Abstract
Antibodies (Abs) directed at the Galα1,3Galβ1,4GlcNAc-R (αGal) carbohydrate epitope initiate xenograft rejection. Previously, we have shown that bone marrow transplantation (BMT) with lentivirus-mediated gene transfer of porcine α1,3 galactosyltransferase (GalT) is able to induce tolerance to αGal-expressing heart grafts following a lethal dose of irradiation. Here we show the first demonstration of permanent survival of αGal+ hearts following transplantation with autologous, lentivirus-transduced BM using a nonmyeloablative regimen. Autologous BM from GalT knockout (GalT–/–) mice was transduced with a lentiviral vector expressing porcine GalT and transplanted into sublethally irradiated (3 Gy) GalT–/– mice. Chimerism in the peripheral blood cells (PBCs) remained low but was higher in the BM, especially within the stromal cell population. Mice reconstituted with GalT did not produce anti-αGal Abs over time. We immunized these mice with αGal-expressing cells and assessed humoral immune responses. Anti-αGal xenoantibodies were not produced in mice reconstituted with GalT, but normal Ab responses to other xenoantigens were detected. Mice reconstituted with GalT accepted αGal+ heart grafts over 100 days. Transduction with lentiviral vectors results in chimerism at levels sufficient to induce long-term tolerance under nonmyeloablative conditions.
Introduction
The number of cases of organ transplantation has been increasing, however, the shortage of donor organs is a major problem because the number of donors is limited. Xenotransplantation using nonhuman species as organ donors has received much attention as a possible solution to this problem. Pigs are regarded as the most likely species to serve as donors for clinical xenotransplantation.1 All mammals except humans, apes, and old-world monkeys express α1,3 galactosyltransferase (GalT), hence Galα1,3Galβ1, 4GlcNac-R (αGal) is expressed on most tissues including vascular endothelium.2-4 However, as humans have natural antibodies (Abs) against αGal, it is known that xenotransplantation of pig organs into humans induces hyperacute rejection, acute vascular rejection/delayed xenograft rejection, and even chronic rejection against αGal.5-7 In pig-to-primate discordant combination, 80% to 90% of antipig xenogeneic Abs are anti-αGal Abs.8 Therefore, overcoming this response is presently the biggest problem facing xenotransplantation.
To solve this problem, various approaches have been proposed.9-13 Mixed hematopoietic chimerism can induce tolerance in allogeneic and xenogeneic models of transplantation.14,15 Recently, it has been shown that tolerance to αGal can be induced in GalT knockout (GalT–/–) mice that produce anti-αGal Abs. Yang et al16 applied mixed-cell chimerism to achieve permanent acceptance of transplanted organs by injecting bone marrow (BM) from wild-type (WT) donor mice into GalT–/– mice conditioned with irradiation and T-cell depletion. Bracy et al17 have reported use of gene therapy to induce chimerism and tolerance following transplantation of autologous BM cells expressing a retrovirally transduced gene encoding GalT. We have reported that donor hearts were permanently accepted in GalT–/– mice conditioned with lethal irradiation and injected with autologous BM cells transduced with a lentiviral vector expressing GalT.18 Lethal irradiation conditioning, however, is not reasonable for use in clinical applications. We therefore have focused on establishing the conditions under which tolerance can be achieved using nonlethal irradiation and autologous gene therapy as a combined protocol.
Unlike other retroviruses, lentivirus vectors are able to transduce nondividing cells including hematopoietic stem cells.19 These genes are permanently transmitted and expressed as they are integrated into the genome. Here, we show the first application of gene therapy using lentiviral vectors and nonmyeloablative conditioning to overcome anti-αGal antibody-mediated rejection of WT hearts in GalT–/– mice.
Materials and methods
Mice
GalT–/– mice homozygous for the targeted disruption in the GalT do not express the αGal epitope and produce anti-αGal–reactive Abs in their serum. GalT–/– mice were backcrossed 10 times using C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) and were obtained from Dr A. d'Apice (St Vincent's Hospital in Melbourne, Australia). The mice used for these experiments were 3 to 5 months of age at the time of bone marrow transplantation (BMT). All animals received humane care in compliance with the Principles of Laboratory Animal Care, formulated by the National Society for Medical Research, and the Guide for the Care and Use of Laboratory Animals, prepared by the National Institutes of Health.20
Construction of the lentiviral vector
The vector provirus has less than 850 bp of the human immunodeficiency virus genome, lacks long terminal repeat (LTR) enhancers and promoters (self-inactivating [SIN]), but contains the central polypurine tract and central terminal site sequences to increase the rate and extent of proviral integration. Sequences from Simian virus (SV) 40 were added to augment polyadenylation from the 3′ LTR to enhance gene expression and minimize transcriptional read-through to downstream cellular sequences at the site of proviral integration. The vector contains 80 bp from the gag region and lacks a 5′ splice donor site, making the vector independent of rev. The vector contains an MCU3 promoter that is the U3 region from the MND retroviral vector with substitution of the TATA site with that of the human CMV promoter. Titers were measured by flow cytometry following transduction of HIT293T cells. The woodchuck hepatitis virus posttranscriptional regulatory element reported to increase expression of transgenes delivered by viral vectors was included in the construct.21 The porcine α1,3 Galt gene or a control construct expressing the porcine Galt gene in the antisense orientation (REV) was used to transduce BM from GalT–/– mice. The vector was pseudotyped with vesicular stomatitis virus glycoprotein (VSV-G), as described.22
Transduction, bone marrow transplantation, and irradiation
Animals were matched for age and anti-αGal Ab levels prior to BMT. BM donors were killed, and bone marrow cells were flushed from the femora and humeri using phosphate-buffered saline. Transduction of BM from autologous GalT–/– mice was done over a 48-hour period using virus at a concentration of 1 × 107 IU/mL in X-VIVO media (Cambrex Bio Science, Walkersville, MD) supplemented with 50 ng/mL IL3, IL6, and SCF (Stem Cell Technologies, Vancouver, BC, Canada).23 Twenty-four to 36 hours after transduction, bone marrow recipients were prepared by treatment with 3 Gy of whole-body irradiation using a 137Cs irradiator. Sublethally irradiated GalT–/– mice were reconstituted with 8 × 106 to 30 × 106 transduced BM cells within 12 to 24 hours after irradiation by tail vein injection. Blood samples from the tail vein were taken prior to transplantation and at time intervals ranging from 1 week to 15 weeks after transplantation to monitor chimerism and Ab levels.
Immunization
To induce a humoral immune response, mice were immunized by intraperitoneal injection of 1 × 106 rabbit red blood cells (RRBCs). Mice were reimmunized by intraperitoneal injection of 1 × 107 pig kidney (MPK) cells. Immunized control mice for enzyme-linked immunospot (ELISPOT) assay were injected intraperitoneally with 1 × 106 RRBCs 2 weeks before the experiment.
Enzyme-linked immunosorbent assay
Anti-αGal Ab levels and antiporcine xenoantibody levels were measured using the enzyme-linked immunosorbent assay (ELISA).24 For the anti-αGal ELISA, a 5-μg/mL solution of purified αgal(1-3)βgal(1-4) (Dextran, Ramsey, United Kingdom) in Na2CO3 (pH 9.4) was incubated on 96-well plates overnight at 4°C. For the antipig xenoantibody ELISA, primary cultured pig aortic endothelial cells (PAECs) were grown to confluence on the plate prior to the assay. Nonspecific binding was blocked by the addition of 1% bovine serum albumin. Antibodies in the serum were incubated for 1 hour at room temperature. Binding of Abs to anti-αGal or anti-PAECs was detected using either horseradish peroxidase (HRP)–conjugated goat anti–mouse IgM at a concentration of 1 μg/mL; HRP-conjugated goat anti–mouse IgG using a 2-μg/mL solution (Kirkegaard and Perry Laboratories, Gaithersburg, MD); or isotype-specific rat anti–mouse–purified monoclonal antibodies specific for IgG1, IgG2a, IgG2b, and IgG3 (BD Biosciences, San Diego, CA). SureBlue substrate for peroxidase (Kirkegaard and Perry Laboratories) was added to each well, and the reaction was stopped with 1N HCl. Absorbance was read at 450 nm using an HTS 7000 plus BioAssay reader (Perkin Elmer, Norwalk, CT).
Flow cytometry
Flow cytometric analysis was used to detect the surface expression of the αGal epitope on bone marrow cells and peripheral blood cells (PBCs). This assay was used to identify molecular chimerism in various cell lineages, as reconstituted animals expressing a functional porcine Galt gene will express the αGal carbohydrate epitope that can be detected with the IB4-lectin.25 Briefly, mouse cells were incubated for 30 minutes at 4°C with fluorescein isothiocyanate–labeled IB4-lectin (Sigma, St Louis, MO) to detect the αGal carbohydrate and lineage-specific Abs conjugated to phycoerythrin: antimouse CD3 for T cells, anti–mouse CD19 for B cells, anti-Ly6G/C (Gr-1) for granulocytic cells, and anti–mouse Ter-119 for erythroid lineage cells (all from BD Biosciences Pharmingen, San Diego, CA). The cells were washed and analyzed using the FACSCalibur (Becton Dickinson, San Jose, CA). To detect the subclass of T cells, we used anti-CD4 and anti-CD8 Abs (BD Bioscience Pharmingen).
Quantitative polymerase chain reaction
The integration of porcine GalT was assessed by quantitative polymerase chain reaction (PCR) performed on ABI PRISM 7700 Sequence Detector (Perkin Elmer, Foster City, CA). To compensate for differences in the DNA quality and quantity, parallel assays were run on each sample for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and were normalized with respect to GAPDH content. The following synthetic oligonucleotides were used as primers: GAPDH sense 5′-GGC ATG GAC TGT GGT CAT GAG-3′, antisense 5′-TGC ACC ACC AAC TGC TTA GC-3′; lenti-porcine GalT sense 5′-GTT CGC TTC TCG CTT CTG TT-3′, antisense 5′-CCA AAA CAC AAC CAT TAC AGT TGA G-3′. Quantitative amplifications were performed using Quantitect SYBR Green PCR kit (Qiagen, Valencia, CA) according to manufacturer's protocol. The conditions for PCR were initial denaturation for 15 minutes at 95°C, then amplification was performed by 45 cycles at 95°C for 15 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. Duplicate experiments were done for each sample. To exclude nonspecific amplification, samples were electrophoresed on a 1% agarose gel.
Flow cytometric cytotoxicity assay
Flow cytometry was used to measure complement-mediated cytotoxicity. Serum isolated from the PBCs of immunized mice was serially diluted and incubated with MPK cells along with the addition of baby rabbit complement (Serotec, Raleigh, NC). The uptake of propidium iodide was used to measure cell death and was read on the FACSCalibur (Becton Dickinson).
Enzyme-linked immunospot assay
ELISPOT assays were performed according to published procedures.26 Briefly, cell suspensions were prepared from the spleen, peritoneal cavity, and BM cells of GalT–/– mice (GalT BMT and REV BMT), GalT–/– mice immunized with RRBCs (positive control), or WT mice (negative control). These cells were serially diluted and cultured for 24 hours at 37°C in multi-screen HA plates (Millipore, Bedford, MA), coated with 5 μg/mL αGal-BSA (V-Labs, Covington, LA) and blocked with complete media. After washing the wells, goat anti–mouse IgM plus IgG secondary Abs (Southern Biotechnology Associates, Birmingham, AL) were added to detect bound antibodies. Plates were washed and color development was done using 3-amino-9-ethyl carbazole (Sigma). All samples were plated in triplicate.
Heterotopic heart transplantation
Intra-abdominal heterotopic heart transplantation was performed. Donor hearts derived from C57BL/6 mice (αGal+) were transplanted into the abdominal cavity of GalT BMT mice and REV BMT mice. Syngeneic transplantation controls included WT to WT and GalT–/– to GalT–/–, respectively. Ventricular contractions were assessed daily by palpation. Rejection was determined by cessation of cardiac function and was verified by pathologic examination.
Natural anti-αGal Ab levels and the influence of 3-Gy irradiation. (A) ELISA assay demonstrating that IgM and IgG natural anti-αGal Ab levels in naive GalT–/– mice increase with age. Serum was isolated from naive GalT–/– mice and anti-αGal Ab levels were assessed by ELISA (n = 81). OD indicates optical density. (B) Three-gray irradiation decreases B-cell numbers in GalT–/– mice (n = 5 in each group). The percentage of CD4+, CD8+, and B lymphocytes in PBCs was assessed by flow cytometry. CD4+ cells (□), CD8+ cells (▪), CD19+ cells (○). (C) Influence of 3-Gy irradiation on anti-αGal Ab levels were assessed by ELISA. IgM anti-αGal Abs rise at 3 weeks after irradiation in the absence of GalT BMT. Irradiated mice (□), nonirradiated control mice (•;n = 7, respectively). Error bars indicate SE.
Natural anti-αGal Ab levels and the influence of 3-Gy irradiation. (A) ELISA assay demonstrating that IgM and IgG natural anti-αGal Ab levels in naive GalT–/– mice increase with age. Serum was isolated from naive GalT–/– mice and anti-αGal Ab levels were assessed by ELISA (n = 81). OD indicates optical density. (B) Three-gray irradiation decreases B-cell numbers in GalT–/– mice (n = 5 in each group). The percentage of CD4+, CD8+, and B lymphocytes in PBCs was assessed by flow cytometry. CD4+ cells (□), CD8+ cells (▪), CD19+ cells (○). (C) Influence of 3-Gy irradiation on anti-αGal Ab levels were assessed by ELISA. IgM anti-αGal Abs rise at 3 weeks after irradiation in the absence of GalT BMT. Irradiated mice (□), nonirradiated control mice (•;n = 7, respectively). Error bars indicate SE.
Histology
Heart grafts were harvested at the time of rejection and placed into 10% formalin for fixation. Paraffin blocks were prepared, sections were stained with hematoxylin and eosin (HE), and stained sections were examined microscopically (Olympus BX51, Melville, NY). Images were captured using an Olympus MicroFire camera model S99809, uPlan F1 10 ×/0.3 NA objectives, and Picture Frame Application software version 2.1.
Statistical analysis
The results were analyzed by Student t test. P less than .05 was assessed as significant. All data are shown as mean ± SE.
Results
Anti-αGal antibodies are induced by 3-Gy irradiation
Anti-αGal Abs are naturally induced in GalT–/– mice. It has been reported that GalT–/– mice younger than 6 months of age do not produce significant levels of anti-αGal Abs in the absence of immunization.27,28 Anti-αGal xenoantibodies, however, are detected at higher levels in older GalT–/– mice. We examined the levels of anti-αGal IgM and IgG Abs in our colony of GalT–/– mice over time by ELISA (Figure 1A; n = 81). Both IgM and IgG anti-αGal Ab levels increased with age, but a significant mouse-to-mouse variation was noted.
Next, we assessed the influence of 3-Gy irradiation on the number of CD4+, CD8+, and CD19+ lymphocytes in PBCs and anti-αGal Ab levels over time in GalT–/– mice (Figure 1B; n = 5 in each group). CD19+ cells were significantly decreased at 1 week after irradiation compared with the number of CD19+ cells prior to conditioning (P < .001), then rebounded at 2 weeks (P < .01). Both CD4+ and CD8+ cells decreased to a lesser extent at 2 weeks (P < .01). Anti-αGal IgM Ab levels increased substantially by 3 weeks following 3 Gy of irradiation (P < .05 vs nonirradiated control; Figure 1C; n = 7 in each group). Anti-αGal IgG Ab levels did not change. Total IgM and IgG Ab levels were stable after 3-Gy irradiation (data not shown).
Lentiviral gene transfer following sublethal irradiation leads to long-term expression of αGal epitope and inhibition of anti-αGal natural antibody production
In order to assess transduction efficiency in the BM cells used for transplantation, cells were transduced with a lentiviral vector encoding either porcine GalT or its reverse sequence (REV; n = 12 and n = 9, respectively). Cells were incubated in vitro for 5 days to eliminate pseudotransduction, then assessed by PCR using primers specific for GalT and REV to identify genomic integration. GalT and REV were expressed specifically in the transduced cells (Figure 2A). We also confirmed the expression of the αGal carbohydrate produced by GalT using the lectin-IB4 to identify αGal+ cells by flow cytometry. Expression of αGal was detected on 16.07% ± 3.19% of the BM cells (Figure 2B).
Next, we monitored the level of chimerism in various lineages of PBCs over time. Cells were double-stained with lectin-IB4 (αGal) and either CD3 (T cells), CD19 (B cells), Ly6G (granulocytic cells), or TeR119 (erythroid lineage cells) and analyzed by flow cytometry to determine the proportion of αGal+ cells in each lineage (Figure 3A; n = 5). The number of αGal+ cells declined over time and reached a low-level plateau approximately 7 weeks after transduction. The level of chimerism was much lower after sublethal irradiation when compared with our previous report in which BMT was given after lethal irradiation (8% to 75% of cells of all lineages were αGal+ at 7 weeks after BMT).18
Transduction efficiency of BM cells in vitro by lentiviral vectors expressing GalT. (A) Specific integration in the genome assessed by PCR at 5 days after transduction. Genomic DNA was isolated from GalT and REV-transduced cells. Gene-specific primers were used to demonstrate the presence of the GalT and REV genes in transduced cells. The electrophoresis results from representative data are shown. – indicates not transduced, +; transduced. (B) Surface expression of αGal on transduced cells as assessed by flow cytometry using lectin-IB4 to identify αGal+ cells. The boldface line indicates nontransduced bone marrow; the thin line, GalT-transduced bone marrow.
Transduction efficiency of BM cells in vitro by lentiviral vectors expressing GalT. (A) Specific integration in the genome assessed by PCR at 5 days after transduction. Genomic DNA was isolated from GalT and REV-transduced cells. Gene-specific primers were used to demonstrate the presence of the GalT and REV genes in transduced cells. The electrophoresis results from representative data are shown. – indicates not transduced, +; transduced. (B) Surface expression of αGal on transduced cells as assessed by flow cytometry using lectin-IB4 to identify αGal+ cells. The boldface line indicates nontransduced bone marrow; the thin line, GalT-transduced bone marrow.
In vivo chimerism level and anti-αGal Ab levels over time. (A) αGal expression assessed by flow cytometry as lectin-IB4 binding cells in various cell lineages in the peripheral blood of GalT-transduced BMT mice (n = 5). Whole PBCs (Total), T cells (CD3), B cells (CD19), granulocytes (Ly6G/C), erythroid lineage cells (TeR119). Data are shown as mean ± SE. (B) Genomic integration of GalT was assessed by quantitative PCR. Lentiviral vector–specific forward primer and porcine GalT–specific reverse primers were used for quantitative genomic PCR (n = 5 each). Data are shown as mean ± SE. KO indicates knockout. (C) Anti-αGal Ab levels were assessed by ELISA in GalT BMT (□), REV BMT (•), IR controls (▴) (n = 5 each). Xenoantibodies that bind to αGal are not produced in GalT BMT mice. Data are shown as mean ± SE.
In vivo chimerism level and anti-αGal Ab levels over time. (A) αGal expression assessed by flow cytometry as lectin-IB4 binding cells in various cell lineages in the peripheral blood of GalT-transduced BMT mice (n = 5). Whole PBCs (Total), T cells (CD3), B cells (CD19), granulocytes (Ly6G/C), erythroid lineage cells (TeR119). Data are shown as mean ± SE. (B) Genomic integration of GalT was assessed by quantitative PCR. Lentiviral vector–specific forward primer and porcine GalT–specific reverse primers were used for quantitative genomic PCR (n = 5 each). Data are shown as mean ± SE. KO indicates knockout. (C) Anti-αGal Ab levels were assessed by ELISA in GalT BMT (□), REV BMT (•), IR controls (▴) (n = 5 each). Xenoantibodies that bind to αGal are not produced in GalT BMT mice. Data are shown as mean ± SE.
Since the level of chimerism was very low, genomic quantitative PCR was used to further determine whether the microchimerism could be monitored. Similar to the results obtained by flow cytometry, the relative level of genome-integrated GalT (expressed as the log GalT/GAPDH) decreased over time and reached a plateau 7 weeks after BMT (Figure 3B; n = 5 each).
The levels of anti-αGal Abs (IgM and IgG) in the serum were measured by ELISA using purified trisaccharide αGal during a period of 11 to 14 weeks after BMT. The level of anti-αGal IgM Ab increased over time in the control mice (Figure 3C; n = 5 each). However, no increase was observed in the GalT BMT mice, which remained at the background level (P < .05, 5 to 11 weeks vs the other groups). The levels of anti-αGal IgG Ab remained low in all groups. These data demonstrate that lentivirus-mediated GalT BMT inhibits anti-αGal Ab production in GalT–/– mice without myeloablative conditioning.
Expression of αGal epitopes by lentivirus induces specific tolerance to antigen challenge
To stimulate anti-αGal Ab production,29 further experiments were performed as follows. RRBCs, which abundantly express αGal, were intraperitoneally administered at 1 × 106 cells per mouse. A strong antibody response was observed in both the age-matched naive GalT–/– mice and control mice that were treated with 3 Gy of irradiation in the absence of a BMT (data not shown). Serum was collected 8 days after immunization and analyzed to measure anti-αGal Ab levels by ELISA. The levels of IgM and IgG Ab increased in REV and GalT–/– controls (Figure 4A). However, anti-αGal Abs were not induced in WT controls and GalT BMT mice (P < .05 vs REV, irradiation control [IR], or GalT–/– controls). Using the same serum samples with the addition of complement, cytotoxicity against MPK cells that express an abundant amount of αGal was examined using the flow cytometric cytotoxicity assay. As expected, cytotoxicity levels were high in REV, IR, and GalT–/– controls (Figure 4B; P < .01 vs IR, GalT–/– control; P < .05 vs REV). The cytotoxicity toward MPK cells was low in WT mice and GalT BMT mice.
We immunized with MPK cells intraperitoneally at 1 × 107 cells per mouse and the Ab response was assessed by ELISA 8 days after immunization. Both IgM and IgG anti-αGal Ab levels remained low in the mice that received transplants of BM expressing GalT (Figure 4A; P < .05 vs REV, IR, and GalT–/– control). In the cytotoxicity assay using MPK cells, the levels of cytotoxicity increased in both the WT mice and GalT BMT mice, and no significant difference was noted between groups (Figure 4B), suggesting that xenoantibodies other than anti-αGal Abs are involved in this cytotoxicity. Next, we examined the immune response to PAECs assessed by ELISA. This was done to establish whether xenoantibodies directed at xenoantigens other than αGal were produced in GalT BMT mice. After MPK immunization, antipig xenoantibodies were induced in all groups and there was no significant difference in the levels of anti-PAEC Abs in these groups of animals (Figure 4C). These data suggest that our regimen could induce αGal-specific humoral immune response inhibition against immunization by αGal+ xenogeneic cells.
We further assessed the macrochimerism and microchimerism levels in the PBCs (18 weeks to 41 weeks after BMT) and confirmed that chimerism was stable at almost 1.5% [macrochimerism: whole cells = 1.65% ± 0.27%; microchimerism: log (GalT/GAPDH) = 1.26 × 10–6 ± 0.31 × 10–6].
Tolerance induction to Gal-expressing vascularized heart grafts
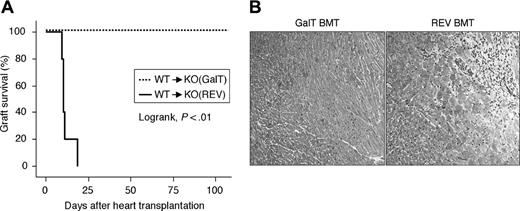
Next, to examine whether the tolerance acquired in this model is maintained during vascularized solid organ transplantation, we performed heart transplantation 4 weeks after BMT (n = 5 each). All of the hearts from the αGal+ WT donor mice were rejected at an average of 12.8 days (9-20 days) after transplantation into the REV BMT mice (Figure 5A). In contrast, all grafts survived in GalT BMT mice during a period of 100 days (log rank test, P < .01). HE staining of the graft hearts in REV control animals that rejected their hearts showed severe focal inflammatory cell infiltration and myocardial necrosis (Figure 5B). In contrast, all mice in the GalT BMT group showed no histologic signs of rejection of the engrafted hearts.
Inhibition of antibody production is specific for αGal. (A) Anti-αGal IgM and IgG Abs in chimeras at 11 to 14 weeks after BMT (before) and following RRBC and MPK cell immunization (shown as RRBC and MPK, respectively) were identified by anti-αGal Ab ELISA. GalT BMT mice did not respond to αGal+ xenogeneic cell immunization. GalT-transduced BMT mice (□), REV (•), IR (▴), WT (▪), KO (○) (n = 5 each). (B) Complement-mediated cytotoxicity assay demonstrating cytotoxicity by non–anti-αGal xenoantibody in GalT BMT mice. (C) Anti-PAEC Abs assessed by ELISA. GalT BMT mice produce xenoantibodies to pig cells. Data are shown as mean ± SE.
Inhibition of antibody production is specific for αGal. (A) Anti-αGal IgM and IgG Abs in chimeras at 11 to 14 weeks after BMT (before) and following RRBC and MPK cell immunization (shown as RRBC and MPK, respectively) were identified by anti-αGal Ab ELISA. GalT BMT mice did not respond to αGal+ xenogeneic cell immunization. GalT-transduced BMT mice (□), REV (•), IR (▴), WT (▪), KO (○) (n = 5 each). (B) Complement-mediated cytotoxicity assay demonstrating cytotoxicity by non–anti-αGal xenoantibody in GalT BMT mice. (C) Anti-PAEC Abs assessed by ELISA. GalT BMT mice produce xenoantibodies to pig cells. Data are shown as mean ± SE.
Permanent graft survival of GalT-mediated BMT mice. BMT mice received a heterotopic heart transplantation from WT (αGal+) mice 4 weeks after BMT (n = 5 for GalT and REV mice). Syngeneic hearts (WT to WT, GalT–/– to GalT–/–; n = 5 each) were not rejected during a period of 100 days (data not shown). (A) Graft survival assessed by Kaplan-Meier method. (B) The rejected grafts in REV BMT mice show severe focal inflammatory cell infiltration and myocardial necrosis in hematoxylin and eosin (HE)–stained sections (original magnification × 100). Grafts from GalT BMT mice that were killed at 100 days after heart transplantation showed no signs of rejection.
Permanent graft survival of GalT-mediated BMT mice. BMT mice received a heterotopic heart transplantation from WT (αGal+) mice 4 weeks after BMT (n = 5 for GalT and REV mice). Syngeneic hearts (WT to WT, GalT–/– to GalT–/–; n = 5 each) were not rejected during a period of 100 days (data not shown). (A) Graft survival assessed by Kaplan-Meier method. (B) The rejected grafts in REV BMT mice show severe focal inflammatory cell infiltration and myocardial necrosis in hematoxylin and eosin (HE)–stained sections (original magnification × 100). Grafts from GalT BMT mice that were killed at 100 days after heart transplantation showed no signs of rejection.
We assessed the levels of anti-αGal Abs over time in these mice that received heart transplants. Both IgM and IgG anti-αGal Ab levels remained low by 15 weeks after BMT in GalT BMT mice (Figure 6A; n = 5 each). However, IgM Ab levels of REV BMT animals demonstrated a significant increase (P < .01 compared with GalT at 3-5 weeks after BMT). To analyze the mechanism by which tolerance is induced in these animals, we performed an IgG subtype ELISA to identify whether significant increases in the levels of specific IgG antibody subtypes that have been reported to be cytoprotective could be demonstrated in these animals.30,31 There was no significant difference in levels of any IgG subtype antibodies during the course of these experiments in either Gal BMT or REV BMT mice (Figure 6B). In the absence of a role for cytoprotective antibodies in the induction of tolerance in our model, we then assessed whether clonal deletion may function to eliminate anti-αGal antibody–producing B cells in these animals. We isolated lymphocytes from the spleen (SPL), peritoneal cavity (PerC), and BM and performed ELISPOT assays. The samples from REV BMT mice showed the presence of anti-αGal Ab–producing cells. No anti-αGal Ab–producing B cells were detected in the GalT BMT mice in those tissues (Figure 6C SPL and BM; P < .005 at 8 × 105 cells vs the other controls). We conclude that clonal deletion or receptor editing occurs in this model.
Bone marrow cells show higher levels of macrochimerism compared with peripheral blood cells
In our model, the PBCs demonstrated low levels of macrochimerism. To determine whether higher levels of chimerism could be identified in the BM, we isolated BM cells and PBCs at 100 days after heart transplantation and chimerism was assessed by flow cytometry in GalT BMT mice (n = 5). BM cells maintain higher levels of chimerism compared with PBCs (Figure 7A; P < .01). Using quantitative PCR, the same tendency was confirmed. Next, we cultured these BM cells for 24 hours, separated the stromal cells (adherent population) from the hematopoietic cells (nonadherent population), and assessed the level of αGal expression and degree of genomic integration in the fractionated populations. Chimerism was higher in stromal cells compared with the hematopoietic cell population (Figure 7B; P < .05 vs whole and hematopoietic cells, n = 5 each).
We analyzed the in vitro transduction efficiency of hematopoietic cells compared with stromal cells after 5 days in culture (n = 10 each). Adhesive cells have higher levels of expression of αGal (P < .001 vs whole and nonadhesive cells) and GalT integration in the genome (Figure 7C). Thus, high levels of chimerism in the BM of GalT BMT chimeras compared with the PBCs is due to the contribution of transduced stromal cells expressing αGal in the BM.
Tolerance induction in GalT BMT mice. (A) Anti-αGal Ab levels were assessed by ELISA at 15 weeks after BMT in mice that received transplants of WT hearts. REV BMT mice received transplants of WT hearts at 4 weeks after BMT and were killed when they rejected the graft; therefore, this data ends at 5 weeks. GalT-transduced BMT mice (□), REV (•) (n = 5 each). (B) Levels of IgG subtypes in the sera were analyzed by ELISA before BMT, 9 weeks after BMT in GalT-transduced BMT mice, and 5 weeks after BMT in REV BMT mice (n = 5 each). □ indicates IgG1; ▦, IgG2a; ▧, IgG2b; and ▪, IgG3. IMM indicates immunized control mice (n = 5 each); HTx indicates heart transplantation. (C) To assess the existence of anti-αGal Ab–producing cells, spleen, BM, and PerCs were collected and assessed using an ELISPOT assay. Anti-αGal Ab–producing B cells are not detected in GalT BMT chimeras. GalT-transduced BMT mice (□), REV (•), WT (▪), immunized GalT–/– mice (○) (n = 5 each). Data are shown as mean ± SE.
Tolerance induction in GalT BMT mice. (A) Anti-αGal Ab levels were assessed by ELISA at 15 weeks after BMT in mice that received transplants of WT hearts. REV BMT mice received transplants of WT hearts at 4 weeks after BMT and were killed when they rejected the graft; therefore, this data ends at 5 weeks. GalT-transduced BMT mice (□), REV (•) (n = 5 each). (B) Levels of IgG subtypes in the sera were analyzed by ELISA before BMT, 9 weeks after BMT in GalT-transduced BMT mice, and 5 weeks after BMT in REV BMT mice (n = 5 each). □ indicates IgG1; ▦, IgG2a; ▧, IgG2b; and ▪, IgG3. IMM indicates immunized control mice (n = 5 each); HTx indicates heart transplantation. (C) To assess the existence of anti-αGal Ab–producing cells, spleen, BM, and PerCs were collected and assessed using an ELISPOT assay. Anti-αGal Ab–producing B cells are not detected in GalT BMT chimeras. GalT-transduced BMT mice (□), REV (•), WT (▪), immunized GalT–/– mice (○) (n = 5 each). Data are shown as mean ± SE.
BM stromal cells show higher levels of transduction and chimerism compared with hematopoietic cells. (A) BM cells and PBCs were collected from killed GalT-transduced BMT mice. αGal expression was assessed by flow cytometry and genomic integration of GalT was assessed by quantitative PCR (n = 5). (B) Chimerism levels in BM stromal cells (SC) were higher than in hematopoietic cells (HC) (n = 5). BM cells were cultured in retronectin-coated plates for 24 hours and αGal expression and genomic integration of GalT were compared in harvested adhesive cells (SC) and nonadhesive cells (HC) (n = 5). (C) Transduced cells were harvested individually as adhesive cells and nonadhesive cells 5 days after transduction. αGal expression and genomic integration of GalT were high in stromal cells (n = 10 each). Data are shown as mean ± SE.
BM stromal cells show higher levels of transduction and chimerism compared with hematopoietic cells. (A) BM cells and PBCs were collected from killed GalT-transduced BMT mice. αGal expression was assessed by flow cytometry and genomic integration of GalT was assessed by quantitative PCR (n = 5). (B) Chimerism levels in BM stromal cells (SC) were higher than in hematopoietic cells (HC) (n = 5). BM cells were cultured in retronectin-coated plates for 24 hours and αGal expression and genomic integration of GalT were compared in harvested adhesive cells (SC) and nonadhesive cells (HC) (n = 5). (C) Transduced cells were harvested individually as adhesive cells and nonadhesive cells 5 days after transduction. αGal expression and genomic integration of GalT were high in stromal cells (n = 10 each). Data are shown as mean ± SE.
Discussion
In this study, we successfully induced tolerance specific for αGal under nonmyeloablative conditions by gene transfer into BM cells using a lentiviral vector encoding porcine GalT. Stable chimerism was obtained in all cell lineages but at levels significantly lower than those we have previously reported following lethal conditioning. Nevertheless, the production of anti-αGal Ab was inhibited, and transplanted αGal+ hearts survived beyond 100 days. This is the first report, to our knowledge, that demonstrates the induction of tolerance to αGal by BMT using gene therapy under nonmyeloablative conditions.
Tolerance induction to tissues expressing αGal plays a key role in successful xenotransplantation, as this carbohydrate is a major target of the immune response to xenografts. Induction of tolerance specific to αGal was reported for the first time in the transplantation of BM of WT mice into GalT–/– mice.16 Chimerism sufficient to induce tolerance was also attained under a nonmyeloablative regimen, demonstrating the feasibility of induction of tolerance by BMT for clinical use.26,32 Ohdan et al33 further showed that chimerism and tolerance can be achieved in a xenotransplantation model using rat heart donors in a mouse recipient. However, BMT in a rat-to-mouse concordant model is significantly different from the pig-to-primate discordant model, in which many more xenoreactive antigens are involved, and there is a large difference in the nonimmune physiologic niche. Furthermore, mixed-cellular chimerism using pig-to-primate BMT may be extremely difficult in clinical applications.34 As a solution to this problem, gene therapy has been applied in the BMT model using viral vectors to express αGal.17,35,36 The concept is to induce expression of αGal on autologous BM cells and to use these cells for BMT to achieve chimerism and tolerance to the αGal carbohydrate. This is thought to have a better practical prospect as it represents an alternative method of preventing hyperacute rejection due to xenoantibodies or anti-αGal Abs. Natural Abs are predominantly directed at the αGal carbohydrate and cannot be blocked with immunosuppressive drugs. The application of chimerism to block hyperacute rejection and use of immunosuppressive drugs later to block T-cell–dependent responses may prolong the survival of porcine xenografts. Moreover, the use of autologous BM cells eliminates the possibility of graft-versus-host disease and increases the probability of chimerism and induction of tolerance with less immunosuppressant.
Several laboratories are studying the application of gene therapy to achieve tolerance to the αGal carbohydrate.17,18,37 Ogawa et al37,38 and Mohiuddin et al39 have reported the induction of tolerance in GalT–/– mice using autologous lymphocytes transduced with an adenovirus vector expressing GalT. Since the transduced splenic lymphocytes demonstrate limited survival, these reports suggest that long-term, high-level chimerism in PBCs is not necessary for stable tolerance induction. Our results are consistent with this data. In our study, chimerism in the BM was higher than that demonstrated in PBCs but detected at levels significantly lower than achieved following lethal irradiation. The stromal cells have higher levels of transduction efficiency. Low-level, stable chimerism in cells such as BM stromal cells, therefore, may be sufficient to induce tolerance and is not readily detectable in the peripheral blood. Although we are not certain which cell lineages are important for maintaining chimerism and induction of B-cell tolerance in this study, mesenchymal stem cells might be one good candidate because they are transduced in total bone marrow suspensions and have been shown to establish chimerism in other models.40,41
The goal of our experimental design was to establish stable chimerism using BMT under nonmyeloablative conditions. At first, we studied the influence of sublethal (3 Gy) irradiation on anti-αGal antibody production. Anti-αGal antibody–producing B cells have been reported to be irradiation resistant.16 It has also been shown that T-cell depletion in vivo induces higher levels of anti-αGal antibodies.42,43 In our study, sublethal irradiation did not change the levels of total IgM and IgG antibodies. However, IgM anti-αGal antibody levels increased significantly beginning at 3 weeks after irradiation. The Gal BMT procedure prevents this irradiation-induced increase in anti-αGal antibody levels.
In our model, tolerance is achieved due to chimerism and is maintained by receptor editing or clonal deletion because anti-αGal antibody–producing B cells were not detected at 100 days after transplantation by the ELISPOT assay. Chimerism was sufficient to tolerize newly formed anti-αGal antibody–producing B cells and naive anti-αGal B cells with this nonmyeloablative regimen.16,26 In the mixed-cell chimerism model, anergy was demonstrated at 2 weeks after bone marrow transplantation and clonal deletion or receptor editing occurs later, resulting in tolerance to αGal.44,45 Our model similarly demonstrates that clonal deletion or receptor editing occurs when tolerance is induced using gene therapy as a means to achieve chimerism. Our model differs from the accommodation model in which young naive GalT–/– mice do not reject transplanted wild-type hearts due to an increase in the levels of cytoprotective IgG2b antibodies.31 The levels of IgG anti-αGal antibodies of all subtypes remain very low in our BMT mice following transplantation of WT hearts.
In conclusion, we described successful gene transfer into BM cells using lentiviral vectors and nonmyeloablative conditioning as a means to achieve chimerism and transplant tolerance. Our studies have focused on the use of lentiviral vectors for this procedure, as they are highly efficient and have undergone several new modifications that have increased the likelihood that they will be used for clinical trials in the near future (reviewed in Logan et al46 ). These new biosafety features include the development of SIN lentiviral vectors in which the promoter and enhancer elements have been deleted from the U3 region of the LTR. This reduces the possibility of insertional mutagenesis and allows the incorporation of promoters selected for broad or lineage-specific expression. Additional safety-enhancement features include the addition of an insulator and the human interferon-β scaffold attachment region into the SIN lentiviral vector backbone to reduce the effect of chromosomal sequences flanking the site of insertion.47 Only recently has it become evident that HIV-based lentiviral vectors have important differences in their pattern of integration when compared with vectors based on murine leukemia virus.48-51 MLV integration occurs preferentially near transcription start sites, while HIV-1 integrations occur within the transcriptional region of the gene but not upstream of the transcriptional start site. Additional studies are needed in this area, since integration patterns have important implications for biosafety and the selection of vectors for gene therapy in clinical trials.52,53 Studies are currently underway in our group to determine whether lentiviral vectors can safely and effectively be used to achieve chimerism and transplant tolerance in nonhuman primates. The application of this procedure in the pig-to-primate model represents the next step toward our long-term objectives and will provide additional information on the biologic events that occur in vivo following lentiviral gene transfer in clinically relevant large animals.
Prepublished online as Blood First Edition Paper, November 15, 2005; DOI 10.1182/blood-2005-03-1172.
Supported by American Heart Association grant 8086-RGW.000007 (M.K.-J.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Fred Dorey for his helpful advice on statistical analysis.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal