.8 (0.014) | 15.1 (0.030) | No | Alive; APRel* |
| 400 | 0.182 | 51.4 (0.094) | 23 (0.042) | 17.3 (0.031) | NA | Died; relapse |
| Group B | ||||||
| 1 | 0.622 | 3.6 (0.022) | 28.3 (0.176) | 44.4 (0.276) | Yes | Alive; in MR |
| 28 | 0.632 | 31.4 (0.198) | 3.5 (0.022) | 52 (0.328) | Yes | Alive; in MR |
| 31 | 0.918 | 16.6 (0.152) | 5 (0.046) | 60.8 (0.558) | Yes | Alive; in MR |
| 237 | 0.817 | 0 (0) | 9.1 (0.074) | 18.2 (0.149) | Yes | Alive; in MR |
| 262 | 0.449 | 18.6 (0.084) | 20.2 (0.091) | 43.7 (0.196) | Yes | Alive; in MR |
| 264 | 0.534 | 27.5 (0.147) | 11.4 (0.061) | 27.9 (0.149) | Yes | Alive; in MR |
| 270 | 1.498 | 13.2 (0.198) | 5.1 (0.076) | 67.1 (1.005) | Yes | Alive; in MR |
| 273 | 0.656 | 40.7 (0.267) | 8.8 (0.058) | 35.1 (0.230) | Yes | Alive; in MR |
| 335 | 0.874 | 39.9 (0.349) | 13.3 (0.116) | 37.7 (0.330) | Yes | Alive; in MR |
| 393 | 0.641 | 19 (0.122) | 7.5 (0.048) | 60.8 (0.390) | Yes | Alive; in MR |
Group, Patient no. . | LC30, × 109/L . | CD8 CD3+CD8+, % (× 109/L) . | CD4 CD3+CD4+, % (× 109/L) . | NK30, % (× 109/L) . | MR at 3 mo . | Outcome . |
|---|---|---|---|---|---|---|
| Group A | ||||||
| 12 | 0.032 | 18.3 (0.006) | 17.7 (0.006) | 56.7 (0.018) | Yes | Died; GVHD |
| 17 | 0.052 | 17.8 (0.009) | 5.1 (0.003) | 65.7 (0.034) | No | Died; relapse |
| 27 | 0.171 | 23.3 (0.040) | 17.9 (0.031) | 33.9 (0.058) | No | Alive; in MR |
| 36 | 0.030 | 1.54 (0.001) | 2.23 (0.007) | 86.3 (0.026) | NA | Died; viral encephalitis |
| 73 | 0.102 | 20.6 (0.021) | 19.4 (0.020) | 60.2 (0.061) | No | Died; relapse |
| 258 | 0.117 | 13.1 (0.015) | 3 (0.004) | 61.9 (0.072) | No | Died; IP |
| 283 | 0.278 | 48.7 (0.135) | 10.9 (0.030) | 22.7 (0.063) | No | Alive; MRel |
| 285 | 0.172 | 31.5 (0.054) | 17.4 (0.030) | 36.5 (0.063) | NA | Died; IP |
| 286 | 0.199 | 70.3 (0.140) | 6.8 (0.014) | 15.1 (0.030) | No | Alive; APRel* |
| 400 | 0.182 | 51.4 (0.094) | 23 (0.042) | 17.3 (0.031) | NA | Died; relapse |
| Group B | ||||||
| 1 | 0.622 | 3.6 (0.022) | 28.3 (0.176) | 44.4 (0.276) | Yes | Alive; in MR |
| 28 | 0.632 | 31.4 (0.198) | 3.5 (0.022) | 52 (0.328) | Yes | Alive; in MR |
| 31 | 0.918 | 16.6 (0.152) | 5 (0.046) | 60.8 (0.558) | Yes | Alive; in MR |
| 237 | 0.817 | 0 (0) | 9.1 (0.074) | 18.2 (0.149) | Yes | Alive; in MR |
| 262 | 0.449 | 18.6 (0.084) | 20.2 (0.091) | 43.7 (0.196) | Yes | Alive; in MR |
| 264 | 0.534 | 27.5 (0.147) | 11.4 (0.061) | 27.9 (0.149) | Yes | Alive; in MR |
| 270 | 1.498 | 13.2 (0.198) | 5.1 (0.076) | 67.1 (1.005) | Yes | Alive; in MR |
| 273 | 0.656 | 40.7 (0.267) | 8.8 (0.058) | 35.1 (0.230) | Yes | Alive; in MR |
| 335 | 0.874 | 39.9 (0.349) | 13.3 (0.116) | 37.7 (0.330) | Yes | Alive; in MR |
| 393 | 0.641 | 19 (0.122) | 7.5 (0.048) | 60.8 (0.390) | Yes | Alive; in MR |
Patients in group A: LC30 count less than 0.3 × 109/L; group B: greater than or equal to 0.3 × 109/L.
MR indicates molecular remission; IP, interstitial pneumonitis; MRel, molecular relapse; APRel, advanced-phase relapse; NA, not applicable.
Patient experienced relapse after second transplantation but achieved MR after salvage therapy with combination DLI and imatinib
Distribution of NK30 counts did not differ between BMT and PBSCT recipients (4 of 7 patients vs 6 of 13 patients, respectively, with NK30 less than 100/μL; P = 1.0) or between chronic- and advanced-phase CML (6 of 14 vs 4 of 6 patients, respectively, with NK30 less than 100/μL; P = .63) Only 3 patients were treated with imatinib in this group of 20 patients; none had received imatinib in the 3 months before transplantation.
Discussion
Allogeneic SCT is the only treatment with known curative potential for CML. Long-term survival rates associated with HLA-matched related-donor transplants range from 50% to 75% in patients with chronic-phase CML.15-18 Survival rates after transplantation for advanced-phase CML are approximately 40% and less than 20% for patients who undergo transplantation while in blast crisis.19-21 The actuarial survival for CP patients in our study was 85% ± 5%, and it was significantly higher (91% ± 4%) in a more recent cohort of patients receiving PBSCT, comparing favorably with published results. In contrast, predictably poor results were seen in our patients with advanced-phase CML (actuarial survival, 36% ± 10%). Historically, between 75% and 90% of CML patients in complete hematologic remission after transplantation test negative for residual leukemia as measured by RT-PCR amplification of BCR-ABL transcripts.4,22 We also observed high rates of molecular remission, with 91% (51 of 56 patients) of our surviving patients without detectible disease at a median follow-up of 64.5 months (range, 5.5-135 months).
Since 1993, we have progressively modified our transplantation regimen in patients with leukemia undergoing allogeneic SCT from HLA-identical siblings in attempts to improve transplantation outcomes. This analysis represents results from 6 consecutive studies using a TBI-based, T cell-depleted transplantation approach. The major changes over this period have been the adoption of PBSCT in place of BMT as a source of stem cells, a trend toward reducing the dose and duration of posttransplantation immunosuppression, and a trend toward giving increased transplantation doses of CD34 cells and reduced doses of CD3 cells (Table 1). A particular feature of all protocols has been the elective transfusion of up to 6 × 107 CD3 cells/kg within the first 100 days after transplantation in patients who have not already developed grade 2 or greater GVHD and who have hematologic, karyotypic, or molecular evidence of persisting leukemia, to enhance the GVL effect of the T cell-depleted transplant. To identify transplantation approaches and prognostic variables associated with better outcomes in CML, we performed univariate and multivariate analyses for major transplantation outcomes (OS, current LFS, TRM). The opportunity in CML to quantitate residual disease after SCT by RT-PCR to detect BCR-ABL mRNA transcripts also enabled us to study factors modifying the graft-versus-leukemia (GVL) effect. For this purpose, the pace and completeness of molecular response was measured by BCR-ABL RT-PCR at 3, 6, and 12 months after transplantation.
Our results highlight a powerful predictive effect of the day-30 posttransplantation lymphocyte count (LC30) on transplantation outcome. Because there were no deaths before day 30, all patients were evaluable by this parameter. When treated as a dichotomous variable (absolute lymphocyte count above or below the median of 0.30 × 109/L), the LC30 emerged as a unique, independent, and powerful factor for predicting OS, current LFS, TRM, and the pace of minimal residual disease disappearance. The only other factor significantly affecting outcome in multivariate analysis was the stage of disease at transplantation.
These data confirm previous observations indicating the significance of lymphocyte recovery as a prognostic factor for outcome after allogeneic stem cell transplantation.23-27 Posttransplantation lymphopenia has been associated with increased incidence of CMV infection,23,28 fungal infection,23 adenovirus infection,29 and respiratory virus infection.30 Donor-derived lymphocytes are also important in the eradication of leukemia after allogeneic SCT,31-33 and the quality of lymphocyte recovery, therefore, affects relapse in acute leukemia.26,27 According to Powles et al,26 adequate lymphocyte reconstitution can eliminate recipient hematopoiesis in the early posttransplantation phase, thereby reducing the risk for relapse. Other studies indicate that higher lymphocyte counts in the first few months correlate with an increased incidence of GVHD.23,25,28 In our patients, the more rapid control of residual disease after SCT in patients who achieved higher-than-median LC30 is further evidence linking immune recovery with the GVL effect. However, in contrast to other studies,23,25,28 we did not find any relationship between LC30 and GVHD.
Other studies show that the early recovery of immunity after allogeneic SCT involves T lymphocytes and NK cells and that slow recovery of NK cells and CD8+ cells correlates with an increased risk of relapse.34-37 In earlier studies, we found that cytotoxic T-cell and NK-cell function correlated with a GVL effect in CML.33,34 In the early posttransplantation period, most peripheral blood lymphocytes are NK cells,38 which can mediate cytotoxicity without prior sensitization and may be responsible for early GVL effects.34 Lymphocyte subset analysis in 20 patients selected for values above and below the median LC30 revealed that NK cells were the dominant population and that the LC30 reflected the absolute count of NK cells because the 2 values were highly correlated. Patients who had higher NK cell counts on day 30 after transplantation achieved rapid MR and significantly improved transplant outcomes. It is, therefore, possible that early recovery of lymphocyte reflected by NK cells, contributed not only antileukemic activity but also overall immunity, resulting in decreased nonrelapse mortality: 21 of 24 patients who died had LC30 lower than 0.30 × 109/L (Table 3), implicating an association of low NK cell numbers with poor transplantation outcome.
We cannot assume from these results that lymphocyte or NK cell counts are the prime determinants of early transplantation outcome. They may be surrogates for other measures of T-cell and NK-cell function, engraftment, or pathologic processes that ultimately result in early death and failure to eradicate residual disease. We found that the CD34 cell dose correlated strongly with the LC30 and also with the absolute NK cell count and that it was the second most powerful predictive factor for OS and current LFS and the pace and completeness of residual disease eradication (Tables 4, 5). Several studies have identified CD34 cell dose as a critical factor affecting transplantation outcome, including our own earlier studies in the T cell-depleted transplantation protocols described here.39-41 Higher CD34 doses are generally associated with reduced TRM and relapse rates and superior survival and LFS rates.42-47 However, the infusion of high doses of CD34 cell has also been associated with an increased rate of acute and or chronic GVHD.43,45,48 We observed superior OS, current LFS, and TRM when patients received more-than-median (4.5 × 106/kg) CD34 cell doses but no adverse effect of higher CD34 cell dose on either acute or chronic GVHD (data not shown).
How CD34 dose, LC30, and NK cell count are related is unclear. Kim et al23 showed that lymphocyte recovery after allogeneic SCT was affected by the stem cell and lymphocyte content of the graft. Because our transplants were T-cell depleted and because the T-cell dose was strictly controlled, it is unlikely that the CD34 dose effect was confounded by an effect from transplanted lymphocytes. Furthermore, the LC30 was higher in protocols in which smaller T-cell numbers were transplanted, and CD3+ lymphocyte counts on day 30 did not correlate with the LC30 and CD34 doses. Rather, the CD34 dose was linked to NK-cell recovery. It is, therefore, possible that higher CD34 doses established an earlier donor NK cell population, resulting in superior antileukemic activity after transplantation. Whatever the mechanism, the possible relationship between CD34 dose and immune recovery is of practical importance: given that the CD34 cell dose can be regulated (especially when abundant stem cell sources such as PBSCT are used), transplantation outcome and disease control could be improved by ensuring that patients receive CD34 cell doses on the order of 5 × 106/kg or greater. Finally, it should not be forgotten that this analysis is limited by its retrospective nature and the diversity of treatment approach. Although the samples available for NK analysis were randomly stored, it is possible that our data were affected by an inadvertent selection bias. A prospective study including day-30 lymphocyte subset analysis on a larger patient cohort would be required to further validate these findings.
Prepublished online as Blood First Edition Paper, August 30, 2005; DOI 10.1182/blood-2005-05-1897.
Supported by a grant from the Dr Mildred Scheel Stiftung für Krebshilfe, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal