A critical element for treatment of hemophilia B via gene therapy is sustained transgene expression. This study describes novel AAV vectors, optimized for expression in the liver, that generate sustained, therapeutic levels of factor IX expression in nonhuman primates.
Although sustained expression of factor IX has been achieved in animal models, this has not translated into a successful therapy for humans in 2 recent phase ½ trials.1,2 Lack of sustained expression may have resulted from a combination of low transduction activity of the adeno-associated virus type 2 (AAV2) vector used in those studies and previous patient exposure to the virus through natural infection, with the latter triggering a cell-mediated immune response to the transduced cells.
Historically, increasing the transduction activity of the vector was thought to be critical for a successful outcome via gene therapy. Additionally, a vector with a low expression level requires the use of more vector, increasing the likelihood of triggering an immune response to the transduced cells. In this publication, Nathwani and colleagues bring together several elements that individually were shown to enhance transduction activity of, and transgene expression from, AAV vectors. The result is a novel codon-optimized, liver-restricted, mini-human factor IX (hFIX) expression cassette that is efficiently packaged as a double-stranded, transcriptionally active cassette in either an AAV5 or an AAV8 particle.
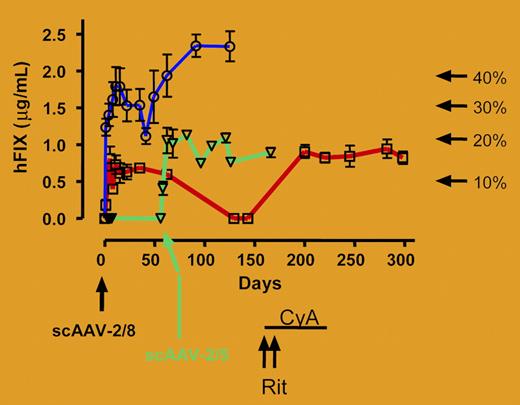
Testing of these vectors in mice, and more importantly in monkeys, demonstrates significantly more activity than current FIX-encoding vectors (purple line in figure). Indeed, rhesus monkeys exhibit complications observed in humans treated for hemophilia B with protein concentrates, such as inhibitory antibody formation to FIX. In this study, inhibitor formation is observed and determined to be the result of xenoprotein (human FIX) expression in nonhuman primates. However, the authors also describe a novel method to blunt the inhibitory antibodies with rituximab and cyclosporine, potentially offering a new approach for treating the formation of inhibitors to other therapeutic proteins (red line in figure).FIG1
Human FIX concentration in rhesus plasma was determined at the indicated time points after administration of 1 × 1012 vg/kg (M1-sc, □/red line; M2-sc, ○/blue line) of scAAV2/8-LP1-hFIXco into the mesenteric vein of 2 rhesus macaques. Treatment of M1-sc with rituximab (Rit × 2 doses) and oral cyclosporine (CyA) is shown. M4-sc (▿/green line) was initially transduced with 4 × 1011 vg/kg scAAV2/8-LP1-hFIXco but did not respond and was and subsequently retransduced with 1 × 1012 vg/kg scAAV2/5-LP1-hFIXco as indicated by the arrow.
Human FIX concentration in rhesus plasma was determined at the indicated time points after administration of 1 × 1012 vg/kg (M1-sc, □/red line; M2-sc, ○/blue line) of scAAV2/8-LP1-hFIXco into the mesenteric vein of 2 rhesus macaques. Treatment of M1-sc with rituximab (Rit × 2 doses) and oral cyclosporine (CyA) is shown. M4-sc (▿/green line) was initially transduced with 4 × 1011 vg/kg scAAV2/8-LP1-hFIXco but did not respond and was and subsequently retransduced with 1 × 1012 vg/kg scAAV2/5-LP1-hFIXco as indicated by the arrow.
A question remains: will this new vector be able to avoid triggering an immune response in humans that would prevent long-term FIX expression? While no data in this study directly address this point, several facts suggest sustained expression is more likely with these vectors than with the AAV2 vector used in the 2 clinical trials. The transduction efficiency and overall expression level from this new vector are much higher than from earlier vectors, allowing for production of therapeutic levels of FIX at a lower vector dose that did not induce transaminitis with AAV2 vectors. Furthermore, seroprevalence to either AAV5 or AAV8 is less compared with AAV2, decreasing the risk of preexisting immunity.3,4
How long will such therapy last? The authors demonstrate that vector copy number is not stable and does decrease over time. The implication is that readministration of vector will be necessary to maintain therapeutic levels of FIX. This likely would be blocked as a result of humoral response to the initial administration of vector. With this scenario in mind, the authors demonstrate that administration of vector with another capsid can avoid this preexisting immunity. Injection of an AAV5-based vector carrying the same modified expression cassette mediated successful long-term transduction in a macaque with immunity to AAV8 (green line in figure). Of interest, similar levels of transduction are observed in nonhuman primates with AAV5 and AAV8 vectors, despite significant capsid-specific difference in transduction of the murine liver. This indicates that there are species-specific differences in tropism of AAV sero-types that ought to be considered when developing gene therapy strategies in the future. Despite repeated setbacks over the past few years, preclinical progress in the treatment of hemophilia B using gene therapy is very encouraging and will eventually be converted to clinical success. ▪
Comment on Nathwani et al, page 2653

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal