Abstract
To evaluate the prognostic impact of minimal residual disease (MRD), quantitative real-time polymerase chain reaction (RQ-PCR) of clonal IGH rearrangements was performed in 29 patients with mantle cell lymphoma (MCL) treated with high-dose radiochemotherapy and autologous stem cell transplantation (ASCT). Fourteen of 27 patients evaluable for MRD after ASCT achieved complete clinical and molecular remission, whereas 13 patients had detectable MRD within the first year after ASCT. Molecular remission after ASCT was strongly predictive for improved outcome, with a median progression-free survival (PFS) of 92 months in the MRD-negative group compared with 21 months in the MRD-positive group (P < .001). Median overall survival (OS) was 44 months in the MRD-positive group and has not been reached in the MRD-negative group (P < .003). In multivariate analysis, molecular remission and bulky disease were independent prognostic factors for PFS (P = .001 and P = .021, respectively). While cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP)–like cytoreduction had only modest influence, ara-C–containing mobilization and myeloablative radiochemotherapy significantly reduced MRD. Quantitative MRD measured in the stem cell products of 27 patients was not predictive for molecular remission. We conclude that sequential quantitative monitoring of residual disease after ASCT is a powerful indicator for treatment outcome in MCL and defines subgroups of patients with a significantly different prognosis.
Introduction
Mantle cell lymphoma (MCL) is characterized by advanced stage of disease at diagnosis and an aggressive clinical course with a short median overall survival of 3 to 4 years after standard treatment.1,2 Biologically, MCL is characterized by the chromosomal translocation t(11;14)(q13;q32), leading to CCND1 protein overexpression. The translocation is detectable by molecular cytogenetic methods in more than 90% of patients with MCL. Current treatment strategies include combinations of the monoclonal anti-CD20 antibody rituximab with different chemotherapy regimens3-5 and more intensive treatment protocols, including high-dose radiochemotherapy followed by autologous or allogeneic hematopoietic stem cell transplantation.
Autologous stem cell transplantation (ASCT) as part of first-line treatment leads to a substantial prolongation of disease-free survival, at least in younger patients with MCL. 5-9 However, a significant proportion of these patients will ultimately relapse, but prognostic markers allowing individual risk estimation are lacking. Contamination of the autologous stem cell product by lymphoma cells is considered a major reason for recurrence of the disease after ASCT. Antibody-based immunologic purging methods of autologous stem cells fail to produce tumor cell–free stem cell products in most patients with MCL.10,11
Molecular monitoring of minimal residual disease (MRD) by polymerase chain reaction (PCR) amplification of clone-specific immunoglobulin heavy chain (IGH) gene rearrangements or BCL2-IGH fusion genes has been shown to be of prognostic value after ASCT in patients with follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL), respectively.12,13 Moreover, the recent development of assays for quantitative molecular MRD assessment has allowed comparison of the relative impact of different treatment modalities (conventional chemotherapy, ASCT, and monoclonal antibodies) on the tumor load and provided insights into the kinetics of tumor regrowth after cytotoxic treatment in FL and CLL.4,13,14 Similarly, clonal IGH rearrangements and the MCL-specific BCL1-IGH fusion gene are suitable molecular targets for molecular assessment of MRD in MCL.15,16 However, because of limited patient numbers and lack of relevant patient populations achieving MRD negativity, a useful prognostic algorithm could not be established by qualitative PCR in MCL,17 and there are no data on the prognostic impact of quantitative MRD monitoring after ASCT.
We therefore addressed the question of a potential clinical role for quantitative MRD monitoring after ASCT for MCL by sensitive quantitative real-time PCR (RQ-PCR). We evaluated the prognostic impact of MRD on disease control and compared the effect of different treatment modalities (standard chemotherapy, ara-C–containing mobilization chemotherapy, and myeloblative radiochemotherapy with or without peritransplantation-administered rituximab) on MRD load in MCL. Finally, we were able to study the kinetics of lymphoma regrowth after ASCT by longitudinal MRD assessment.
Patients and methods
In the present study, 29 patients with advanced MCL and a PCR-detectable clonal IGH rearrangement in diagnostic peripheral blood (PB), bone marrow (BM), or lymph node samples were selected for MRD assessment. Median age of patients was 56 years (range, 38-68 years), and 28 of 29 patients had advanced stage III-IV disease. Patient characteristics are summarized in Table 1.
Patient characteristics
. | . | Patients with MRD follow-up . | . | |
|---|---|---|---|---|
| Variable . | Patients . | MRD positive . | MRD negative . | |
| No. | 29* | 13 | 14 | |
| Age, y (range) | 56 (38-68) | 56 (41-61) | 56 (38-68) | |
| Men/women, no. | 24/5 | 13/0 | 10/4 | |
| Disease stage, no. | ||||
| II | 1 | 0 | 1 | |
| III | 3 | 2 | 1 | |
| IV | 25 | 11 | 12 | |
| Induction treatment, no. | ||||
| COP | 2 | 1 | 1 | |
| CHOP | 26 | 12 | 12 | |
| PmM | 1 | 0 | 1 | |
| Disease status before ASCT, no. | ||||
| CR | 7 | 1 | 6 | |
| PR | 17 | 10 | 6 | |
| Second PR | 4 | 2 | 1 | |
| MR | 1 | 0 | 1 | |
| Disease status after ASCT, no. | ||||
| CR | 22 | 6 | 14 | |
| PR | 7 | 7 | 0 | |
| Rituximab exposure, no. | 12 | 3 | 9 | |
| LDH abnormal, no. | 4† | 0† | 4 | |
| Extranodal site, no. | 15‡ | 8† | 6† | |
| BM involvement, no. | 19 | 10 | 7 | |
| Bulky disease, no. | 12† | 7 | 5† | |
| Spleen enlargement, no. | 12† | 6 | 5† | |
. | . | Patients with MRD follow-up . | . | |
|---|---|---|---|---|
| Variable . | Patients . | MRD positive . | MRD negative . | |
| No. | 29* | 13 | 14 | |
| Age, y (range) | 56 (38-68) | 56 (41-61) | 56 (38-68) | |
| Men/women, no. | 24/5 | 13/0 | 10/4 | |
| Disease stage, no. | ||||
| II | 1 | 0 | 1 | |
| III | 3 | 2 | 1 | |
| IV | 25 | 11 | 12 | |
| Induction treatment, no. | ||||
| COP | 2 | 1 | 1 | |
| CHOP | 26 | 12 | 12 | |
| PmM | 1 | 0 | 1 | |
| Disease status before ASCT, no. | ||||
| CR | 7 | 1 | 6 | |
| PR | 17 | 10 | 6 | |
| Second PR | 4 | 2 | 1 | |
| MR | 1 | 0 | 1 | |
| Disease status after ASCT, no. | ||||
| CR | 22 | 6 | 14 | |
| PR | 7 | 7 | 0 | |
| Rituximab exposure, no. | 12 | 3 | 9 | |
| LDH abnormal, no. | 4† | 0† | 4 | |
| Extranodal site, no. | 15‡ | 8† | 6† | |
| BM involvement, no. | 19 | 10 | 7 | |
| Bulky disease, no. | 12† | 7 | 5† | |
| Spleen enlargement, no. | 12† | 6 | 5† | |
Two of 29 patients had insufficient DNA samples for MRD analysis within the first year after ASCT.
Status was not documented in 1 patient.
Status was not documented in 2 patients.
Patients were treated according to a local treatment protocol aimed at investigating the role of early sequential high-dose therapy in patients with advanced MCL7,18 or a prospective trial of the German Lymphoma Study Group (GLSG).4 The protocols had been approved by the responsible institutional review boards. Informed consent was provided according to the Declaration of Helsinki. All patients received initial standard treatment of 2 to 6 cycles cyclophosphamide, vincristine, prednisolone (COP, n = 2), prednimustine, mitoxantrone (PmM, n = 1), or cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP, n = 26).
Stem cell mobilization was performed with 1 (n = 10), 2 (n = 18), or 3 (n = 1) cycles of DexaBEAM (dexamethasone 3 × 8 mg days 1-10, bischloroethylnitrosourea [BCNU] 60 mg/m2 day 2, etoposide 75 mg/m2 days 4-7, cytarabine 100 mg/m2 every 12 hours days 4-7, melphalan 20 mg/m2 day 3) followed by daily G-CSF administration (10 μg/kg subcutaneously), as previously described.7 In one patient, high-dose cytarabine (cytarabine 2 g/m2 every 12 hours days 1-2 and mitoxantrone 10 mg/m2 days 2-3) was used in addition to 1 cycle of DexaBEAM for mobilization.
Myeloablative therapy consisted in 25 patients of fractionated total body irradiation (TBI) of 12 Gy (2 Gy administered twice daily on 3 consecutive days) followed by high-dose cyclophosphamide (60 mg/kg daily on 2 consecutive days [TBI/Cy]). Three patients received BEAM as a conditioning regimen (BCNU 300 mg/m2, etoposide 800 mg/m2, cytarabine 1600 mg/m2, and melphalan 140 mg/m2), and in one patient busulfan 4 × 4 mg/kg plus cyclophosphamide 2 × 60 mg/m2 was used. In 10 of 29 patients, 2 doses of the monoclonal anti-CD20 antibody rituximab (375 mg/m2) were added to the myeloablative regimen on days –8 and –2 (R-TBI/Cy) as part of a pilot study.18 Two patients each received 2 doses of rituximab before ASCT, deviating from the treatment protocol.
Twenty-five patients underwent transplantation while in first remission (11 in CR, 14 in PR), whereas 4 patients had a history of relapse or disease progression and underwent transplantation while in second PR with chemosensitive disease. Stem cell products from 8 patients underwent CD34+ selection with the Isolex300 system (Nexell Therapeutics, Irvine, CA) according to the manufacturer's instructions.19 All other stem cell products were retransfused unmanipulated.
Histologic, immunohistochemical, and cytogenetic analyses
The diagnosis of MCL was established according to WHO criteria.20 Conventionally and immunohistochemically stained paraffin sections of lymph node biopsies were reviewed. Minimal requirements for immunohistochemistry included positivity for CD20 (monoclonal, 1:80 dilution; DAKO, Hamburg, Germany) and CD5 and negativity for CD23 and CD10 (monoclonal; DAKO). The proliferation marker Ki-S5 (monoclonal against Ki-67; Department of Hematopathology, University of Kiel) was used to evaluate the proliferation index. Histology slides and immunohistochemical stainings (CD20, CD5, CD23, Ki-S5) were evaluated according to the Annecy criteria,21 and MCLs were subclassified according to the different cytologic subtypes. Histology slides were reviewed by a member of the European Mantle Cell Lymphoma Study Group (M.T.).
In 23 patients, paraffin sections were available for histologic review. Twenty-one patients had classic MCL; of those, one had a classic variant with pleomorphic sections and one had a blastoid variant of MCL.
The presence of a t(11;14)(q13;q32) translocation was confirmed in 20 of 29 patients by t(11;14) PCR, t(11;14) fluorescence in situ hybridization (FISH), or conventional cytogenetics. In 9 patients for whom samples were unavailable to detect t(11;14) by these methods, MCL was verified by cyclin D1 staining (n = 5) or by histologic review only (n = 4).
Molecular genetic analyses
DNA from PB, BM, fresh lymph nodes, or autologous stem cell products was extracted with the Qiagen Blood Mini Kit (Qiagen, Hilden, Germany). Samples were analyzed by t(11;14) and IGH-FR1 multiplex PCR, as published.16,22,23 Gene scanning and sequence analyses were performed on an ABI PRISM 377 automated sequencer (Applied Biosystems, Foster City, CA). Sequences were obtained by using the BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems).
RQ-PCR
For RQ-PCR of IGH rearrangements, 2 consensus probes complementary to germline JH1, JH4, and JH5(JH1/4/5 probe) and JH6(JH6 probe) were used in combination with 4 germline reverse primers (JH1, JH4, JH5, and JH6, respectively). PCR reactions were performed following a published protocol.24 Specificity was obtained by allele-specific (ASO-) forward primers matching the individual VH-N-D-N-JH region. All RQ-PCR reactions were performed on an ABI PRISM 7700 thermal cycler (Applied Biosystems).
Three hundred nM of each primer (300 nM primer concentration was shown to be the assay-specific optimal concentration in all patients), 200 nM JH probe, sterile water (Merck, Darmstadt, Germany), and 500 ng genomic DNA were used for RQ-PCR. After 2 steps of 2 minutes at 50°C for UNG activation and 10 minutes at 95°C for AmpliTaq Gold (Applied Biosystems) activation, DNA was subjected to 45 cycles of PCR with denaturation at 95°C for 15 seconds, followed by a combined annealing/extension step at 59°C to 63°C for 30 seconds. Annealing/extension temperatures were selected individually for each primer/probe combination. RQ-PCR conditions were optimized for the highest normalized reporter signal (RN) and the lowest threshold cycle (CT). We tested the sensitivity of our assays by analyzing 10-fold serial dilutions from diagnostic samples in polyclonal DNA derived from equivalent amounts of 5 healthy donors. For determining the quantitative MRD levels, target copy numbers were related to the number of target copies at diagnosis. Experiments were performed at least in triplicate for each sample. To equalize differences in the amount and quality of DNA, albumin copies were quantitated according to the method of Pongers-Willemse et al25 as internal reference in all samples and were used for subsequent normalization of MRD values. MRD levels were given as a fraction of numbers of MCL cells per total number of mononuclear BM or PB cells analyzed per PCR assay.
Investigation time points. Diagnostic PB or BM and follow-up samples were collected at 3-month intervals and were analyzed by RQ-PCR. Overall, 174 samples were investigated with 119 follow-up samples from months 3, 6, 9, and 12 after ASCT. Only samples with a minimum sensitivity of 1 × 10–4 were included in survival analysis.
Definition of molecular response. Molecular CR was defined as the absence of PCR-detectable neoplastic cells in PB or BM at any time point after ASCT investigated by RQ-PCR and with a sensitivity of at least 10–4.
Statistical analysis
Distribution of variables between groups was compared using Fisher exact test or χ2 test; the Mann-Whitney U test was used to estimate the significance of differences in continuous parameters. The end point to determine the prognostic significance of variables studied was progression-free survival (PFS), calculated as the interval between transplantation and first documented relapse or end of observation. Overall survival (OS) was calculated from the time of transplantation to date of death from any cause. The probabilities of PFS and OS were estimated by the method of Kaplan and Meier, and the differences were considered statistically significant if P was below .05. Comparison between curves was performed using the log-rank test.
The influence of potential prognostic factors on PFS and OS was analyzed using a Cox proportional hazards model. Factors shown to have significant influence on the outcome variables in univariate analysis were tested in a stepwise model selection using Akaike's information criterion (AIC) considering up to 2-way interactions between variables.26
All statistical analyses were performed using either GraphPad Prism version 3.02 for Windows (GraphPad Software, San Diego, CA) or the open source R implementation of the S statistical language (http://www.r-project.org, version 2.0.1).
Results
Twenty-nine patients with MCL were monitored for MRD by clone-specific quantitative ASO IGH RQ-PCR. We studied 174 samples (110 PB, 64 BM) from routine staging time points before and after high-dose radiochemotherapy followed by ASCT. All patients with pretreatment PB samples (n = 14) available for PCR demonstrated peripheral blood involvement by RQ-PCR before induction. From 10 patients without morphologic BM infiltration at diagnosis, BM samples of 4 patients were available for PCR analysis. RQ-PCR detected BM infiltration in the range of 0.4% to 4% in all 4 patients.
Correlation between PB and BM MRD levels
Forty-four paired PB and BM samples from different time points were analyzed in parallel and were compared with respect to MRD level. Eighteen paired samples were MRD positive in BM and PB, and 20 samples were negative in both. In 3 patients in whom MRD was negative in PB, low-level MRD could be detected in the corresponding BM; and in 3 patients with MRD-negative BM, residual cells were detectable in the corresponding PB samples.
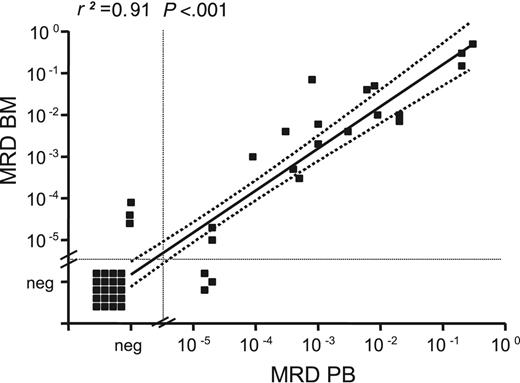
MRD levels in paired BM/PB samples correlated with a correlation coefficient (r2) of 0.91 (P < .001; Figure 1). Median MRD levels were slightly higher in BM than in PB but did not differ significantly (median BM MRD level, 6.5 × 10–3 vs 2.0 × 10–3 in PB; P = .328). Despite the small number of samples in the regression analysis, we concluded from the congruency of our data that PB is comparable to BM for MRD assessment.
MRD levels in paired BM and PB samples. MRD levels in 44 paired BM and PB samples collected during and after treatment were determined by quantitative IGH RQ-PCR. A strong correlation between MRD levels in BM and PB was observed (r2 = 0.91). Regression coefficient refers to samples with positive MRD levels.
MRD levels in paired BM and PB samples. MRD levels in 44 paired BM and PB samples collected during and after treatment were determined by quantitative IGH RQ-PCR. A strong correlation between MRD levels in BM and PB was observed (r2 = 0.91). Regression coefficient refers to samples with positive MRD levels.
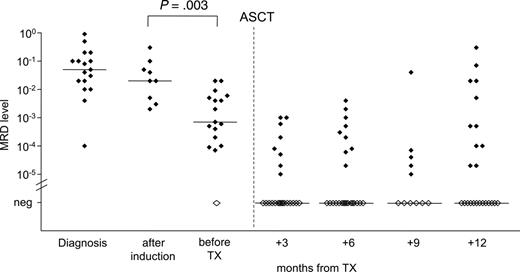
MRD quantification during the course of treatment. Quantification of residual lymphoma cells of 29 patients was performed during the course of treatment. DexaBEAM resulted in significant lymphoma cell depletion by 1 to 2 log (P = .003). Filled symbols indicate MRD-positive samples; open symbols indicate MRD-negative samples.
MRD quantification during the course of treatment. Quantification of residual lymphoma cells of 29 patients was performed during the course of treatment. DexaBEAM resulted in significant lymphoma cell depletion by 1 to 2 log (P = .003). Filled symbols indicate MRD-positive samples; open symbols indicate MRD-negative samples.
Treatment response and prognostic relevance of MRD
The overall clinical remission rate after induction and stem cell mobilization was 96%, with 24% (7 of 29) CR and 76% (21 of 29) PR. One patient had a minimal response (MR) before ASCT. After ASCT 22 of 29 (76%) patients achieved CR, and 7 of 29 (24%) achieved PR. Median PFS after ASCT was 41 months; median OS for all patients was 65 months.
Monitoring of MRD kinetics during treatment revealed that induction therapy with CHOP or CHOP-like regimens did not significantly reduce the tumor load in PB or BM compared with pretreatment MRD levels (P = .29) (Figure 2). However, the application of DexaBEAM for stem cell mobilization resulted in a significant lymphoma cell depletion by 1 to 2 log (P = .003), with a median MRD level of 2 × 10–2 before DexaBEAM (range, 2 × 10–3 to 3 × 10–1) and 7 × 10–4 (range, 10–5 to 2 × 10–2) thereafter. All but one patient who received 2 cycles of DexaBEAM for mobilization had measurable MRD in PB or BM before ASCT (Figure 2).
MRD assessment after ASCT was performed in 27 of 29 patients for whom DNA from at least 2 of the 4 selected time points (3, 6, 9, and 12 months after ASCT) was available (median of 3 different time points investigated per patient). In 2 patients, poor DNA quality of follow-up samples did not allow valid MRD quantification. Overall, 119 samples (75 PB, 44 BM) were available for MRD assessment afterASCT. In 20 of 27 patients, BM was available; in 19 patients, samples from both sources were examined; in 7 patients, PB served as the only DNA source for MRD assessment.
After ASCT, 14 of 27 (52%) patients achieved complete clinical and molecular remission (MRD negativity in all of at least 2 samples tested during the first posttransplantation year), whereas 13 of 27 (48%) patients had detectable MRD at least once after ASCT. Of these patients, 6 achieved CR and 7 achieved PR.
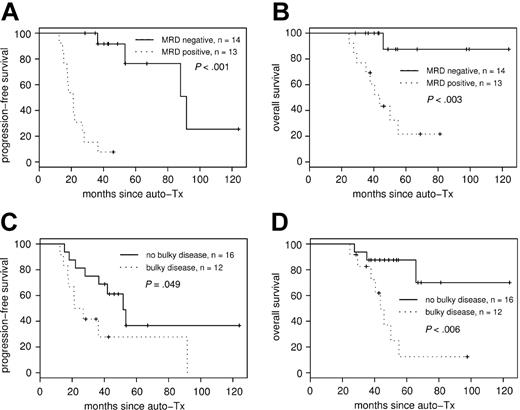
Consistently negative MRD status within the first year of ASCT had a strong impact on PFS (median PFS, 92 months in the MRD-negative group compared with 21 months in the MRD-positive group (P < .001; hazard ratio [HR], 31.4; 95% CI, 4.0-248) (Figure 3A). Overall survival also differed significantly, with 44 months in the MRD-positive group, whereas median OS in the MRD negative group has not been reached after a median observation time of 48 months (P < .003; HR, 11.9; 95% CI, 1.5-93.7) (Figure 3B).
Only 4 of 14 (29%) MRD-negative patients have relapsed so far, whereas all 13 patients with positive MRD status measured at any time point within the first year of ASCT have relapsed, regardless of clinical remission status after transplantation. In the MRD-positive group, only 3 of 13 patients transiently achieved MRD-negative status. Negativity occurred in 2 of them at early time points (months 3 and 6 after ASCT) but converted to MRD positivity by the next available sampling time point (months 9 and 12, respectively). In the third patient, MRD level fluctuated at the sensitivity level of the IGH RQ-PCR assay in the range of 10–5. All other patients were continuously MRD positive at all investigated time points.
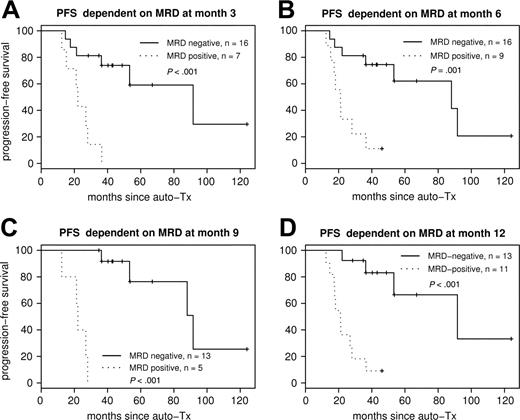
Figure 4 shows the Kaplan-Meier plots for PFS according to MRD status at 3, 6, 9, and 12 months after ASCT. Each sampling time point within the first year was highly predictive for PFS.
Both MRD risk groups were similar with respect to sex, age, LDH level, spleen enlargement, extranodal involvement, and bulky disease (Table 1). Twelve patients received 2 doses of peritransplantation rituximab as in vivo purging before ASCT. Three (25%) of these remained MRD positive after ASCT, whereas 10 of 15 (67%) rituximab-naive patients had detectable MRD (P = .054).
MRD negativity retained its prognostic value in rituximabnaive patients, as demonstrated by separate analyses of those 15 patients who never received rituximab. In the rituximab-naive group, results were concordant with a significantly prolonged PFS in the MRD-negative group (92 months) compared with 20 months in the MRD positive patients (P = .001).
PFS and OS according to MRD status after transplantation and bulky disease at diagnosis. MRD status after ASCT correlates to PFS (A) and OS (B). Achievement of molecular remission after ASCT strongly predicts improved PFS, with median PFS of 92 months for the MRD-negative group compared with 21 months in the MRD-positive group (P < .001). Bulky disease at diagnosis was associated with adverse PFS (C) and OS (D).
PFS and OS according to MRD status after transplantation and bulky disease at diagnosis. MRD status after ASCT correlates to PFS (A) and OS (B). Achievement of molecular remission after ASCT strongly predicts improved PFS, with median PFS of 92 months for the MRD-negative group compared with 21 months in the MRD-positive group (P < .001). Bulky disease at diagnosis was associated with adverse PFS (C) and OS (D).
PFS according to MRD status after ASCT. PFS dependent on MRD status at different sampling time points: MRD at month 3 (A), MRD at month 6 (B), MRD at month 9 (C), and MRD at month 12 (D) after ASCT. Each sampling time point within the first year is highly predictive for PFS.
PFS according to MRD status after ASCT. PFS dependent on MRD status at different sampling time points: MRD at month 3 (A), MRD at month 6 (B), MRD at month 9 (C), and MRD at month 12 (D) after ASCT. Each sampling time point within the first year is highly predictive for PFS.
In addition to molecular remission, clinical variables such as extranodal involvement, bulky disease, elevated LDH level, splenomegaly, and histologic BM involvement were tested as predictors for PFS and OS (Table 2) by univariate analysis. Among these, only bulky disease at diagnosis was associated with adverse PFS (Figure 3C) and OS (Figure 3D). Furthermore, treatment response parameters such as achievement of CR before and after ASCT and peritransplantation rituximab application evolved to be of prognostic significance for PFS, and CR after ASCT was predictive for OS (Table 2).
Predictive factors of PFS and OS after ASCT
. | Univariate analysis, P . | . | Multivariate analysis, P . | . | ||
|---|---|---|---|---|---|---|
| Variable . | PFS . | OS . | PFS . | OS . | ||
| LDH | .312 | .795 | NA | NA | ||
| Histologic BM infiltration | .079 | .457 | NA | NA | ||
| Extranodal involvement | .936 | .914 | NA | NA | ||
| Bulky disease | .049 | .006 | .021 | .060 | ||
| Splenomegaly | .347 | .834 | NA | NA | ||
| CR before ASCT | .006 | .060 | >.999 | NA | ||
| CR after ASCT | .003 | .047 | .220 | .320 | ||
| Rituximab exposure | .024 | .267 | .340 | NA | ||
| Molecular response | <.001 | .002 | <.001 | .051 | ||
. | Univariate analysis, P . | . | Multivariate analysis, P . | . | ||
|---|---|---|---|---|---|---|
| Variable . | PFS . | OS . | PFS . | OS . | ||
| LDH | .312 | .795 | NA | NA | ||
| Histologic BM infiltration | .079 | .457 | NA | NA | ||
| Extranodal involvement | .936 | .914 | NA | NA | ||
| Bulky disease | .049 | .006 | .021 | .060 | ||
| Splenomegaly | .347 | .834 | NA | NA | ||
| CR before ASCT | .006 | .060 | >.999 | NA | ||
| CR after ASCT | .003 | .047 | .220 | .320 | ||
| Rituximab exposure | .024 | .267 | .340 | NA | ||
| Molecular response | <.001 | .002 | <.001 | .051 | ||
NA indicates not applicable.
Parameters significant for PFS and OS in univariate analysis were each tested as a single variable in a multivariate Cox regression model. Only bulky disease and MRD status after ASCT were observed to be independent prognostic factors for PFS (bulky disease: P = .028; HR, 4.1; 95% CI, 1.2-14.6; MRD status: P < .001; HR, 39.3; 95% CI, 4.6-339.2). For OS, the model retaining only MRD status as an independent prognostic factor was associated with P < .05 (P = .019). However, looking at the residual plot and the fact that the interaction between MRD status and bulky disease is eliminated in the first step of the model selection process, the model retaining both MRD status and bulky disease as independent risk factors probably represented the contribution of risk factors to OS more accurately, though it was not statistically significant (bulky disease: P = .08; HR, 4.2; 95% CI, 0.85-20.5; MRD status: P = .05; HR, 7.4; 95% CI, 0.9-60.3).
MRD in the stem cell harvests
To address the prognostic significance of quantitative MRD levels in the stem cell products, we analyzed 50 leukapheresis (LP) samples from 28 patients for the presence of MRD. All but 2 (4%) LP samples had significant levels of lymphoma cell contamination, with a median MRD level at first separation of 4.5 × 10–3 (range, MRD negative to 2.0 × 10–1). In one patient, stem cell collection after the second cycle of DexaBEAM resulted in a PCR-negative stem cell product; the other patient had a blastoid variant of MCL stage III and no histologic BM infiltration.
Stem cell products from 8 patients underwent CD34+ selection with the Isolex300 system. CD34+ selection induced a 1 to 2 log tumor cell reduction, with median MRD load of 1.3 × 10–2 (range, 1.3 × 10–3 to 1.5 × 10–1) before purging compared with median MRD levels of 6.7 × 10–4 (range, 2.6 × 10–5 to 3.7 × 10–2) after purging, but the difference was not significant (P = .097). In 2 of 8 patients, purging resulted in a MRD-negative CD34+ fraction.
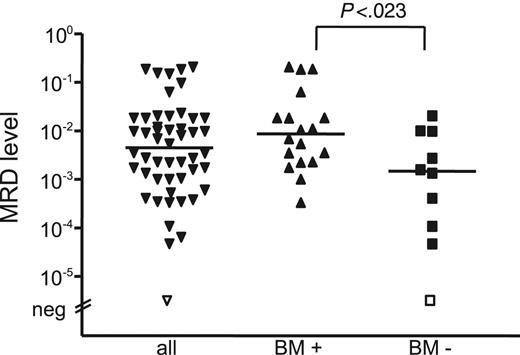
MRD levels in stem cell products were significantly different, depending on histologic BM infiltration, with a lower median MRD load of 1.4 × 10–3 (range, MRD negative to 2.0 × 10–2) in patients without BM infiltration (n = 10) compared with 9 × 10–3 (range, 3.3 × 10–4 to 2.0 × 10–1) in patients with BM involvement (n = 18) (P = .023) (Figure 5). The 2 different MRD risk groups did not differ significantly with regard to MRD levels of the stem cell products (P = .104). All except 3 patients received stem cell products with significant MRD levels, 12 of 14 in the favorable group and 12 of 13 in the unfavorable MRD group. Hence, it appears that the amount of reinfused MCL cells does not predict achievement of molecular remission and does not influence outcome after ASCT.
Molecular relapse
MRD kinetics until clinical relapse could be followed in 10 patients (2 MRD-negative patients with late relapse and 8 MRD-positive patients). Two patients who had molecular remission and experienced late clinical relapse 5 to 6 years after ASCT had low-level MRD over a period of 12 and 19 months, respectively, with slowly increasing MRD levels preceding clinical relapse. In contrast, dynamics of MRD increase in patients in the MRD-positive group was more rapid, with a 1 to 2 log increase within a short period of 6 to 12 months (median, 7 months) early after ASCT, reflecting different growth kinetics of the disease.
MRD levels of stem cell products. The MRD level of stem cell products was significantly different, depending on histologic BM infiltration.
MRD levels of stem cell products. The MRD level of stem cell products was significantly different, depending on histologic BM infiltration.
Discussion
High-dose therapy with subsequent ASCT in first clinical remission has a sustained impact on improved PFS and OS in patients with MCL.5,7,9 Disease status at transplantation was revealed to be the most significant factor affecting survival.5,7,27 However, even after dose-intensified therapies, most patients with MCL will ultimately relapse. Contamination of the harvested stem cells with circulating lymphoma cells is considered to contribute to relapse, whereas the other most relevant factor is the inability to eradicate lymphoma cells in the body by pretransplantation chemotherapy and even by myeloablative radiochemotherapy with subsequent ASCT.10-12
Until now, few reports have addressed the role of residual disease in small series of patients with MCL. The aims of our study were, therefore, to establish a highly reproducible and sensitive method for MRD detection that allows MRD assessment within larger series of patients, to investigate whether MRD is a critical factor influencing the prognosis of patients with MCL after high-dose treatment and ASCT, and to assess the impact of contaminating lymphoma cells in the stem cell product on clinical and molecular outcome.
RQ-PCR using allele-specific primers in combination with consensus probes has been shown to be a useful tool for MRD monitoring within clinical trials in acute lymphoblastic leukemia.24 According to this approach, we used a quantitative IGH RQ-PCR assay based on the design of individual allele-specific forward primers matching the clonal VH-DH-JH region for MRD quantification of MCL within a clinical study protocol including autologous transplantation. By this approach, we were able to estimate tumor cell load over a wide dynamic range of 5 log levels up to a highly reproducible sensitivity level of 10–5.
At first we addressed the question of the comparability of PB and BM as sources for MRD assessment in our series. By analyzing 44 paired PB and BM samples, we demonstrated that concordant results were achieved with respect to MRD positivity and negativity and to MRD levels. This contradicts reports in patients with FL in whom higher sensitivity of bone marrow, with 1 to 1.5 log higher MRD levels compared with PB, have been reported.28,29 The comparability of PB and BM for MRD detection is an important factor concerning the applicability of MRD within multicenter clinical studies with respect to the easier access to PB. However, because of the increasing use of rituximab, which induces strong peripheral B-cell depletion, the question of comparability of PB and BM as sources for MRD assessment should be addressed in further studies.
Quantitative MRD assessment during treatment allowed evaluation of the effects of single treatment elements. As depicted in Figure 2, CHOP did not significantly reduce MRD levels. This is in line with observations by Andersen et al,5 who conclude that even dose-intensified Maxi-CHOP induction might not be a sufficient regimen in MCL. In contrast, in patients with FL, tumor cell reduction of 1 to 2 log levels can be induced by CHOP alone.30
The contribution of an ara-C–containing regimen such as DexaBEAM mobilization on cytoreduction and PFS cannot be precisely determined by clinical results.8 With the use of quantitative PCR monitoring, we were able to show that DexaBEAM had a major impact on tumor cell reduction. Beyond this, our data suggest that high-dose chemotherapy, including TBI, has a decisive impact on the elimination of MCL cells. Although peritransplantation rituximab might have augmented the cytoreductive effects of TBI/CY, it is unlikely that the large prognostic benefit of the patients achieving MRD negativity after ASCT was due mainly to rituximab because induction of molecular response by treatment with rituximab and CHOP alone does not translate to prolonged PFS.3,4 Moreover, in our study, MRD negativity was associated with similarly long PFS in patients not receiving peritransplantation rituximab.
MRD negativity after ASCT was highly predictive of a favorable prognosis in MCL. Early after transplantation, patients with unfavorable outcomes could be distinguished from those with long-lasting PFS by the combined MRD information of months 3 to 12 after ASCT. To date, only 4 of 15 patients who became MRD negative experienced clinical disease recurrence after a median follow-up of more than 7 years. Thus, achieving MRD negativity is clearly a desirable goal in the treatment of MCL. However, additional prospective clinical studies are reasonable because our patient series was heterogenous with respect to induction treatment, stem cell mobilization, and transplantation protocol.
Our results are in contrast to published data suggesting that achievement of molecular remission in MCL is possible after ASCT in only 12% to 25% of patients without any impact on prognosis.10,12,32 These differences may in part be explained by the fact that most published studies investigated patients undergoing ASCT in second or higher remission, when the impact of ASCT on prognosis might be less pronounced. Only one study investigated MRD by PCR after first-line high-dose treatment followed by ASCT and demonstrated that achievement of molecular remission is possible in a relevant fraction of patients (5 of 13; 38%).5 However, no differences in outcome according to MRD status could be demonstrated for this small number of patients. We observed a sustained molecular response after up-front sequential high-dose radiochemotherapy followed by ASCT in 52% of patients with MCL. In our MCL series, most (86%) patients underwent transplantation in first clinical remission, and most (76%) of those achieved CR. This is in line with results from different clinical studies suggesting that autologous transplantation early in the clinical course leads to improved prognosis.5,7-9 Moreover, the use of a potent cytoreductive regimen for mobilization might have contributed to the favorable results observed here.
Furthermore, most previous reports of MRD detection in MCL relied on qualitative PCR approaches that seem unsuitable for PCR-based risk stratification in patients with MCL. Only one study documented the applicability of a quantitative IGH RQ-PCR assay in 6 patients with MCL, and in 3 it was after up-front ASCT.17 However, molecular remission did not translate to improved survival in this small group of patients.
Achievement of molecular remission after ASCT was strongly associated with the presence of clinical CR (14 of 22 patients) and was not found in patients who achieved PR. Moreover, a fluctuating MRD status with intermittent MRD negativity, a common feature in patients with FL, was rarely seen in patients with MCL (3 of 27 patients). Transient MRD negativity in 2 patients occurred only at early time points after ASCT, probably because of only brief lymphoma control by transplantation. In the third patient, a fluctuating MRD state occurred within the sensitivity level of the IGH RQ-PCR assay, reflecting the technical limitations of MRD detection below 10–5.
In contrast to recent published results of MRD quantitation in patients with FL, in whom low levels of BCL2-IGH+ cells in the BM at diagnosis were the best predictors for the achievement of complete clinical and molecular response,30 the different prognostic groups in MCL defined by MRD assessment did not differ with regard to MRD levels before treatment (Figure 2). Differences in MRD kinetics of histologic entities reflect the variable biologic composition of distinct non-Hodgkin lymphoma and underscore the necessity of careful evaluation of the prognostic value of MRD assessment according to histologic subtype and precise definition of sampling time points and critical MRD levels for prognostic evaluation.
When we attempted to determine clinical factors of prognostic value, only bulky disease and achievement of CR before and after ASCT were of prognostic relevance in univariate analysis (Table 2). In multivariate analysis, bulky disease and MRD status after ASCT were identified as independent prognostic factors for PFS. Interestingly, looking at the distribution of residuals, the same regression model containing MRD status after ASCT and bulky disease also most accurately described OS. Considering the relatively low number of patients, the statistical power might have been insufficient to indicate the relevance of bulky disease and MRD status as independent risk factors for OS.
Within the 2 MRD risk groups, we observed differences with respect to MRD kinetics at relapse. Patients with MRD positivity after ASCT tended to experience rapidly progressive MRD within a short period of time, whereas patients in the MRD-negative group remained so or demonstrated low-level MRD preceding clinical relapse over a long period. Based on MRD kinetics in the 2 MRD risk groups, it seems unlikely that the differences in OS and PFS were caused by different sensitivity to chemotherapy. It is tempting to speculate that both MRD risk groups differed with respect to proliferation kinetics, the strongest predictor of survival in MCL, as reported in a recent study of gene expression analysis.32
In previous studies, tumor cell contamination of the stem cell product was found to be related to a high relapse rate.11,31 Immunologic purging failed to eradicate PCR-detectable disease in most patients with MCL.10 This is in line with our results indicating that purging by CD34+ selection did not induce a sufficient tumor cell depletion, leaving 6 of 8 purged LP products with PCR-detectable disease. In contrast to other reports33,34 suggesting a role for retransfused residual cells in disease recurrence, our data show that MCL cells conferred with the graft had no major prognostic impact in the treatment protocol used here.
In conclusion, the current study shows that longitudinal monitoring of MRD by quantitative RQ-PCR is a powerful indicator of treatment outcome in patients with MCL who undergo sequential high-dose treatment, including ASCT with or without peritransplantation rituximab. MRD elimination after ASCT is highly predictive of a favorable prognosis, making molecular remission a desirable goal in the treatment of MCL. While CHOP-like cytoreduction had only modest influence, both cytarabine-containing mobilization and myeloablative radiochemotherapy significantly reduced MRD. In the context of the treatment regimen used here, the prognostic influence of the tumor load of the autograft seems to be limited. These findings are important for prospective clinical studies in which MRD assessment may serve as an early surrogate marker to evaluate new treatment strategies in MCL.
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-07-2845.
Supported by Deutsche Krebshilfe grant 70-2405-Kn2 (C.P., M.K.), the Lymphoma Research Foundation (R.S.), and the European Community within the European MCL Network LSHC-CT 2004-503351 (M.D.).
C.P. participated in the development of the PCR assay, was responsible for data collection and interpretation, and drafted the manuscript; C.S. was responsible for sample collection; M.T. was responsible for the pathologic review; S.G., L.H., and R.S. were responsible for cytogenetic and molecular cytogenetic data collection and interpretation; T.R. participated in statistical analysis; M.B., M.R., and B.G. optimized and performed the PCR analysis; M.U. is the statistician of the German Low Grade Lymphoma Study Group (GLSG) and, together with M.D., provided clinical data from study patients and assisted in data interpretation; W.H. is the coordinator of the GLSG and was responsible for the clinical trial design and conduct; P.D. conducted the trial with autologous stem cell transplantation in mantle cell lymphoma and was responsible for clinical data assessment from patients in Kiel; and M.K. was responsible for the overall conduct of the minimal residual disease study and participated in manuscript preparation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This article is dedicated to Günther Brittinger on the occasion of his 75th birthday. We thank Nadine Rambadt, Ariane Stuhr, and Ingrid Bolz for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal