Signals from the microenvironment have a profound influence on the maintenance and/or progression of hematopoietic and epithelial cancers. Mesenchymal or marrow-derived stromal cells, which constitute a large proportion of the non-neoplastic cells within the tumor microenvironment, constitutively secrete the chemokine stromal cell-derived factor-1 (SDF-1/CXCL12). CXCL12 secretion by stromal cells attracts cancer cells, acting through its cognate receptor, CXCR4, which is expressed by both hematopoietic and nonhematopoietic tumor cells. CXCR4 promotes tumor progression by direct and indirect mechanisms. First, CXCR4 is essential for metastatic spread to organs where CXCL12 is expressed, and thereby allows tumor cells to access cellular niches, such as the marrow, that favor tumor-cell survival and growth. Second, stromal-derived CXCL12 itself can stimulate survival and growth of neoplastic cells in a paracrine fashion. Third, CXCL12 can promote tumor angiogenesis by attracting endothelial cells to the tumor microenvironment. CXCR4 expression is a prognostic marker in various types of cancer, such as acute myelogenous leukemia or breast carcinoma. Promising results in preclinical tumor models indicate that CXCR4 antagonists may have antitumor activity in patients with various malignancies. Collectively, these observations reveal that CXCR4 is an important molecule involved in the spread and progression of a variety of different tumors. As such, CXCR4 antagonists, although initially developed for treatment of AIDS, actually may become effective agents for the treatment of neoplastic disease.
Chemokine system overview
The human chemokine system currently includes more than 40 chemokines and 18 chemokine receptors. Chemokine receptors are defined by their ability to induce directional migration of cells toward a gradient of a chemotactic cytokine (chemotaxis). Chemokine receptors are a family of 7 transmembrane domain, G-protein-coupled cell surface receptors that are designated CXCR1 through CXCR5, CCR1 through CCR11, XCR1, and CX3CR1, based on their specific preference for certain chemokines. Chemokines are small secreted proteins that can be segregated into 2 main subfamilies based on whether the 2 conserved cysteine residues present in all chemokines are separated by an intervening amino acid, respectively accounting for CXC or CC chemokines.
Chemokine receptors are present on many different cell types. Initially, these receptors were identified on leukocytes, where they were found to play an important role in the homing of such cells to sites of inflammation.1 However, during the past few years, hematopoietic and nonhematopoietic cells have been found to express receptors for various chemokines that are constitutively expressed in distinct tissue microenvironments. The interactions between such receptors and their respective chemokines help coordinate the trafficking and organization of cells within various tissue compartments.2,3
Lymphocytes trafficking between blood and secondary lymphoid tissues, for example, is a nonrandom process that is regulated by tissue-specific expression of chemokines.4 Circulating blood lymphocytes interact transiently and reversibly with vascular endothelium through adhesion molecules (selectins, integrins) in a process called rolling. Chemokines on the luminal endothelial surface can activate chemokine receptors on the rolling cells, which triggers integrin activation.5 This results in the arrest, firm adhesion, and transendothelial migration into tissues where chemokine gradients direct localization and retention of the cells.6 These steps, collectively referred to as “homing,” are essential for normal development of the organism, organization and function of the immune system, and tissue replacement. There is growing evidence that these physiologic mechanisms of tissue-specific recruitment also are functional in neoplastic cells.
CXCR4 chemokine receptor and its ligand, SDF-1 (CXCL12)
Stromal cell-derived factor-1 (SDF-1), which now is designated as CXCL12,7 is a homeostatic chemokine that signals through CXCR4,8 which in turn plays an important role in hematopoiesis, development, and organization of the immune system. Initial studies on CXCR4 focused on its role in the pathogenesis of HIV infection. The discovery that CXCR4 functions as a coreceptor for entry of T-tropic (X4) HIV viruses into CD4+ T cells9 initiated a broad research effort to elucidate the function of this receptor-ligand pair. Cxcl12 and Cxcr4 gene-deleted mice displayed an identical, lethal phenotype, indicating a monogamous relation between this chemokine and its receptor. The phenotype of these mice is characterized by deficient B-lymphopoiesis and myelopoiesis and by abnormal neuronal and cardiovascular development.10-13 CXCL12 is a highly conserved chemokine that has 99% homology between mouse and man, allowing CXCL12 to act across species barriers. The CXCR4-CXCL12 axis is functional in evolutionarily distant organisms such as zebra fish and mice, in which CXCR4 expression is a prerequisite for germ cell migration to CXCL12-expressing gonads during development.14-16
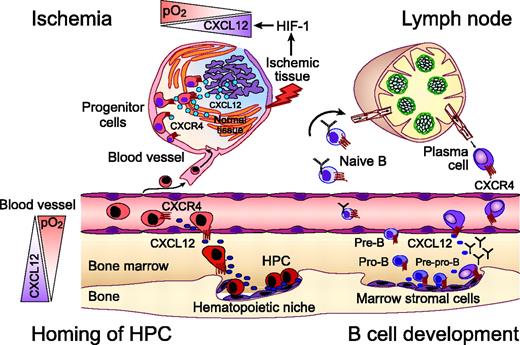
The CXCR4 chemokine receptor in homing of hematopoietic progenitors, B-lymphocyte development, and progenitor recruitment to sites of ischemic tissue damage. Homing and retention of hematopoietic progenitor cells (HPCs) within the marrow microenvironment requires active migration of HPCs to distinct hematopoietic niches. CXCL12 gradients within the marrow induce firm adhesion of circulating HPCs to endothelial cells via CXCR4, followed by transmigration and homing to marrow stromal cells. Regions of hypoxia within the marrow microenvironment display high CXCL12 concentrations that attract HPCs, as indicated by the triangles on the left-hand side. Homing of circulating progenitors, such as cardiac, endothelial, or neural progenitors, to peripheral tissues for repair after ischemic injuries also requires CXCR4. Hypoxia-inducible factor-1 (HIF-1) induces expression of CXCL12 in direct proportion to reduced oxygen tension at sites of injury. As such, CXCL12 recruits CXCR4-expressing circulating progenitors to “conditional” stem cell niches for tissue repair. Early steps of B-lymphocyte development from HPCs are critically regulated by close contact with marrow stromal cells. Both early B-cell precursor (pre-pro-B cells) and end-stage B cells (plasma cells) require CXCR4 for homing to specific niches within the marrow. Other developmental stages of B cells migrate to other niches within the marrow (pro B cells), leave the marrow, and circulate through the secondary lymphatic tissues where B- and T-zone chemokines (CXCL13, CCL19, CCL21) regulate their homing. Plasma cell differentiation is associated with a coordinated switch in chemokine sensitivity with an increased sensitivity to CXCL12, which allows for plasma cell homing to the marrow.
The CXCR4 chemokine receptor in homing of hematopoietic progenitors, B-lymphocyte development, and progenitor recruitment to sites of ischemic tissue damage. Homing and retention of hematopoietic progenitor cells (HPCs) within the marrow microenvironment requires active migration of HPCs to distinct hematopoietic niches. CXCL12 gradients within the marrow induce firm adhesion of circulating HPCs to endothelial cells via CXCR4, followed by transmigration and homing to marrow stromal cells. Regions of hypoxia within the marrow microenvironment display high CXCL12 concentrations that attract HPCs, as indicated by the triangles on the left-hand side. Homing of circulating progenitors, such as cardiac, endothelial, or neural progenitors, to peripheral tissues for repair after ischemic injuries also requires CXCR4. Hypoxia-inducible factor-1 (HIF-1) induces expression of CXCL12 in direct proportion to reduced oxygen tension at sites of injury. As such, CXCL12 recruits CXCR4-expressing circulating progenitors to “conditional” stem cell niches for tissue repair. Early steps of B-lymphocyte development from HPCs are critically regulated by close contact with marrow stromal cells. Both early B-cell precursor (pre-pro-B cells) and end-stage B cells (plasma cells) require CXCR4 for homing to specific niches within the marrow. Other developmental stages of B cells migrate to other niches within the marrow (pro B cells), leave the marrow, and circulate through the secondary lymphatic tissues where B- and T-zone chemokines (CXCL13, CCL19, CCL21) regulate their homing. Plasma cell differentiation is associated with a coordinated switch in chemokine sensitivity with an increased sensitivity to CXCL12, which allows for plasma cell homing to the marrow.
Constitutive secretion of CXCL12 by marrow stromal cells is a major source for CXCL12 in the adult. Stromal cells create cellular niches in which hematopoietic stem cells (HSCs) and progenitors are retained for growth and differentiation.17,18 Chemotactic responsiveness of hematopoietic stem cells is restricted to CXCL12.19 This unique selectivity for CXCL12 may be necessary for retention of HSCs in the hematopoietic microenvironment and marrow-specific homing of circulating HSCs (Figure 1).20,21 Recent clinical trials have demonstrated that CXCR4 antagonists, alone or in combination with granulocyte colony-stimulating factor (G-CSF), can affect rapid mobilization of HSCs, supporting the hypothesis that CXCL12 is essential for HSC retention within the marrow.22 Also, because CXCL12 helps retain B-cell precursors in close contact with protective stromal cells within the hematopoietic microenvironment, its expression is essential for normal B-cell development.23,24 End-stage B cells (plasma cells) also require CXCR4 for homing to CXCL12-rich niches within the marrow (Figure 1).25
Moreover, CXCL12 also may function as a paracrine growth factor for B lymphocytes and other cell types. Initial studies characterized CXCL12 as a pre-B-cell growth-stimulating factor (PBSF), because recombinant CXCL12 supported the proliferation of a stromal cell-dependent B-cell line.26 More recent studies demonstrated that CXCL12 supports the survival or growth of a variety of normal or malignant cell types, including hematopoietic progenitors,27 germ cells,16 leukemia B cells,28 and breast carcinoma cells.29
Another highly important function of the CXCR4-CXCL12 axis is related to tissue repair and regeneration. Repair of ischemic injuries involves the selective recruitment of circulating or resident progenitor cells. Hypoxia-inducible factor-1 (HIF-1), a central mediator of tissue hypoxia, induces CXCL12 expression in ischemic areas in direct proportion to reduced oxygen tension in vivo.30 HIF-1-induced CXCL12 expression on endothelial cells attracts circulating stem and progenitor cells to areas of tissue damage. As such, hypoxia induces a transient, conditional stem cell niche for CXCR4-mediated progenitor cell recruitment for tissue repair (Figure 1). During tissue regeneration the expression of CXCL12 normalizes after regular oxygen tension has been restored.30 Distinct niches of hypoxia are also present in the normal marrow that display increased levels of CXCL12. Progenitor cells colocalize with these niches, suggesting that HIF-1-regulated CXCL12 expression plays an important role in generating concentration gradients of CXCL12 within the marrow microenvironment.30
In addition to inducing CXCL12, HIF-1 enhances the expression and function of CXCR4 on normal and malignant cells.31,32 Under regular oxygen tension, pVHL, the product of the von Hippel-Lindau tumor suppressor gene (VHL), induces degradation of HIF-1. However, during hypoxia, or in cases of renal cell carcinoma (RCC) that harbor mutations in the VHL gene, HIF-1 accumulates and induces expression of CXCR4. In patients with clear cell RCC, tumor cells with inactivating mutations in the VHL gene express higher levels of CXCR4 than tumor cells without such mutations, a characteristic that is associated with poor survival.32,33 This provides a possible mechanism to explain how CXCR4 is induced during tumor cell evolution, which may allow neoplastic cells to egress from areas of low oxygen. As such, the VHL-HIF-1 pathway, which apparently regulates expression of CXCR4 and CXCL12 for the homing of progenitor cells to injured tissues,30 may be adapted by neoplastic cells to allow for their metastatic spread to CXCL12-expressing organs, such as the marrow.34
CXCR4 in the trafficking of hematopoietic malignancies
Chronic lymphocytic leukemia
B-cell chronic lymphocytic leukemia (CLL) is characterized by the accumulation of monoclonal lymphocytes that appear to originate from mature, antigen-experienced B cells. CLL cells accumulate in the blood, marrow, and secondary lymphoid tissues. Despite their apparent longevity in vivo, isolated CLL cells generally undergo spontaneous apoptosis in vitro when cultured under conditions that support the growth of human B-cell lines.28 As such, the mechanism(s) governing the resistance to apoptosis may not be entirely intrinsic to the neoplastic B cell.
In the marrow and lymphoid tissues, CLL cells are in close contact with accessory cells (stromal cells and/or “nurselike cells” [NLCs]) that constitute distinct microenvironments. NLCs are large, round, adherent cells that differentiate in long-term cultures of blood mononuclear cells from patients with CLL28 and can be detected in secondary lymphoid tissues of patients with CLL.35 In vitro, stromal cells or NLCs can support long-term leukemia cell survival.28 By inference, the leukemia cell microenvironment also may enhance the survival of CLL cells in vivo and possibly account for the noted resistance of CLL cells to many forms of cancer chemotherapy.
CLL cells express high levels of CXCR4.36 Coculture of CLL cells with marrow stromal cells that secrete CXCL12 induces the neoplastic B cells to migrate to and then underneath the stromal cells in a CXCR4-dependent fashion.36 Similar to marrow stroma, NLCs attract CLL cells via CXCR4 and protect CLL cells from spontaneous or drug-induced apoptosis in a contact-dependent fashion. These observations support a model proposing that expression of CXCR4 by CLL cells allows for their recirculation between the blood and the marrow or lymphoid tissues, where they receive protective survival signals.
Because CXCL12 not only attracts CLL cells to supportive microenvironments but also directly stimulates CLL cell survival,28 the CXCR4-CXCL12 axis may be an important therapeutic target in CLL. Recently, we demonstrated that small peptide CXCR4 antagonists effectively block CXCL12-induced activation, migration, and signaling of CLL cells.37 Stromal cell-mediated protection from spontaneous or fludarabine-induced apoptosis of CLL cells was partially blocked, suggesting a potential role of CXCR4 antagonists in combination with a B-cell targeted therapy in the treatment of CLL.
Other mature B-cell malignancies
Multiple myeloma. During B-cell differentiation into plasma cells, plasma cells undergo a coordinated change in chemokine responsiveness. As B cells differentiate into plasma cells, they become increasingly sensitive to CXCL12 while losing responsiveness to B- and T-zone chemokines (CXCL13, CCL19, CCL21) through respective down-regulation of CXCR5 and CCR7.38,39 In addition to CXCR4, plasma cells express functional CXCR6, CCR10, and CCR3 chemokine receptors.40 Tokoyoda et al25 recently demonstrated a stage-specific homing of the earliest B-cell precursors (pre-pro-B cells) and plasma cells to the same marrow niches in which stromal cells secrete high levels of CXCL12, suggesting that CXCL12 maintains immature and terminally differentiated B-cell types in the marrow microenvironment (Figure 1). However, recent animal models suggest that CXCR4 is not a stringent requirement for plasma cell homing to the marrow. Gene-targeted mice that selectively lack CXCR4 expression in the B-cell compartment fail to retain B-cell precursors in the marrow, along with an extensive apoptosis of the misplaced B-cell precursors. Despite the lack of CXCR4, substantial numbers of long-lived plasma cells can be found in the marrow of such mice, which may be due to different chemokine requirements for different plasma cell populations generated during immune responses. On the basis of their animal model with B lineage CXCR4 deletion, Nie et al41 proposed that plasma cells generated during early phases of immune responses in secondary lymphoid tissues depend on CXCR4 for marrow homing, whereas a different population of plasma cell precursors can home to the marrow independent of CXCR4 and differentiate to long-lived antibody-secreting cells in situ.
Multiple myeloma (MM) cells home to the BM where they adhere to marrow stromal cells and extracellular matrix (ECM) proteins in the marrow microenvironment through VLA-4 integrins to stromal fibronectin. In addition, myeloma cells display functional CXCR4 chemokine receptors that cooperate with VLA-4 integrins in myeloma cell adhesion and migration.42 This mechanism may allow myeloma cells to home to the marrow microenvironment, where adhesive interactions promote growth, survival, and confer cell adhesion-mediated drug resistance (CAM-DR).43
Other B-cell lymphomas. CXCR4 expression has been demonstrated in B-cell36,44 and T-cell45 non-Hodgkin lymphoma (NHL). Malignant B cells from patients with B-NHL express functional CXCR4 receptors and other chemokine receptors, such as CXCR3, CXCR5, CCR7, CCR5, and others.46-48 The distinct pattern of chemokine receptor expression is thought to be involved in lymphoma cell trafficking and homing and may allow to distinguish different NHL subsets.47 In an animal model, mice were challenged with T-cell hybridoma cells that were engineered to retain CXCR4 within the cytoplasm. In contrast to controls, these CXCR4-deficient hybridoma cells failed to disseminate to CXCL12-expressing target organs.49 In another mouse model of human high-grade NHL, CXCR4 neutralization by monoclonal antibodies inhibited homing of circulating NHL cells and improved survival. Bertolini et al50 therefore suggested CXCR4 neutralization as a novel therapeutic approach in NHL.
CXCR4 in acute leukemias. In contrast to solid tumors that invade into the marrow, acute leukemias originate in the marrow. In the marrow microenvironment, acute leukemia cells are in close contact with marrow stromal cells that provide growth and survival signals through surface-bound or secreted factors. Because CXCR4 plays a critical role for retention of hematopoietic progenitors in the marrow, several groups have examined the role played by CXCR4 in progenitor cell leukemias. Precursor-B-cell acute lymphoblastic leukemia (ALL) expresses functional CXCR4 receptors51 that participate in homing of leukemia cells to the marrow in nonobese diabetic severe combined immunodeficient (NOD/SCID) mice.52,53 In an elegant animal model, Sipkins et al34 provided direct evidence that functional CXCR4 is necessary for the homing of ALL cells to the marrow microenvironment. CXCR4 receptors also are functional in acute myelogenous leukemia (AML),54 even though expression levels vary between different cases of AML. Perhaps for this reason, there may be varying degrees of dependency on CXCR4 for engraftment of AML cells in NOD/SCID mice.55,56
CXCR4 in acute myelogenous leukemia (AML). Despite a general sensitivity to chemotherapy, long-term disease-free survival in AML remains low because a majority of patients relapse from minimal residual disease (MRD). The marrow is considered the primary site for MRD where adhesion to stromal elements may protect AML cells from cytotoxic drugs. Adhesion molecules of the very late antigen (VLA)-4 integrin type on AML cells play a critical role for mediating adhesion to stromal fibronectin.57 CXCR4 may facilitate VLA-4 signaling by directing spontaneous migration of AML cells beneath marrow stromal cells (“pseudoemperipolesis”).58 On adhesion to marrow stromal fibronectin, AML cells become resistant to spontaneous or drug-induced apoptosis in vitro.57 In a mouse model of AML, the combination of anti-VLA-4 antibodies with cytarabine (AraC) induced long-term remissions, whereas AraC alone caused only a minor prolongation of survival.57 Therefore, adhesion molecules and CXCR4 appear to be central regulators of survival signals that account for anti-cancer drug resistance. This concept is supported by the finding that high-level expression of CXCR4 by leukemia cells is an adverse prognostic indicator in AML.59,60
CXCR4 in nonhematopoietic malignancies
There is increasing evidence that epithelial tumor cells exploit mechanisms that normally regulate leukocyte trafficking and homing. The distinct pattern of chemokine receptor expression by tumor cells has a critical role in determining the site(s) of metastatic spread.61 In addition, stromal cells within the tumor microenvironment at primary or metastatic sites apparently can regulate tumor progression (Figure 2). Adhesion to stromal cells supports the growth of neoplastic cells through the high-level expression of CXCL1229 and integrins that can activate CXCR4 and/or growth-promoting tyrosine kinases.62
CXCR4 in breast cancer
The spread of breast cancer follows a distinct metastatic pattern typically involving spread of tumor to regional lymph nodes, lung, liver, and/or marrow. The ligand for CXCR4, CXCL12, is highly expressed by stromal fibroblasts within these tissues.61,63 In contrast to normal breast tissue, breast cancer cells typically express high levels of functional CXCR4 receptors that can direct chemotaxis and invasive responses.61 Treatment with anti-CXCR4 monoclonal antibodies (mAbs) inhibits the metastatic spread to target organs in vivo.61 High-level expression of CXCR4 on neoplastic cells is associated with relatively poor overall survival in patients with breast cancer.64
High-level expression of HER2/neu, which is observed in about 30% of all breast cancers, also is associated with a relatively poor prognosis. Li et al64 recently demonstrated that HER2/neu enhances the expression and function of CXCR4 by inhibiting CXCR4 degradation.
Stromal fibroblasts constitute a major proportion of the cellular microenvironment in breast cancer. Carcinoma-associated fibroblasts, but not normal mammary fibroblasts, express high levels of CXCL12, as demonstrated on the mRNA and protein level29 and by gene expression profiling (Figure 2).63 Expression of CXCL12 in turn promotes the progression of breast cancer by directly enhancing tumor-cell growth and by recruiting endothelial progenitor cells that are required for tumor angiogenesis (Figure 2).29 As such, the high-level expression of CXCL12 by carcinoma-associated fibroblasts apparently can promote breast cancer progression in both a paracrine and an endocrine fashion.
Importance of the CXCR4 chemokine receptor and its ligand, CXCL12, in the tumor microenvironment and for targeted metastasis. Within hypoxic areas of tumors, both CXCL12 expression by fibroblasts and CXCR4 expression on tumor cells increases, which stimulates tumor cell motility and invasiveness. Carcinoma-associated fibroblasts (CAFs), but not normal fibroblasts, stimulate tumor progression by CXCL12 secretion. Two major mechanisms by which fibroblast-derived CXCL12 promotes tumor genesis have been identified. First, CXCL12 promotes tumor cell growth in a paracrine fashion by directly stimulating tumor cell growth via CXCR4. Second, CXCL12 from CAFs induces recruitment of endothelial progenitors, which allow for tumor angiogenesis (endocrine effect of CXCL12). Targeted metastasis to the marrow or other sites of high CXCL12 expression involves CXCR4 activation on circulating tumor cells that “hijack” the CXCR4-CXCL12 axis for homing to microenvironments that normally are restricted to hematopoietic progenitor cells (HPCs). CXCL12 gradients attract CXCR4-positive tumor cells to marrow niches where marrow stromal cells secrete high levels of CXCL12. As a consequence, tumor cells can displace HPCs from their protective microenvironment, resulting in hematopoietic dysfunction. Moreover, tumor cells may invade adjacent tissues, resulting in bone destruction.
Importance of the CXCR4 chemokine receptor and its ligand, CXCL12, in the tumor microenvironment and for targeted metastasis. Within hypoxic areas of tumors, both CXCL12 expression by fibroblasts and CXCR4 expression on tumor cells increases, which stimulates tumor cell motility and invasiveness. Carcinoma-associated fibroblasts (CAFs), but not normal fibroblasts, stimulate tumor progression by CXCL12 secretion. Two major mechanisms by which fibroblast-derived CXCL12 promotes tumor genesis have been identified. First, CXCL12 promotes tumor cell growth in a paracrine fashion by directly stimulating tumor cell growth via CXCR4. Second, CXCL12 from CAFs induces recruitment of endothelial progenitors, which allow for tumor angiogenesis (endocrine effect of CXCL12). Targeted metastasis to the marrow or other sites of high CXCL12 expression involves CXCR4 activation on circulating tumor cells that “hijack” the CXCR4-CXCL12 axis for homing to microenvironments that normally are restricted to hematopoietic progenitor cells (HPCs). CXCL12 gradients attract CXCR4-positive tumor cells to marrow niches where marrow stromal cells secrete high levels of CXCL12. As a consequence, tumor cells can displace HPCs from their protective microenvironment, resulting in hematopoietic dysfunction. Moreover, tumor cells may invade adjacent tissues, resulting in bone destruction.
CXCR4 in lung cancer
Small-cell lung cancer (SCLC) is an aggressive, rapidly metastasizing neoplasm with a high propensity for marrow involvement. Even with combination chemotherapy and radiotherapy treatments, the 5-year survival is only about 5% because of rapid development of drug resistance. SCLC preferentially metastasizes to the marrow, whereas other lung cancers have a tendency to metastasize to the osseous tissue. Because of this distinct metastatic pattern and high constitutive CXCL12 expression within the marrow microenvironment, we hypothesized that SCLC cells might use the CXCR4 receptor for marrow metastasis. Indeed, we and others found that CXCR4 is the major chemokine receptor commonly expressed by primary SCLC cells or SCLC cell lines.65,66 In SCLC cells, CXCR4 activation induces migratory and invasive responses and adhesion to marrow stromal cells in a CXCR4- and integrin-dependent fashion. Moreover, signaling via CXCR4 on SCLC cells induces activation and signaling of tumor-associated integrins that apparently play an important role in tumor progression.67 Adhesion to marrow stromal cells or extracellular matrix proteins via integrins can protect SCLC cells from chemotherapy-induced apoptosis.62,67 Stromal cell protection of SCLC cells can be inhibited by CXCR4 antagonists, suggesting that CXCR4 antagonists interfere with survival signal from the microenvironment.67 Collectively, these studies indicate that expression of CXCR4 by SCLC cells cooperates with integrins that regulate the adhesion of tumor cells to the marrow microenvironment, which in turn confers drug resistance and tumor cell growth. Moreover, CXCR4 may direct the distinct metastatic pattern observed in patients with SCLC.
The neoplastic cells in NSCLC also may express CXCR4, but at levels that are lower than that expressed by SCLC cells.65 Interestingly, hypoxia could induce a significant increase in the expression level of CXCR4 on NSCLC cells through the VHL-HIF-1 pathway,68 supporting the notion that HIF-1-mediated up-regulation of CXCR4 may be a common response of tumor cells to hypoxia.
CXCR4 in other nonhematopoietic malignancies
Over the past 5 years, several other nonhematopoietic neoplasias have been described to express CXCR4 chemokine receptors. CXCR4 activation by CXCL12 induces migration and/or survival of the neoplastic cells, including tumor cells from brain neoplasm (neuronal and glial tumors),69,70 neuroblastoma cells,71 colorectal cancer,72 prostate cancer,73 melanoma,74 renal cell cancer, ovarian cancer,75 and others. In patients with colorectal cancer76 and melanoma,74 CXCR4 expression of primary tumor cells correlates with recurrence, metastasis, and survival. The importance of von Hippel-Lindau tumor suppressor pVHL for CXCR4 expression in renal cell carcinoma32,33 has been discussed in the section about CXCR4 and CXCL12. These studies also describe CXCR4 expression by the tumor cells as a prognostic factor in RCC.
Targeting the microenvironment with CXCR4 antagonists
Genetic lesions and aberrant signaling networks within cancer cells have been the main focus in cancer biology over the past decades. As a consequence of this oncogene- and tumor suppressor-centric view, most current cancer therapies target the tumor cells, which frequently acquire resistance owing to their inherent genomic instability. Because signals from the tumor microenvironment may make pivotal contributions to the progression of hematopoietic and epithelial malignancies, increasing emphasis is being placed on targeting the tumor cell microenvironment.
The discovery that CXCR4 functions as a coreceptor for X4 HIV-1 viruses that evolve during the course of HIV-1 infection generated a high interest in the development of small molecular CXCR4 receptor antagonists for treatment of HIV. With the emergence of the physiologic functions of CXCR4, other potential applications of CXCR4 antagonists are becoming apparent. As noted, the CXCR4-CXCL12 axis may play a central role in the spread and progression of many different types of tumors. Agents that target this receptor or its ligand therefore could conceivably mobilize tumor cells from their protective microenvironments, antagonize the paracrine growth effects, and/or make tumor cells more accessible to conventional therapy. Currently, CXCR4 antagonists are being evaluated in clinical trials for mobilization of hematopoietic stem cells and in preclinical studies for treatment of neoplastic or autoimmune diseases.
Several CXCR4 antagonists have been described of which 2 (AMD3100 and ALX40-4C) have been administered to human subjects.22 Mobilization of hematopoietic progenitors in 26 healthy volunteers by the CXCR4 antagonist AMD3100 has been reported by Liles et al.77 A single injection at doses up to 240 μg/kg/d subcutaneously induced a rapid, generalized leukocytosis associated with a transient up to 10-fold increase in peripheral blood CD34+ cells. Administration was well tolerated with only mild, transient toxicity. In addition, Devine et al78 recently reported AMD3100 as a safe and effective agent for the mobilization of CD34+ cells in patients with multiple myeloma or lymphoma who have received prior chemotherapy. In another clinical study, 2 of 40 HIV-infected individuals treated with AMD3100 experienced premature ventricular contractions, which resulted in the discontinuation of this trial.79 Another CXCR4 antagonist termed T140 is a 14-residue polypeptide that was downsized from a naturally occurring horseshoe crab self-defense peptide. Comparative studies on T140 and AMD3100 found that each inhibits CXCR4 via different mechanisms.80,81 AMD3100 and ALX40-4C have weak partial agonist (CXCL12-like) activity, inducing CXCL12-like G-protein activation in CXCR4-expressing cells upon binding of these CXCR4 antagonists.81 In contrast, T140 functions as an inverse agonist80,81 and does not induce signaling on binding to CXCR4. Because CXCR4 activation caused by a CXCR4 antagonist could be a disadvantage to the treatment of diseases in which CXCR4 activation provides a survival signal (such as in CLL or breast cancer cells), T140-derived CXCR4 antagonists might have an advantage over AMD3100 or ALX40-4C in the treatment of neoplastic disease. However, potential side effects of CXCR4 antagonists have to be considered, particularly if they would be administered in combination with chemotherapy. The mobilization of normal hematopoietic progenitor cells by CXCR4 antagonists is a major concern, because this could increase the toxicity of cytotoxic drugs to normal hematopoiesis. A tumor cell-targeted therapy in combination with CXCR4 antagonists could avoid this potential hazard. Moreover, resistance of tumor cells to CXCR4 antagonists may represent a potential problem. As reported by Labrosse et al,82 single amino acid substitutions of CXCR4 confer resistance to AMD3100. Such structural changes may occur during tumor development, potentially decreasing the efficacy of CXCR4 antagonists.
In any case, both AMD3100- and T140-derived CXCR4 antagonists appear to have activity in animal tumor models, providing rationale for future clinical trials of these agents in patients with various cancers.83-86 The key role of CXCR4 in the context of crosstalk between tumor cells and their respective microenvironment suggests that CXCR4-targeted therapeutic approaches may become clinically relevant in the near future.
Prepublished online as Blood First Edition Paper, November 3, 2005; DOI 10.1182/blood-2005-08-3182.
Supported by Deutsche José Carreras Leukämiestiftung (grant no. DJCLS R02/08) (J.A.B.) and Deutsche Krebshilfe (grant no. 10-1688-Bu) (J.A.B.).
We apologize that because of space limitation we were not able to discuss and cite a number of additional studies of other investigators that are related to CXCR4 in neoplastic diseases.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal