Varicella-zoster virus (VZV) disease occurs in 30% of allogeneic hematopoietic cell transplant recipients who had a history of VZV infection. A safe and effective prevention strategy has not been established. In a double-blind controlled trial, 77 hematopoietic cell transplant recipients at risk for VZV reactivation were randomized to acyclovir 800 mg twice daily or placebo given from 1 to 2 months until 1 year after transplantation. VZV disease at 1 year was the primary end point; VZV disease after discontinuation of prophylaxis, VZV-specific T-cell immunity, herpes simplex virus (HSV) infection, cytomegalovirus (CMV) disease, survival, and safety were secondary end points. Acyclovir significantly reduced VZV infections at 1 year after transplantation (HR, 0.16; 95% CI, 0.035-0.74; P = .006). In the postintervention observation period, this difference was not statistically significant (2 years: HR, 0.52; 95% CI, 0.21-1.3; 5 years: HR, 0.76; 95% CI, 0.36-1.6). There was no statistically significant difference in reconstitution of VZV-specific T-helper cell responses, HSV infections, CMV disease, chronic graft-versus-host disease, and overall survival between the groups. Acyclovir was well tolerated. Post-study VZV disease predominantly occurred in patients with continued need for systemic immunosuppression. In conclusion, acyclovir effectively and safely prevents VZV disease during the first year after hematopoietic cell transplantation. Periods of prophylaxis longer than 12 months may be beneficial for those hematopoietic cell transplant recipients on continued immune suppression.
Introduction
Reactivation of varicella-zoster virus (VZV) disease is common after allogeneic hematopoietic cell transplantation (HCT), occurring in more than 30% of patients.1-7 Most cases occur between the third and twelfth month after transplantation. During this time, the care of these patients is predominantly managed by their referring physicians rather than at specialized cancer centers. Morbidity, including pain, scarring, post-herpetic neuralgia, and secondary infection occur in up to one third of patients with VZV infection, and hospitalization may be required with disseminated disease.1,7-9 VZV disease can occasionally also be fatal after allogeneic HCT, even if treated with acyclovir, with most fatalities occurring during the first year after transplantation.1-3 Furthermore, hepatic or gastrointestinal VZV disease may occur with few or no skin lesions, which often delays the diagnosis and leads to a high mortality.1,10-23 Severe ocular complications and VZV myelitis may also occur.1,24,25 Finally, patients with VZV reactivation disease, especially when disseminated, may expose susceptible individuals to VZV.26 This can cause major disruptions in health care settings that treat immunosuppressed susceptible individuals.26 Although effective antiviral treatment with acyclovir is available for VZV infection and disease,8,27,28 it may not be completely effective in preventing mortality,1,3,17 and treatment does not reduce initial morbidity.
Two randomized placebo-controlled trials showed that acyclovir given for the first 6 months after transplantation significantly reduced the incidence of VZV disease during prophylaxis.23,29 However, there were a substantial number of infections that occurred after discontinuation of acyclovir, and VZV-specific immune reconstitution was suppressed in acyclovir recipients. Currently, the Centers of Disease Control and Prevention (CDC) and the American Society of Blood and Marrow Transplantation (ASMBT) recommend not to use long-term prophylaxis for prevention of VZV disease (DIII recommendation).26
The purpose of this study was to determine if 800 mg of acyclovir given orally twice a day for 1 year after transplantation is a safe, practical, and effective means of preventing reactivation VZV disease. Additional goals were to determine the effect on herpes simplex virus (HSV) infections, to assess VZV-specific immune reconstitution during the period of prophylaxis, and to determine the risk of VZV disease following long-term prophylaxis.
Patients and methods
Patients and study design
Patients undergoing allogeneic marrow transplantation for hematologic malignancy who were 10 years of age or older with a history of chicken pox (prior to transplantation) were included in this trial. Patients were randomized after engraftment when they could tolerate oral medication to receive acyclovir (Zovirax) prophylaxis or placebo. Randomization was stratified for the presence of acute graft-versus-host disease (GVHD), defined as grades 2 to 4. Patients received either oral acyclovir as an 800-mg tablet twice daily or an oral placebo tablet twice daily (Figure 1). Patients with a serum creatinine level higher than 265.2 μM (3.0 mg/dL) and all patients who had experienced herpes zoster infection after marrow transplantation were ineligible. Patients who discontinued study drug due to adverse events were not rechallenged. Study drug was started between day 30 and day 100 (Table 1) and continued until 1 year after transplantation.
Patient characteristics
. | Acyclovir . | Placebo . |
|---|---|---|
| No. patients | 38 | 39 |
| Mean age at transplantation, y (range) | 29 (10-47) | 32 (14-65) |
| Sex, no. (%) | ||
| Male | 16 (42.1) | 21 (53.8) |
| Female | 22 (57.3) | 18 (46.2) |
| Race, no. (%) | ||
| Caucasian | 36 (94.7) | 37 (94.9) |
| Underlying diseases, no. (%) | ||
| Acute leukemia | 10 (26.3) | 17 (43.6) |
| Chronic leukemia | 20 (52.7) | 17 (43.6) |
| Lymphoma | 4 (10.5) | 2 (5.1) |
| Aplastic anemia | 4 (10.5) | 1 (2.6) |
| Other | 0 (0) | 2 (5.1) |
| Donor type, no. (%) | ||
| HLA matched, related | 30 (78.9) | 31 (79.5) |
| HLA mismatched, related | 5 (13.2) | 7 (17.9) |
| HLA matched, unrelated | 3 (7.9) | 1 (2.6) |
| Acute GVHD, grade 2-4, at randomization, no. (%) | 15 (39.5) | 13 (33.3) |
| Median time of randomization, d (range) | 59 (10-91) | 62 (16-83) |
| Median start time of study drug, d (range) | 63 (31-98) | 63 (29-101) |
| Relapse of underlying disease during first 3 y, no. (%) | 17 (45) | 16 (41) |
| Survival at 1 y after transplantation, no. (%) | 32 (84.2) | 26 (67) |
. | Acyclovir . | Placebo . |
|---|---|---|
| No. patients | 38 | 39 |
| Mean age at transplantation, y (range) | 29 (10-47) | 32 (14-65) |
| Sex, no. (%) | ||
| Male | 16 (42.1) | 21 (53.8) |
| Female | 22 (57.3) | 18 (46.2) |
| Race, no. (%) | ||
| Caucasian | 36 (94.7) | 37 (94.9) |
| Underlying diseases, no. (%) | ||
| Acute leukemia | 10 (26.3) | 17 (43.6) |
| Chronic leukemia | 20 (52.7) | 17 (43.6) |
| Lymphoma | 4 (10.5) | 2 (5.1) |
| Aplastic anemia | 4 (10.5) | 1 (2.6) |
| Other | 0 (0) | 2 (5.1) |
| Donor type, no. (%) | ||
| HLA matched, related | 30 (78.9) | 31 (79.5) |
| HLA mismatched, related | 5 (13.2) | 7 (17.9) |
| HLA matched, unrelated | 3 (7.9) | 1 (2.6) |
| Acute GVHD, grade 2-4, at randomization, no. (%) | 15 (39.5) | 13 (33.3) |
| Median time of randomization, d (range) | 59 (10-91) | 62 (16-83) |
| Median start time of study drug, d (range) | 63 (31-98) | 63 (29-101) |
| Relapse of underlying disease during first 3 y, no. (%) | 17 (45) | 16 (41) |
| Survival at 1 y after transplantation, no. (%) | 32 (84.2) | 26 (67) |
While patients were still in Seattle (approximately until day 100 after transplantation), they were monitored every 2 weeks for the presence of adverse events or problems suspected to be resulting from the drug study. Upon departure from Seattle, study participants were given information about typical signs and symptoms of herpes zoster infection. After leaving Seattle, follow-up consisted of monthly phone calls to the study participants and their local physicians. During these calls, information was collected regarding illness or problems, adherence, and laboratory values. Patients were questioned concerning clinical symptoms (and virologic evidence when possible) of VZV. Patients and their physicians were requested to contact the primary investigator immediately if VZV disease was suspected or if an adverse reaction was suspected.
After 1 year, patients were followed for an additional year for occurrence of VZV disease and survival. Follow-up consisted of phone calls to the patients and/or their physicians at 3-month intervals. After that, long-term VZV disease and survival data after the primary treatment phase of the study were obtained using the long-term follow-up database. This database includes results from annual surveys on symptoms and complications, transcripts of telephone consultations, copies of clinic notes, hospital admission and discharge reports, and death reports.
The study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center, and informed consent was obtained from all patients.
Use of acyclovir before randomization
All patients who were seropositive to HSV prior to transplantation were given prophylactic acyclovir (250 mg/m2 every 12 hours intravenously) from 7 days before transplantation until day 30 after transplantation.
Determination of VZV serology and viral infection
VZV serostatus prior to transplantation was tested by enzyme immunoassay (EIA) (VZV STAT, Whittaker Bioproducts, Walkersville, MD). For suspected VZV infections that occurred in Seattle, scraping material from lesions was inoculated into standard viral cultures. Testing at off-site locations was done at local laboratories. HSV testing was done weekly from throat cultures during the first 100 days after transplantation; subsequently, results from local testing were used.
VZV disease was defined by typical clinical symptoms, and a culture or histopathology was requested for confirmation. Study drug was discontinued in those patients who developed active VZV, and they were treated with open-label acyclovir. HSV infection was defined as a positive viral culture from throat washing, urine, or mucocutaneous lesion. If patients on study drug developed active HSV infection and oral acyclovir was used for treatment that required treatment with acyclovir, study drug was continued throughout the treatment course. If intravenous acyclovir was required for the treatment of active HSV infection, study drug was discontinued during the course of intravenous acyclovir and restarted afterward. Susceptibility testing for breakthrough isolates was not performed.
VZV- and HSV-specific T-cell immunity
VZV- and HSV-specific CD4+ T-cell responses were tested at the time of study entry and when VZV occurred or at the end of prophylaxis, whichever occurred first. Examination of cell-mediated immune responses included VZV-specific lymphocyte proliferation as previously described.30 Results were expressed as the stimulation index, calculated as the mean cpm of cells exposed to VZV or HSV antigen divided by cpm of cells exposed to control antigen.30 A stimulation index of 3 or greater was considered a positive response.30,31
Adverse effects
All patients receiving at least one dose of study drug were assessed for toxicity. Evaluations occurred at a minimum of every 3 months through the first year of study and included a physical exam and vital signs, complete blood count, platelet count, serum aminotransferase level, bilirubin level, blood urea nitrogen level, serum creatinine level, and urinalysis. Patients also were questioned concerning rash, nausea, vomiting, gagging, or other unusual symptoms. Severity and relatedness of adverse events with the study drug were assessed during the double-blind phase by the study investigators (R.A.B., J.D.M.).
Statistical analysis
The projected incidence of VZV by 1 year after transplantation in the placebo group was 30%.1 Assuming a reduction of 85% in the VZV disease rate with acyclovir (ie, VZV rate = 4.5%), 40 patients in each arm provided 81% power to detect this difference with a type 1 error rate (alpha) of 5% using the log-rank test. Patients were stratified for the presence of grade 2 to 4 GVHD. The analysis was performed on all patients who were randomized and received at least one dose of study medication (modified intent-to-treat analysis, Figure 1). The primary end point was VZV disease at 1 year after transplantation (study drug was started between day 30 and day 100). Secondary end points were VZV infections after 1 year, HSV infections, CMV disease, toxicity, survival, and VZV- and HSV-specific immune reconstitution. Results were analyzed at 10 months after randomization and at 1 year after transplantation using time-to-event analyses. Since results were virtually identical, primary and secondary end-point results at 1 year after transplantation are presented.
Incidence of VZV, HSV, CMV infection, and chronic GVHD were estimated with cumulative incidence curves, treating death and second transplantation before the event of interest as competing risk events. Survival was estimated with Kaplan and Meier curves. To compare risks of VZV, HSV, CMV infection, chronic GVHD, and death between the 2 arms, hazard ratios and the corresponding 95% confidence intervals were estimated using Cox proportional hazards regression models. The number of patients who need to be treated to prevent one additional VZV event was estimated using the method proposed by Altman and Anderson.32 For all patients alive at 1 year, risk factors for VZV disease after 1 year were examined using Cox proportional hazard regression analyses. Demographic characteristics were compared by chi-square or Fisher exact test. Proportions of patients demonstrating immune reconstitution were compared by Fisher exact test. All reported P values are 2 sided.
Results
Patient characteristics
Eighty-three patients were randomized to the study; 41 to receive acyclovir and 43 to receive placebo. Three patients in each group never started the drug and were excluded from further analysis (Figure 1). Table 1 summarizes characteristics of the remaining 38 patients in the acyclovir group and 39 patients in the placebo group (modified intent-to-treat group). There were no significant differences in any of the demographic characteristics between the groups (Table 1).
VZV disease
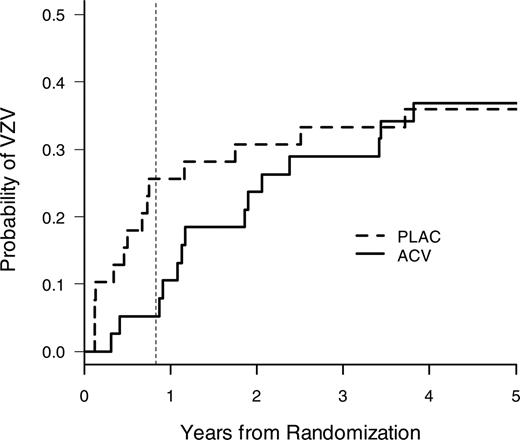
VZV disease at 1 year after transplantation was the primary end point of the study. A highly significant reduction of VZV disease was seen in acyclovir recipients (Figure 2, Table 2). Both breakthrough cases of VZV in the acyclovir group in the modified intent-to-treat analysis occurred in patients who were off acyclovir. An additional on-treatment analysis among patients who took the study drug for at least 30 days showed a complete elimination of VZV disease during the first year (data not shown). A subset analysis of disseminated VZV disease also showed a reduction with acyclovir during the first year (acyclovir, 0 of 38; placebo, 4 of 39 [10.3%]).
VZV disease in the 2 study groups
VZV . | Acyclovir, no. (%) . | Placebo, no. (%) . | HR . | 95% CI . | P . |
|---|---|---|---|---|---|
| By year 1* | 2 (5) | 10 (26) | 0.16 | 0.035, 0.74 | .006 |
| By year 2 | 8 (21) | 12 (31) | 0.52 | 0.21, 1.3 | .15 |
| By year 3 | 11 (29) | 13 (33) | 0.65 | 0.29, 1.5 | .30 |
| By year 5† | 14 (37) | 14 (36) | 0.76 | 0.36, 1.6 | .46 |
VZV . | Acyclovir, no. (%) . | Placebo, no. (%) . | HR . | 95% CI . | P . |
|---|---|---|---|---|---|
| By year 1* | 2 (5) | 10 (26) | 0.16 | 0.035, 0.74 | .006 |
| By year 2 | 8 (21) | 12 (31) | 0.52 | 0.21, 1.3 | .15 |
| By year 3 | 11 (29) | 13 (33) | 0.65 | 0.29, 1.5 | .30 |
| By year 5† | 14 (37) | 14 (36) | 0.76 | 0.36, 1.6 | .46 |
Shown are results from the intent-to-treat analysis and among patients who received at least 30 days of study drug.
HR indicates hazard ratio; CI, confidence interval.
Year refers to year after transplantation
One additional case occurred in each group between 6 and 10 years after transplantation
Time to first VZV disease in the modified intent-to-treat population. Acyclovir prophylaxis significantly reduced VZV disease at the end of the active treatment period (1 year) (Table 2). The vertical dotted line indicates 1 year after transplantation (note: patients were randomized approximately 2 months after transplantation).
Time to first VZV disease in the modified intent-to-treat population. Acyclovir prophylaxis significantly reduced VZV disease at the end of the active treatment period (1 year) (Table 2). The vertical dotted line indicates 1 year after transplantation (note: patients were randomized approximately 2 months after transplantation).
Overall, 23 of 28 patients (82.1%) with VZV disease during the first 5 years had GVHD. Acyclovir also significantly reduced the incidence of VZV disease during the first year in the subset of patients with GVHD (HR, 0.17; 95% CI, 0.04, 0.78). All patients who developed VZV disease and for whom treatment results are available (64%) responded to treatment with intravenous acyclovir.
After discontinuation of prophylaxis, VZV disease occurred in both groups (Figure 2, Table 2). Late disseminated VZV disease occurred in 2 patients in the acyclovir group (14 and 44 months after transplantation) and in one patient in the placebo group (33 months after transplantation); the remaining cases were of dermatomal distribution. A risk factor analysis for VZV disease after 1 year showed that the most important risk factors for late VZV disease were continued immunosuppression and unrelated and HLA-mismatched donor status (Figure 3, Table 3), and it was the only significant risk factor in the multivariate analysis (results not shown). The treatment group (acyclovir vs placebo) was not statistically significantly associated with late VZV disease (Table 3).
Risk factors for VZV disease after 1 year (univariate analysis)
. | . | Modified intent-to-treat cohort . | . | . | ||
|---|---|---|---|---|---|---|
| Variable . | Comparison . | HR . | 95% CI . | P . | ||
| Study medication | Acyclovir vs placebo | 1.9 | 0.67, 5.2 | .22 | ||
| Ongoing use of immunosuppressive drugs* | Yes vs no | 3.1 | 1.2, 8.3 | .02 | ||
| Sex | Male vs female | 1.2 | 0.48, 3.1 | .68 | ||
| Age at transplantation | 10 years and older | 1.2 | 0.76, 1.8 | .47 | ||
| HLA match | MM/UR vs matched | 3.5 | 1.1, 11 | .05 | ||
| Ongoing use of immunosuppressive drugs* or MM/UR donor status | Immune suppressive or MM/UR vs others | 3.5 | 1.3, 9.9 | .01 | ||
. | . | Modified intent-to-treat cohort . | . | . | ||
|---|---|---|---|---|---|---|
| Variable . | Comparison . | HR . | 95% CI . | P . | ||
| Study medication | Acyclovir vs placebo | 1.9 | 0.67, 5.2 | .22 | ||
| Ongoing use of immunosuppressive drugs* | Yes vs no | 3.1 | 1.2, 8.3 | .02 | ||
| Sex | Male vs female | 1.2 | 0.48, 3.1 | .68 | ||
| Age at transplantation | 10 years and older | 1.2 | 0.76, 1.8 | .47 | ||
| HLA match | MM/UR vs matched | 3.5 | 1.1, 11 | .05 | ||
| Ongoing use of immunosuppressive drugs* or MM/UR donor status | Immune suppressive or MM/UR vs others | 3.5 | 1.3, 9.9 | .01 | ||
MM indicates HLA-mismatched donor; UR, unrelated donor.
Ongoing immunosuppression was defined as continued use of systemic immunosuppressive drugs
Number of patients to treat
Giving patients acyclovir prophylaxis until 1 year after transplantation prevented one case of VZV disease at 1 year for every 3.9 patients treated (95% CI 2.3, 13). If one extrapolates the same efficacy of acyclovir that was seen in the intent-to-treat analysis to patients at high risk for late VZV disease (continued immunosuppression or unrelated or HLA-mismatched donors, Table 4), one case of VZV disease would be prevented for every 3.8 patients treated in the second year after transplantation (95% CI, 2.1, 18).
Secondary end points (shown are results in the acyclovir group versus the placebo group)
. | Modified intent-to-treat analysis . | . | . | ||
|---|---|---|---|---|---|
| End point* . | HR . | 95% CI . | P . | ||
| Survival† | |||||
| By 1 year | 0.43 | 0.16, 1.1 | .07 | ||
| By 2 years | 0.65 | 0.29, 1.5 | .30 | ||
| By 3 years | 0.58 | 0.27, 1.2 | .15 | ||
| By 5 years | 0.55 | 0.26, 1.2 | .11 | ||
| VZV-free survival† | |||||
| By 1 year | 0.33 | 0.14, 0.74 | .005 | ||
| By 2 years | 0.59 | 0.31, 1.1 | .10 | ||
| By 3 years | 0.61 | 0.34, 1.1 | .09 | ||
| By 5 years | 0.64 | 0.37, 1.1 | .10 | ||
| Chronic GVHD by 1 year | 1.4 | 0.68, 0.29 | .36 | ||
| HSV infection by 1 year | 0.64 | 0.21, 2.0 | .42 | ||
| CMV disease by 1 year | 1.2 | 0.31, 4.3 | .84 | ||
. | Modified intent-to-treat analysis . | . | . | ||
|---|---|---|---|---|---|
| End point* . | HR . | 95% CI . | P . | ||
| Survival† | |||||
| By 1 year | 0.43 | 0.16, 1.1 | .07 | ||
| By 2 years | 0.65 | 0.29, 1.5 | .30 | ||
| By 3 years | 0.58 | 0.27, 1.2 | .15 | ||
| By 5 years | 0.55 | 0.26, 1.2 | .11 | ||
| VZV-free survival† | |||||
| By 1 year | 0.33 | 0.14, 0.74 | .005 | ||
| By 2 years | 0.59 | 0.31, 1.1 | .10 | ||
| By 3 years | 0.61 | 0.34, 1.1 | .09 | ||
| By 5 years | 0.64 | 0.37, 1.1 | .10 | ||
| Chronic GVHD by 1 year | 1.4 | 0.68, 0.29 | .36 | ||
| HSV infection by 1 year | 0.64 | 0.21, 2.0 | .42 | ||
| CMV disease by 1 year | 1.2 | 0.31, 4.3 | .84 | ||
Years refer to year after transplantation
Hazard ratios less than 1 indicate improved survival relative to placebo
Secondary end points
Secondary end points are shown in Table 4. Most cases of HSV disease occurred before randomization and start of study drug (median, 2 months in both groups, Table 1). In the intent-to-treat analysis, there was a trend toward reduced incidence of HSV, but it did not reach statistical significance (Table 4); however, the overall number of cases was small. An analysis of HSV infections that occurred between randomization and day 100 (the time period when weekly surveillance cultures were obtained) showed that no HSV occurred in acyclovir recipients, compared to 4 infections in placebo patients.
Risk of late VZV disease relative to use of immunosuppression. Cumulative incidence of VZV disease after 1 year in all patients and those who are still on systemic immunosuppression or received a transplant from an unrelated or HLA-mismatched donor versus patients not on immunosuppression or with an HLA-matched related donor.
Risk of late VZV disease relative to use of immunosuppression. Cumulative incidence of VZV disease after 1 year in all patients and those who are still on systemic immunosuppression or received a transplant from an unrelated or HLA-mismatched donor versus patients not on immunosuppression or with an HLA-matched related donor.
Immune reconstitution
All patients had serologic evaluation prior to study entry. Very few patients with a positive history for VZV were actually found to be seronegative at the time of study entry (acyclovir group: 89.5% positive, 7.9% seronegative, 2.6% equivocal; placebo group: 92.3% seropositive, 2.6% seronegative, 5.1% equivocal). VZV- and HSV-specific CD4+ T-cell responses are shown in Figure 4. The proportion of patients with positive VZV- and HSV-specific CD4+ T-cell responses was not different between placebo and acyclovir recipients. The median stimulation index was not different between the study arms for both VZV and HSV at baseline and follow-up testing (data not shown). Among patients who had baseline and follow-up testing performed, 6 of 12 patients recovered VZV-specific responses while receiving placebo (4 following VZV disease), compared to 5 of 14 while on acyclovir (P = .7).
Adverse effects
There were no significant differences between adverse events between the 2 groups. Gastrointestinal adverse effects occurred in 3 of 38 patients in the acyclovir group, compared to 0 of 39 patients in the placebo group (P = .1); 2 of the 3 cases were considered possibly related and one definitely related (gagging). Other adverse events included pancytopenia (n = 1), increased liver function tests (n = 1), and hospitalization due to Guillain Barré syndrome (n = 1) in the acyclovir group and neutropenia (n = 1) in the placebo group. All nongastrointestinal adverse events were considered unrelated or unlikely related. Symptoms in the patient with Guillain Barré syndrome improved while continuing study drug.
Discussion
This study demonstrated that acyclovir given at 800 mg twice daily until 1 year after transplantation is a safe and highly effective strategy to prevent clinical VZV infections in hematopoietic cell transplant recipients. This acyclovir regimen allowed VZV-specific immune reconstitution and did not lead to a statistically significant rebound VZV disease after drug discontinuation. VZV disease after drug discontinuation occurred predominantly in patients with continued need for systemic immunosuppressive drugs.
Although the modified intent-to-treat analysis showed a few cases of VZV disease in the acyclovir group, all these cases occurred in patients who were unable to take the drug. Per study design these patients were not rechallenged but permanently taken off study drug. An on-treatment analysis of patients who were able to take the drug showed a complete elimination of VZV disease. The number of patients needed to treat to prevent one case of disease is 3.9. In this study, most cases of VZV disease occurred in patients with GVHD. However, VZV disease also may occur in allogeneic hematopoietic cell transplant recipients without GVHD as well as in autologous transplant recipients, and GVHD was not a risk factor for disease in all studies.3,7,13,33,34
Acyclovir was effective in preventing the morbidity of herpes zoster, thereby also decreasing the need for the often-necessary hospitalization and intravenous treatment, as well as late complications such as post-herpetic neuralgia and secondary infections. Although not seen in this study, acyclovir prophylaxis is likely to prevent visceral VZV disease that presents without skin manifestations. This complication occurs in approximately 1 in 250 VZV-seropositive allograft recipients, and a review of the literature indicates that this highly fatal complication continues to be a significant clinical problem.1,10-23,25 An additional indirect benefit is the risk reduction for susceptible VZV-seronegative individuals.
VZV- and HSV-specific T-cell reconstitution at start of study drug and at 1 year after transplantation or VZV disease (whichever occurred first). A positive result was defined as a stimulation index of 3 or greater. Shown is the proportion of patients with a positive response. None of the differences are statistically significant. Phytohemagglutinin (PHA) responses were present in all but one patient in both study arms.
VZV- and HSV-specific T-cell reconstitution at start of study drug and at 1 year after transplantation or VZV disease (whichever occurred first). A positive result was defined as a stimulation index of 3 or greater. Shown is the proportion of patients with a positive response. None of the differences are statistically significant. Phytohemagglutinin (PHA) responses were present in all but one patient in both study arms.
Acyclovir was well tolerated. Only mild gastrointestinal adverse effects were considered related to the study medications. This is consistent with the excellent safety record of acyclovir in transplant recipients, which has been demonstrated with prolonged administration of doses significantly higher than that used in this study.23,29,35-37
After this study was started valacyclovir and famciclovir became available. These compounds have a higher bioavailability and efficacy.36 However, since acyclovir at the dose used in this study was 100% effective in preventing VZV disease when it was taken (even in patients with GVHD), it is unclear how these compounds would improve outcome. It is conceivable that once-a-day dosing would be possible with these compounds or perhaps a more complete suppression of HSV. Both questions would require further study.
One important goal of this study was to determine if “rebound” VZV reactivation disease occurred with the prolonged administration of acyclovir. Earlier studies that used 6 months of prophylaxis had shown an increase of disease after drug discontinuation.23,34 This was associated with a delay of VZV- and HSV-specific T-cell reconstitution in one study.23 The present study showed that VZV disease after 1 year predominantly occurred in patients with continued immunosuppression and those with an HLA-mismatched or unrelated donor. Contrary to the earlier study by Ljungman et al,23 prior use of acyclovir was not associated with an increased risk of late VZV infection. If anything, the acyclovir effect appeared to continue for some time, although the difference between the groups lost statistical significance at 2 years and thereafter. This, in addition to the finding that VZV-specific immune reconstitution did not appear to be negatively affected, suggests that the risk of late VZV disease is predominantly driven by the underlying immunosuppression. Using qualitative assays, VZV- and HSV-specific T-cell recovery occurred in both groups in similar proportions. This suggests that subclinical VZV reactivation continues to occur with this twice-a-day acyclovir regimen and that this antigen exposure is sufficient for immune reconstitution.38 We speculate that extended use of this acyclovir in these patients might be beneficial. Extended antimicrobial prophylaxis in patients with continued immunosuppression is presently recommended for prevention of Pneumocystis jiroveci pneumonia.26 The median duration of immunosuppressive treatment for chronic GVHD is approximately 2 years overall and even longer after peripheral blood stem cell (PBSC) transplantation.39 Continued risk for late VZV reactivation occurs predominantly in this clinical setting (Figure 4). Prospective studies are needed to examine this question. An alternative strategy to reduce VZV disease is vaccination. A recent study in autologous transplant recipients who received posttransplant inactivated varicella vaccination, clinical VZV was reduced by 57%.40 No data exists on clinical efficacy of this approach in allogeneic transplant recipients treated for GVHD.
As expected, the present strategy was not effective in preventing CMV disease. An effect on HSV was detected but did not reach statistical significance. This is not surprising since the overwhelming majority of HSV infection occurred before randomization, and there were too few cases to show a significant difference in this study population. The study was not powered to detect differences in late HSV infections. When we analyzed the cases that occurred on study, they almost exclusively occurred in patients who had discontinued acyclovir for various reasons while true HSV breakthrough infection was very rare. There was a trend toward better overall survival, and VZV-free survival was improved in acyclovir recipients.
This study was conducted in bone marrow transplant recipients after myeloablative conditioning. Recent studies demonstrate that the risk of VZV disease is similarly high in patients who receive PBSC31,41,42 transplantation and even higher in recipients of cord blood transplants.4 VZV-specific immune reconstitution was similar after PBSC transplantation when compared to marrow transplantation.31 Furthermore, the risk of VZV disease appears to be similar after nonmyeloablative conditioning regimens.43 Thus, the risk of VZV disease appears to be similar in patients undergoing transplantation with alternative stem cell sources or conditioning regimens, suggesting that VZV prophylaxis may be similarly beneficial.
In conclusion, acyclovir prophylaxis effectively prevented VZV disease after allogeneic transplantation. This regimen is highly effective, safe, inexpensive, and did not appear to interfere with VZV-specific immune reconstitution. It was not associated with “rebound” VZV disease after drug discontinuation. Since most of the severe cases of VZV disease occur during the first year after transplantation,1-3,44 we believe this strategy deserves consideration for standard prophylaxis in this setting. Moreover, extended use of acyclovir during the period of immunosuppression has the potential of being useful for the long-term reduction of VZV disease, although defining the time of drug discontinuation may be difficult. Based on a recent study,44 prophylaxis may have to be continued for several months after discontinuation of immunosuppressive drugs or until reconstitution of VZV-specific immunity is documented. Studies are needed to examine such strategies.
Prepublished online as Blood First Edition Paper, November 10, 2005; DOI 10.1182/blood-2005-09-3624.
Supported by the Burroughs Wellcome Fund (clinical trial) and the National Institutes of Health (CA 18029, CA 15704) (final data analysis and follow-up data collection).
Joel D. Meyers died in 1991.
M.B. analyzed and interpreted the data, supervised review of long-term followup data, and wrote the manuscript. H.W.K. performed the statistical analysis and reviewed the manuscript. M.E.D.F. was responsible for long-term follow-up data collection and reviewed the manuscript. J.D.M. designed the study and analyzed adverse events. R.A.B. designed the study, supervised the clinical trial and immune reconstitution studies, analyzed adverse events, interpreted the data, and critically reviewed the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Karin Rogers, RN, for coordinating the clinical trial; Christie Dahlgren, RN, for chart review; Chris Davis, MS, and Gary Schoch, MS, for database services; Terry Stevens-Ayers, BA, for data analysis; and John Digel, BS, for technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal