Kim and colleagues demonstrate that aging is associated with diminished numbers of IL-7Rα–expressing T cells and that these cells have decreased responsiveness to IL-7, with important implications for the clinical development of IL-7 as an immunomodulatory agent.
Interleukin 7 (IL-7) is a pleiotropic cytokine critical for the efficient development of T and B lymphocytes. It is now clear that the importance of this cytokine for T cells does not end in the thymus. The availability of IL-7 is indispensable for the maintenance of mature T cells and for the expansion of T cells that occurs during lymphopenia. Furthermore, exogenous IL-7 can markedly enhance T-cell recovery following lymphocyte depletion, and this cytokine is being developed therapeutically as an immunorestorative agent and as an adjuvant to immune-based therapies. In spite of a rapid increase in our understanding of the biology of IL-7, little is known about the regulation of IL-7 availability in mature T-cell populations. Emerging data would imply that control of IL-7 receptor expression is critical, as has been demonstrated in developing thymocytes.1 IL-7 uses the common cytokine receptor chain γc and the IL-7Rα chain that it shares with thymic stromal lymphopoietin. Since γc is ubiquitously expressed on mature T cells, intact IL-7 signaling is critically dependent on the presence of IL-7Rα. Indeed, this receptor component is not present on all mature T-cell subsets, suggesting that regulation of expression is important. In contrast to other cytokines such as IL-2, T-cell receptor and IL-7 signaling results in down-regulation of the IL-7Rα chain, and it has been suggested that this allows for efficient sharing of an important limiting resource for T cells.2 In addition, murine models have indicated that maintenance of IL-7Rα on a small subset of effector T cells determines the generation of the central memory pool.3 Thus, the existing data would indicate that the tight control of IL-7α expression plays a critical role in efficient maintenance of mature T cells.FIG1
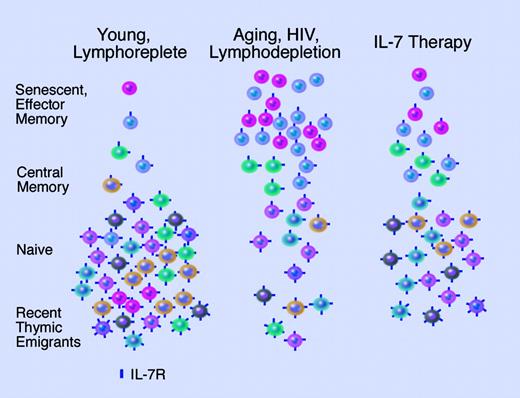
Kim and colleagues demonstrate that aging, like HIV, is associated with the accumulation of T cells expressing low levels of IL-7Rα. While this limits the portion of cells capable of responding to IL-7, diversification of the repertoire may still occur due to preferential expansion of T cells expressing higher levels of IL-7Rα remaining in the naive pool. Illustration by Marie Dauenheimer.
Kim and colleagues demonstrate that aging, like HIV, is associated with the accumulation of T cells expressing low levels of IL-7Rα. While this limits the portion of cells capable of responding to IL-7, diversification of the repertoire may still occur due to preferential expansion of T cells expressing higher levels of IL-7Rα remaining in the naive pool. Illustration by Marie Dauenheimer.
Recently, it has been demonstrated that low levels of IL-7Rα are characteristic of HIV infection,4 and murine studies have indicated that this may be due to chronic antigen exposure.5 This has obvious implications for the potential use of IL-7 as a treatment following lymphopenia, but it is not entirely clear what the impact of changes in expression of IL-7Rα will have on therapeutic responses. In this issue of Blood, Kim and colleagues demonstrate that, like HIV, the immunodeficiency of aging is associated with an increased frequency of IL-7Rαlow cells and that these T cells are phenotypic of senescent, antigen-experienced cells. Of importance, this paper also provides the novel observation that the decreased expression of IL-7 receptor results in decreased survival and proliferation in response to IL-7 in vitro. For these cells, it appears that other cytokines such as IL-15 may be important for survival. Finally, as would be predicted, the repertoire of the antigen-experienced, IL-7Rαlow subset was reduced in both elderly and young individuals, but young individuals showed greater diversity in the effector memory IL-7Rαhigh subset. As pointed out by the authors, this may have implications for the pool of T cells expanded by therapeutic IL-7 in the aged population. However, naive T cells also express high levels of IL-7Rα, contain the most diverse repertoire, and, although reduced in number in the elderly, may be preferentially expanded by IL-7 (see figure). Ultimately, clinical trials will be needed to determine whether the quantitative decrease in responses to IL-7 that may result from an increased number of IL-7Rαlow T cells will hinder clinical benefit or if the preferential expansion of higher quality T cells with a diverse repertoire will overcome the diminished number of cells responding to IL-7 therapy. The paper by Kim et al provides important clues. ▪
Comment on Kim et al, page 2855

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal