Abstract
The involvement of the small GTPase Rap1b in platelet integrin α2β1-dependent outside-in signaling was investigated. Platelet adhesion to 4 different specific ligands for integrin α2β1, monomeric collagen, decorin, and collagen-derived peptides CB8(II) and CB11(II), induced a robust and rapid activation of Rap1b. This process did not require secreted ADP or thromboxane A2 production but was critically regulated by phospholipase C (PLC)–derived second messengers. Both Ca2+ and protein kinase C were found to organize independent but additive pathways for Rap1b activation downstream of integrin-α2β1, which were completely blocked by inhibition of PLC with U73122. Moreover, integrin α2β1 engagement failed to trigger Rap1b activation in murine platelets lacking CalDAG-GEFI, a guanine nucleotide exchange factor regulated by Ca2+ and diacylglycerol, despite normal phosphorylation and activation of PLCγ2. In addition, CalDAG-GEFI–deficient platelets showed defective integrin α2β1-dependent adhesion and spreading. We found that outside-in signaling through integrin α2β1 triggered inside-out activation of integrin αIIbβ3 and promoted fibrinogen binding. Similarly to Rap1b stimulation, this process occurred downstream of PLC activation and was dramatically impaired in murine platelets lacking the Rap1 exchange factor CalDAG-GEFI. These results demonstrate that Rap1b is an important element in integrin-dependent outside-in signaling during platelet adhesion and regulates the cross talk between adhesive receptors.
Introduction
Rap proteins are ubiquitously expressed small GTPases that are supposed to regulate several adhesive receptors, particularly members of the integrin family.1 The ability of integrins to interact with specific ligands is regulated by intracellular signals that switch them from a low- to a high-affinity conformation.2,3 This process, called inside-out signaling, has been shown to involve active Rap1 protein.4-7 More recently, Rap1 has also been found to regulate cell-cell adhesion mediated by E-cadherin.8,9 Upon interaction with their adhesive ligands, activated integrins initiate intracellular signaling pathways promoting and regulating many cellular responses, such as spreading, migration, and differentiation. This process, called outside-in signaling, typically involves several intracellular effectors, including tyrosine kinases, lipid metabolizing enzymes, and small GTPases of the Rho family such as Rac and Cdc42.2,3 By contrast, the involvement of Rap proteins in integrin-directed outside-in signaling is still controversial, as conflicting results have been reported.10-12
Circulating platelets express high levels of the Rap proteins Rap1b and, to a lower extent, Rap2b.13 At the site of vessel wall damage, platelet integrins mediate fundamental processes, such as adhesion to the subendothelial matrix and thrombus growth.3 The main integrin receptors on the platelet surface are integrins α2β1 and αIIbβ3. Together with the FcR γ-chain–associated GPVI, integrin α2β1 serves as a collagen receptor14 and is also responsible for platelet interaction with the proteoglycan decorin.15 It is now clear that inside-out activation of integrin α2β1 results from the stimulation of platelets with many agonists16-19 and that integrin α2β1-dependent platelet adhesion initiates outside-in signaling pathways involving the tyrosine kinases Src and Syk, the phospholipase Cγ2 (PLCγ2), and the small GTPases Rac and Cdc42.15,20-22 In particular, PLCγ2 and Rac have been proposed to play a role in platelet spreading through integrin α2β1.21,22
Although no data have so far correlated integrin α2β1 with the small GTPases of the Rap family, increasing evidence indicates an involvement of Rap1b in the inside-out activation of the other main platelet integrin, the platelet fibrinogen receptor integrin αIIbβ3. In murine megakaryocytes, expression of constitutively active Rap1b increases fibrinogen binding to integrin αIIbβ3.23 Moreover, platelets from mice lacking CalDAG-GEFI, a Ca2+- and DAG-activated guanine nucleotide exchange factor for Rap proteins, show a dramatic impairment of agonist-induced activation of integrin αIIbβ3, fibrinogen binding, and platelet aggregation.24 Similarly, platelets from Rap1b knockout mice display defective function and a tendency to bleed resulting from impaired integrin αIIbβ3 activation and reduced aggregation.25 Conversely, in human platelets, all of the known agonists capable of inducing fibrinogen binding and platelet aggregation can also stimulate GTP loading on Rap1b, and pharmacologic inhibition of agonist-induced Rap1b activation typically results in the prevention of aggregation.26-29
Although the role of Rap1b in inside-out regulation of platelet integrin αIIbβ3 is well documented, nothing is known about the possible involvement of this small GTPase in integrin outside-in signaling related to platelet adhesion. In this work, we report and characterize the stimulation of Rap1b induced by platelet adhesion through integrin α2β1, providing evidence that this small GTPase is also involved in integrin-dependent cell activation. Moreover, we demonstrate that integrin α2β1-dependent activation of Rap1b is required for inside-out stimulation of integrin αIIbβ3 in adherent platelets, suggesting a previously unknown role for Rap1b in the cross talk between different integrin receptors.
Materials and methods
Materials
Apyrase, PGE1, acetylsalicylic acid (aspirin), fibrinogen, TRITC-conjugated phalloidin, creatine phosphate (CP), creatine phosphokinase (CPK), A3P5PS, and o-phenylenediamine dihydrochloride (OPD) were from Sigma (Milan, Italy). GSH-Sepharose 2B and 32P-orthophosphate were from Amersham Biosciences (Cologno Monzese, Italy). U73343 and U73122 were from Alexis (Vinci-Biochem, Vinci, Italy). BAPTA-AM and Ro31-8220 were from Calbiochem (La Jolla, CA). Bicinchoninic acid assay and EZ-link sulfo-NHS-biotin were from Pierce (Pero, Italy). Microtiter plates and 60 mm–diameter polystyrene dishes were from Orange Scientific (Milan, Italy). AR-C69931MX was a generous gift from AstraZeneca (Charnwood, United Kingdom). Convulxin was provided by K. J. Clemetson (Theodor Kocher Institute, University of Berne, Switzerland). Decorin, collagen type I, and CNBr-derivated peptides CB8(II) and CB11(II) were purified and prepared as described elsewhere.15,30 The rabbit polyclonal antibodies against Rap1 (121) and PLCγ2 (Q20) were from Santa Cruz Biotechnology (Tebu-Bio, Magenta, Italy). Antiphosphotyrosine and anti-FcR γ-chain antibodies were from Upstate Biotechnology (Lake Placid, NY). The rabbit polyclonal antibody against phosphoserine PKC substrates was from Cell Signaling Technology (Celbio, Pero, Italy). The cDNA for the Rap binding domain of RalGDS (RBD) was kindly provided by Dr J. L. Bos (University of Utrecht, The Netherlands).
Preparation of human and murine platelets
Blood was withdrawn from healthy volunteers using 10% citric acid–citrate–dextrose as anticoagulant and centrifuged at 120g for 15 minutes at room temperature. Platelet-rich plasma (PRP) was collected and, upon addition of 0.2 U/mL apyrase and 1 μM PGE1, centrifuged at 300g for 15 minutes. Platelet pellet was then washed with PIPES buffer (20 mM PIPES, 137 mM NaCl, pH 6.5) containing 0.2 U/mL apyrase, 1 μM PGE1, and 5.5 mM glucose and finally resuspended in HEPES buffer (10 mM HEPES, 137 mM NaCl, 2.9 mM KCl, 12 mM NaHCO3, pH 7.4). Platelet count was adjusted to 1 × 109/mL, and cells were allowed to rest for 30 minutes at room temperature before being diluted and used for the different assays. Treatment of platelets with specific inhibitors was as follows: 5 mM CP plus 40 U/mL CPK for 1 minute, 3 U/mL apyrase for 1 minute; 10 μM U73343 or 10 μM U73122 for 3 minutes; 5 or 10 μM Ro31-8220 for 5 minutes; 500 nM AR-C69931MX for 2 minutes, 200 μM A3P5PS for 2 minutes, 1 mM aspirin for 30 minutes, 10 μM indometacin for 30 minutes, 10 μM SQ29548 for 10 minutes, 15 or 30 μM BAPTA-AM for 30 minutes.
Murine platelets were prepared from blood collected from abdominal vena cava using 50 U/mL heparin as anticoagulant. Blood was centrifuged at 120g for 15 minutes, and 0.2 U/mL apyrase and 1 μM PGE1 were added to the isolated PRP. To increase platelet yield, the red blood cell pellet was washed with CGS (13 mM citrate, 120 mM NaCl, 30 mM glucose, pH 7.4) and centrifuged at 120g for 15 minutes. The upper phase was collected and pooled with the PRP. Platelets were then recovered by centrifugation at 500g for 15 minutes and resuspended in HEPES buffer. Platelet count was adjusted to 1 × 109/mL and, upon addition of 5.5 mM glucose, cells were allowed to rest for 30 minutes at room temperature.
Adhesion assay
Adhesion assays were performed essentially as previously described.15 Typically, 60-mm polystyrene dishes were coated overnight at room temperature with 0.1 μM (50 μg/mL) collagen type I diluted in 0.1 M acetic acid, 0.1 μM CB8(II) or CB11(II) peptides, 100 μg/mL decorin, 20 μg/mL convulxin, or 0.5% BSA, all diluted in PBS. Dishes were then washed 3 times with 5 mL PBS, blocked with 2 mL of 5% BSA in PBS for 2 hours at room temperature, and then washed 3 more times with PBS. Human or murine platelets were diluted to 2 × 108/mL in HEPES buffer containing 2 mM MgCl2 and 1 mM RGDS. An aliquot (0.5 mL) was added to the coated dishes and incubated at room temperature for 30 minutes unless differently stated. Nonadherent cells were removed, and dishes were washed 3 times with 5 mL PBS. Adherent platelets were recovered by scraping in 1 mL ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.4, 200 mM NaCl, 2.5 mM MgCl2, 1% Nonidet P-40, 10% glycerol, 1 mM PMSF, 1 μM leupeptin, 0.1 μM aprotinin, 0.1 nM Na3VO4). Upon incubation on ice for 10 minutes, lysed cells were transferred to a test tube and clarified by centrifugation at 18 000g (13 000) rpm in a microcentrifuge for 10 minutes at 4°C. The protein content of the cleared supernatants was determined by bicinchoninic acid assay, and aliquots of each sample containing the same amount of proteins (and, thus, deriving from the same number of adherent platelets) were used for Rap1b activation assay, immunoprecipitation, or immunoblotting analysis. For some samples, characterized by a low percent of platelet adhesion, multiples dishes were prepared, and lysed adherent platelets were pooled to obtain an amount of proteins comparable to that of other samples. To calculate the number of adherent platelets, 0.5 mL of the platelet suspension (containing 1 × 108 cells and corresponding to the number of cells added to each dish) was lysed with RIPA buffer 2 × and subjected to protein determination.
When murine platelets were analyzed, a fluorescence microscopy–based method for the simultaneous evaluation of platelet adhesion and spreading was adopted to minimize the number of required cells. Briefly, glass coverslips were coated with 50 μg/mL monomeric collagen type I in 0.1 M acetic acid and incubated with 1 mL murine platelets (3 × 107/mL) for 30 minutes at room temperature in the presence of 2 mM MgCl2 and 1 mM RGDS. Adherent platelets were fixed, permeabilized, and stained by TRITC-conjugated phalloidin. Platelets were viewed on a fluorescence microscope (Olympus BX51; Olympus, Segrate, Italy), and digital images (× 40) were acquired. The number of adherent cells as well as the average cell area (as an index of platelet spreading) were determined using ImageJ software (http://rsb.info.nih.gov/ij/download.html). For each specimen, 5 different fields were analyzed by 2 independent observers.
Rap1b activation assay
Measurement of Rap1b activation was essentially performed as described elsewhere.26 Briefly, 0.2 mg purified recombinant GST-tagged Rap binding domain of RalGDS (GST-RalGDS-RBD) was coupled with 0.1 mL GSH-Sepharose 2B (75% slurry) by incubation for 2 hours at 4°C. Aliquots of lysed adherent platelets obtained from the same number of cells were incubated for 45 minutes at 4°C with 30 μL of the GST-RalGDS-RBD coupled to GSH-Sepharose 2B. Precipitated active Rap1b was washed 3 times with 1 mL ice-cold RIPA buffer, resuspended with 25 μL SDS sample buffer, and revealed by immunoblotting with anti-Rap1 antibody. Aliquots of the total cell lysates from adherent platelets were also analyzed by immunoblotting with anti-Rap1 antibody to prove that the activation assay had been performed with samples containing the same number of cells and proteins. All of the immunoblots reported are representative of similar results obtained in at least 3 different experiments.
Fibrinogen binding assay
This assay was developed to measure the binding of biotin-labeled fibrinogen induced by integrin α2β1-mediated platelet adhesion and to evaluate the effect of pharmacologic inhibitors that might also affect the extent of platelet adhesion. The assay is based on the simultaneous measurement of different parameters and on the combination of data from different determinations. In particular, for each sample of differently treated platelets, the following evaluations were performed: measurement of the number of adherent platelets on collagen- (or other integrin α2β1-specific ligands) and on BSA-coated dishes in the absence and presence of RGDS (to calculate the specific adhesion) and measurement of the binding of biotinylated fibrinogen to platelets adherent to integrin α2β1 ligands and to BSA in the absence and presence of RGDS (to calculate the specific binding). By combining these different data, it was possible to calculate the specific binding for an equivalent number of adherent platelets, which was therefore independent of the effect of the pharmacologic treatments of cell adhesion.
Experiments were performed on 96-well microtiter plates typically coated overnight at room temperature with 50 μL of 50 μg/mL collagen type I diluted in 0.1 M acetic acid or with 0.5% BSA diluted in PBS as negative control. The wells were washed 3 times with 0.5 mL PBS and then blocked for 2 hours at room temperature with 1% BSA in PBS. Human fibrinogen was biotinylated as previously described.31 To evaluate binding of biotinylated-fibrinogen to adherent platelets, 50 μL of washed, aspirin-treated platelets (2 × 108/mL) diluted in HEPES buffer containing 2 mM MgCl2, 0.1 mM CaCl2, and 10 μg/mL biotin-fibrinogen was incubated for 30 minutes with coated wells in the presence or in the absence of 1 mM RGDS peptide. Pretreatment with specific inhibitors was performed as described in “Preparation of human and murine platelets.” Upon removal of nonadherent platelets, the wells were washed 3 times with PBS and incubated for 2 hours with peroxidase-conjugated avidin diluted 1:20 000 in PBS containing 2 mM MgCl2, 0.1 mM CaCl2, 1% BSA. Upon 3 washes with PBS, fibrinogen binding was revealed by a colorimetric reaction using OPD as substrate and quantified using an enzyme-linked immunosorbent assay (ELISA) plate reader at 490/655 nm. The extent of platelet adhesion was evaluated using biotin-labeled platelets. Platelet pellet obtained from PRP was diluted in PIPES buffer and incubated with 0.1 mg/mL EZ-link sulfo-NHS-biotin for 1 hour at room temperature. Platelets were then centrifuged and washed again with PIPES buffer to discharge any unbound biotin residue and were finally resuspended in HEPES buffer. BSA- or collagen-coated wells were incubated for 30 minutes with 50 μL biotin-labeled platelets diluted to 2 × 108/mL in HEPES buffer containing 2 mM MgCl2, 0.1 mM CaCl2, and 10 μg/mL fibrinogen. Every assay was performed both in the presence and in the absence of 1 mM RGDS. Upon incubation, nonadherent platelets were removed and the wells were washed 3 times with PBS and then incubated with 1:20 000 avidin-peroxidase, as described in “Adhesion assay.” The measure of platelet adhesion was obtained through a colorimetric reaction using OPD.
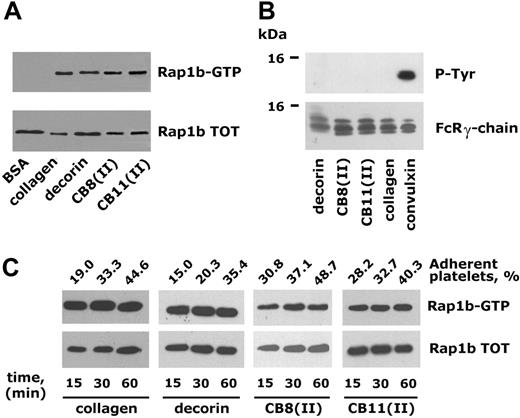
Integrin α2β1-dependent platelet adhesion triggers Rap1b activation. (A) Rap1b activation in platelets adherent to different ligands for integrin α2β1 Human platelets were incubated with immobilized BSA, monomeric collagen, the small proteoglycan decorin, or the collagen-derived peptides CB8(II) and CB11(II), as indicated, for 30 minutes. Active Rap1b (Rap1b-GTP) was precipitated with immobilized GST-tagged RalGDS-RBD and identified by immunoblotting with anti-Rap1 antibody. An identical amount of proteins from each cell lysate was also subjected to immunoblotting analysis with the anti-Rap1 antibody to verify the level of the protein in the different samples (Rap1b TOT). (B) None of the analyzed ligands activate platelet GPVI. The GPVI-associated FcR γ-chain was immunoprecipitated from platelets adherent to decorin, CB8(II), CB11(II), monomeric collagen, and convulxin and analyzed by immunoblotting with antiphosphotyrosine antibody (P-Tyr). The same nitrocellulose membranes were then reprobed with anti-FcR γ-chain (bottom panel). (C) Time course of integrin α2β1-dependent platelet adhesion and Rap1b activation. Human platelets were incubated with the 4 different integrin α2β1 ligands for 15, 30, or 60 minutes, as indicated. The percentage of adherent platelets was determined by a colorimetric assay and is reported above the top panel. The immunoblots show active Rap1b (Rap1-GTP) isolated by the pulldown assay with GST-RalGDS-RBD and the level of total Rap1b (Rap1b TOT) present in the lysates used for the Rap1b activation assays.
Integrin α2β1-dependent platelet adhesion triggers Rap1b activation. (A) Rap1b activation in platelets adherent to different ligands for integrin α2β1 Human platelets were incubated with immobilized BSA, monomeric collagen, the small proteoglycan decorin, or the collagen-derived peptides CB8(II) and CB11(II), as indicated, for 30 minutes. Active Rap1b (Rap1b-GTP) was precipitated with immobilized GST-tagged RalGDS-RBD and identified by immunoblotting with anti-Rap1 antibody. An identical amount of proteins from each cell lysate was also subjected to immunoblotting analysis with the anti-Rap1 antibody to verify the level of the protein in the different samples (Rap1b TOT). (B) None of the analyzed ligands activate platelet GPVI. The GPVI-associated FcR γ-chain was immunoprecipitated from platelets adherent to decorin, CB8(II), CB11(II), monomeric collagen, and convulxin and analyzed by immunoblotting with antiphosphotyrosine antibody (P-Tyr). The same nitrocellulose membranes were then reprobed with anti-FcR γ-chain (bottom panel). (C) Time course of integrin α2β1-dependent platelet adhesion and Rap1b activation. Human platelets were incubated with the 4 different integrin α2β1 ligands for 15, 30, or 60 minutes, as indicated. The percentage of adherent platelets was determined by a colorimetric assay and is reported above the top panel. The immunoblots show active Rap1b (Rap1-GTP) isolated by the pulldown assay with GST-RalGDS-RBD and the level of total Rap1b (Rap1b TOT) present in the lysates used for the Rap1b activation assays.
Immunoprecipitation
Immunoprecipitation was performed essentially as previously described15 using 2 μg anti-FcR γ-chain or anti-PLCγ2. Immunoprecipitated proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% or on 10% to 20% acrylamide gels, transferred to nitrocellulose membranes, tested with antiphosphotyrosine antibody, and then reprobed with the same antibody used for immunoprecipitation.
Measurement of pleckstrin phosphorylation
Pleckstrin phosphorylation in adherent and nonadherent platelets was measured by autoradiography using 32P-labeled cells as well as by immunoblotting with antiphosphoserine PKC-substrates antibody as previously described.31
Results
Integrin α2β1-mediated platelet adhesion promotes activation of Rap1b
To investigate the involvement of Rap1b in the outside-in signaling through integrin α2β1, washed human platelets were allowed to adhere to dishes coated with 4 different ligands: monomeric type I collagen, the collagen-derived peptides CB8(II) and CD11(II), and the small proteoglycan decorin. Fibrillogenesis of monomeric type I collagen was prevented by maintaining the protein in 0.1 M acetic acid during the coating procedure. Peptides CB8(II) and CB11(II), obtained by digestion of collagen type II with BrCN, have been shown to be unable to form fibrils in solution.30 Previous studies have documented that these 4 different substrates represent specific ligands for the platelet integrin α2β1.15,20,32 Upon incubation with immobilized substrates for 30 minutes, adherent platelets were lysed, and active GTP-bound Rap1b was precipitated using GST-tagged RalGDS-RBD. Figure 1A shows that Rap1b was specifically activated in platelets adherent to all the 4 ligands for integrin α2β1 but not in platelets nonspecifically interacting with immobilized BSA, which were used as negative control. Although all the 4 ligands tested have been previously recognized as specific interactors of integrin α2β1, we directly verified that GPVI, the other platelet receptor for collagen, was not recruited under our experimental conditions. This control was particularly relevant because coating of plastic surfaces with monomeric collagen or collagen-derived peptides could originate a disorganized network of molecules reproducing a fibrillar-like structure. We therefore evaluated the tyrosine phosphorylation of GPVI-associated FcR γ-chain in adherent platelets. Figure 1B shows that tyrosine phosphorylated FcR γ-chain was immunoprecipitated exclusively from platelets adherent to the GPVI-specific ligand convulxin but not from platelets adherent to monomeric collagen, collagen-derived peptides CB8(II) and CB11(II), or decorin. These results confirmed that activation of Rap1b upon platelet interaction to all the tested ligands was mediated by outside-in signaling through integrin α2β1.
In time-course experiments, we verified that the amount of adherent platelets to all the tested ligands increased progressively in a comparable manner (Figure 1C). However, when comparable numbers of adherent platelets were analyzed (as documented by the comparable levels of total Rap1b detected in the cell lysates), the amount of active Rap1b did not increase over time. This result indicated that in the population of platelets adhering through integrin α2β1, activation of Rap1b occurred rapidly and did not further increase with prolonged incubation except as a consequence of the increase of the total number of adherent platelets.
Activation of Rap1b downstream of integrin α2β1 is mediated by phospholipase C–derived second messengers
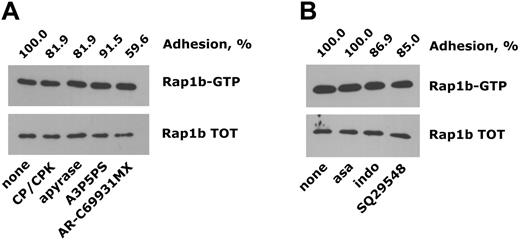
We next investigated the signaling pathways mediating Rap1b activation downstream of integrin α2β1. These studies were mainly performed using monomeric collagen as integrin α2β1 ligand, which represents the cheapest and easiest reagent to obtain, but the reported results were also randomly confirmed in studies with the other selective ligands. We initially determined whether activation of Rap1b in platelets adherent to monomeric collagen was a direct consequence of integrin α2β1-mediated outside-in signaling or was due to the action of secondary soluble messengers released by adherent platelets. Figure 2A shows that in platelets adherent through integrin α2β1, activation of Rap1b was not dependent on secreted ADP, because it was not altered by the presence of the ADP scavengers apyrase or CP/CPK or by incubation with selective antagonists of the P2Y1 and P2Y12 ADP receptors. The percentage of adherent platelets was not remarkably altered by neutralization of extracellular ADP but was evidently and consistently reduced in the presence of AR-C69931MX, suggesting that the Gi-coupled P2Y12 receptor for ADP may contribute to consolidate integrin α2β1-dependent platelet adhesion to collagen, as previously suggested.16,17 Figure 2B shows that prevention of thromboxane A2 synthesis by the cyclooxygenase inhibitors aspirin or indomethacin or blockade of the thromboxane A2 receptor by SQ29548 only minimally reduced the extent of platelet adhesion to monomeric collagen and did not affect Rap1b activation in adherent platelets.
Integrin α2β1-induced activation of Rap1b does not require platelet-derived secondary agonists. Platelet adhesion to monomeric collagen was analyzed using washed platelets preincubated with the ADP scavengers CP/CPK or apyrase or with the ADP receptor antagonists A3P5PS or AR-C69931MX (A) or upon treatment with the cyclooxygenase inhibitors aspirin (asa) and indomethacin (indo) or with the thromboxane A2 receptor antagonist SQ29548 (B), as described in “Materials and methods.” The immunoblots show both active Rap1b (Rap1b-GTP) and total Rap1b (Rap1b TOT) isolated from the same number of adherent platelets. The effect of the different treatments on the extent of platelet adhesion is reported on the top of the panels (adhesion, %), considering as 100% the adhesion of nontreated platelets.
Integrin α2β1-induced activation of Rap1b does not require platelet-derived secondary agonists. Platelet adhesion to monomeric collagen was analyzed using washed platelets preincubated with the ADP scavengers CP/CPK or apyrase or with the ADP receptor antagonists A3P5PS or AR-C69931MX (A) or upon treatment with the cyclooxygenase inhibitors aspirin (asa) and indomethacin (indo) or with the thromboxane A2 receptor antagonist SQ29548 (B), as described in “Materials and methods.” The immunoblots show both active Rap1b (Rap1b-GTP) and total Rap1b (Rap1b TOT) isolated from the same number of adherent platelets. The effect of the different treatments on the extent of platelet adhesion is reported on the top of the panels (adhesion, %), considering as 100% the adhesion of nontreated platelets.
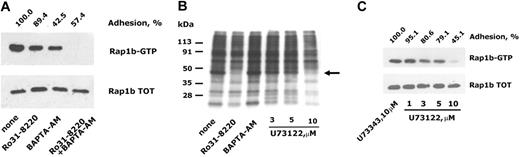
Because previous work has shown that integrin α2β1-mediated platelet adhesion causes activation of PLCγ2,15,20,21 we investigated the involvement of PLC-derived second messengers in adhesion-dependent activation of Rap1b. Figure 3A shows that treatment of platelets with the PKC inhibitor Ro31-8220 or with the intracellular calcium-chelating agent BAPTA-AM caused a partial and comparable reduction of Rap1b activation. Interestingly, however, BAPTA-AM treatment resulted in a much stronger inhibition of platelet adhesion to monomeric collagen than Ro31-8220. The simultaneous inhibition of PKC and chelation of intracellular calcium resulted in a total suppression of Rap1b activation in adherent platelets. This result indicates that PKC and Ca2+ mediate 2 distinct but additive pathways for integrin α2β1-dependent activation of Rap1b and suggests that Rap1b activation lies downstream of PLC in collagen-adherent platelets. The phosphorylation of PLCγ2 downstream of integrin α2β1 has been widely demonstrated, but the real activation of PLC has been poorly documented. Using 32P-labeled cells, we evaluated the phosphorylation of pleckstrin, the main substrate for PKC in platelets adherent to ligands of integrin α2β1. The autoradiography illustrated in Figure 3B shows the occurrence of pleckstrin phosphorylation in adherent platelets, which could be prevented, as expected, by the PKC inhibitor Ro31-8220 but not by BAPTA-AM. This finding indicates that PKC (and thus PLC) is actually activated downstream of integrin α2β1. In addition, Figure 3B shows that adhesion-dependent pleckstrin phosphorylation was inhibited in a dose-dependent manner by the PLC inhibitor U73122, which was maximally effective at 10 μM. Importantly, U73122, but not the inactive related compound U73343, was able to prevent in a similar dose-dependent manner the activation of Rap1b in adherent platelets (Figure 3C) and to cause a progressive inhibition of the extent of platelet adhesion itself. These findings confirm that PLC-derived second messengers are essential for integrin α2β1-dependent activation of Rap1b.
Role of phospholipase C and Rap1b in the cross talk between integrin α2β1 and integrin αIIbβ3
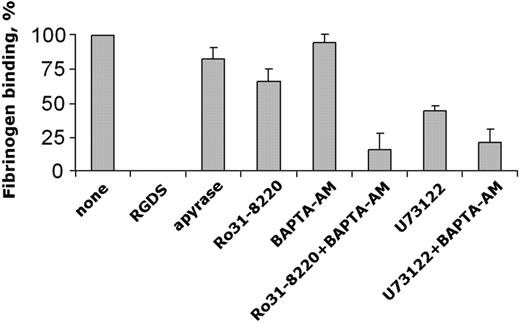
Previous findings have suggested a role for Rap1b in the regulation of the function of several integrins, including the platelet fibrinogen receptor, integrin αIIbβ3.4-7,23-25 Having demonstrated that Rap1b is activated downstream of integrin α2β1, we investigated whether, under the same conditions, integrin α2β1-dependent platelet adhesion could result in the stimulation of integrin αIIbβ3. For this purpose, we developed an assay to measure the specific binding of biotinylated fibrinogen to platelets adherent to monomeric collagen. This assay was designed to allow the evaluation of the specific binding of fibrinogen to the same number of adherent platelets, and thus it was suitable to evaluate the effect of specific pharmacologic inhibitors independent of their possible effect on platelet adhesion itself. Using aspirin-treated platelets, we found that platelet adhesion to monomeric collagen through integrin α2β1 induced the activation of integrin αIIbβ3, detectable as RGDS-sensitive binding of fibrinogen (Figure 4). Secreted ADP did not significantly contribute to this process, as indicated by experiments performed in the presence of apyrase. Similarly, binding of biotinylated fibrinogen to collagen-adherent platelets was not affected by chelation of intracellular Ca2+ with BAPTA-AM and was only slightly reduced upon inhibition of PKC with Ro31-8220. Interestingly, however, concomitant neutralization of PLC-derived second messengers by Ro31-8220 and BAPTA-AM caused a marked inhibition of specific fibrinogen binding to collagen-adherent platelets. Therefore, pharmacologic prevention of integrin αIIbβ3 activation was seen under conditions also preventing activation of Rap1b. An inhibitory effect on fibrinogen binding was also observed upon direct blockade of PLC by U73122 (Figure 4), confirming that integrin α2β1-mediated activation of integrin αIIbβ3 required PLC-derived second messengers. U73122 appeared to be less efficient than the combination of Ro31-8220 and BAPTA-AM in inhibiting fibrinogen binding. Because U73122, at the concentration used, was found to totally inhibit PLC activation, as demonstrated by the lack of PKC-directed pleckstrin phosphorylation (Figure 3B), we considered the possibility that an influx of extracellular Ca2+ could sustain the residual fibrinogen binding. In fact, we found that the intracellular Ca2+ chelator BAPTA-AM was able to cause a further inhibition of fibrinogen binding in U73122-treated platelets, lowering the level of integrin αIIbβ3 activation to that observed in platelets incubated with Ro31-88220 and BAPTA-AM (Figure 4). Although a minimal residual specific fibrinogen binding was constantly detected under conditions in which activation of Rap1b was totally prevented, our results document an important, functional link among PLC, Rap1b, and activation of integrin αIIbβ3 downstream of integrin α2β1 engagement.
Role of PLC-derived second messengers on integrin α2β1-dependent activation of Rap1b. (A) Washed platelets were left untreated (none) or incubated with the PKC inhibitor Ro31-8220, with the intracellular Ca2+ chelating agent BAPTA-AM, or with both compounds, as indicated in “Materials and methods.” Platelet adhesion to monomeric collagen was evaluated after 30 minutes of incubation and is reported on the top of the panel, considering as 100% the adhesion of untreated platelets. Activated Rap1b in each sample was isolated by the pulldown assay using lysates from an identical number of adherent platelets and identified by immunoblotting (Rap1b-GTP). The bottom panel shows the level of total Rap1b present in identical aliquots of the platelet lysates used for the pulldown assay. (B) Analysis of PLC activation. 32P-labeled platelets were incubated with immobilized monomeric collagen for 30 minutes. Adherent platelets were lysed and total proteins separated by SDS-PAGE on a 5% to 15% acrylamide gradient gel. Phosphorylation of pleckstrin, the main platelet substrate for PKC, was visualized by autoradiography. The migration of phosphorylated pleckstrin is indicated by the arrow on the right, while the migration of molecular mass markers is reported on the left. Some samples of 32P-labeled platelets were preincubated with Ro31-8220, BAPTA-AM, or with the indicated concentrations of U73122, as indicated on the bottom, and analysis of pleckstrin phosphorylation was performed on the same number of adherent platelets. (C) Inhibition of PLC prevents Rap1b activation. Washed platelets were treated with increased concentrations of the PLC inhibitor U73122 or with 10 μM U73343, an inactive related compound, and then incubated with immobilized monomeric collagen. The effect of treatment with U73122 on the extent of platelet adhesion and on the accumulation of active Rap1b in the same number of adherent platelets (Rap1b-GTP), as well as the level of total Rap1b in adherent cells (Rap1b total), are reported.
Role of PLC-derived second messengers on integrin α2β1-dependent activation of Rap1b. (A) Washed platelets were left untreated (none) or incubated with the PKC inhibitor Ro31-8220, with the intracellular Ca2+ chelating agent BAPTA-AM, or with both compounds, as indicated in “Materials and methods.” Platelet adhesion to monomeric collagen was evaluated after 30 minutes of incubation and is reported on the top of the panel, considering as 100% the adhesion of untreated platelets. Activated Rap1b in each sample was isolated by the pulldown assay using lysates from an identical number of adherent platelets and identified by immunoblotting (Rap1b-GTP). The bottom panel shows the level of total Rap1b present in identical aliquots of the platelet lysates used for the pulldown assay. (B) Analysis of PLC activation. 32P-labeled platelets were incubated with immobilized monomeric collagen for 30 minutes. Adherent platelets were lysed and total proteins separated by SDS-PAGE on a 5% to 15% acrylamide gradient gel. Phosphorylation of pleckstrin, the main platelet substrate for PKC, was visualized by autoradiography. The migration of phosphorylated pleckstrin is indicated by the arrow on the right, while the migration of molecular mass markers is reported on the left. Some samples of 32P-labeled platelets were preincubated with Ro31-8220, BAPTA-AM, or with the indicated concentrations of U73122, as indicated on the bottom, and analysis of pleckstrin phosphorylation was performed on the same number of adherent platelets. (C) Inhibition of PLC prevents Rap1b activation. Washed platelets were treated with increased concentrations of the PLC inhibitor U73122 or with 10 μM U73343, an inactive related compound, and then incubated with immobilized monomeric collagen. The effect of treatment with U73122 on the extent of platelet adhesion and on the accumulation of active Rap1b in the same number of adherent platelets (Rap1b-GTP), as well as the level of total Rap1b in adherent cells (Rap1b total), are reported.
Integrin α2β1-dependent activation of integrin αIIbβ3. Aspirin-treated human platelets were allowed to adhere to monomeric collagen through integrin α2β1, and specific binding of biotinylated fibrinogen to adherent platelets was measured as described in “Materials and methods.” When indicated, platelets were pretreated with 1 mM RGDS, 3 U/mL apyrase, 10 μM Ro31-8220, 30 μM BAPTA-AM, or 10 μM U73122, alone or in combination, before incubation with immobilized monomeric collagen. Specific binding of fibrinogen has been calculated for the same number of adherent platelets untreated or treated with the different inhibitors. Data are reported considering as 100% the specific fibrinogen binding to control platelets (none) and represent the means ± SD of 3 different experiments performed in duplicate.
Integrin α2β1-dependent activation of integrin αIIbβ3. Aspirin-treated human platelets were allowed to adhere to monomeric collagen through integrin α2β1, and specific binding of biotinylated fibrinogen to adherent platelets was measured as described in “Materials and methods.” When indicated, platelets were pretreated with 1 mM RGDS, 3 U/mL apyrase, 10 μM Ro31-8220, 30 μM BAPTA-AM, or 10 μM U73122, alone or in combination, before incubation with immobilized monomeric collagen. Specific binding of fibrinogen has been calculated for the same number of adherent platelets untreated or treated with the different inhibitors. Data are reported considering as 100% the specific fibrinogen binding to control platelets (none) and represent the means ± SD of 3 different experiments performed in duplicate.
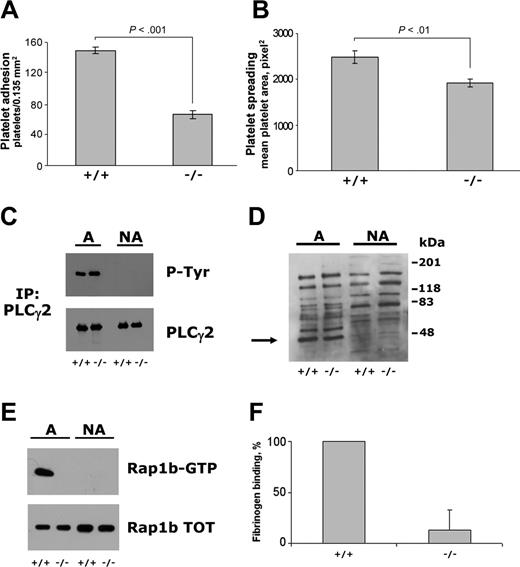
To investigate further the possible role of Rap1b in the cross talk between integrins α2β1 and αIIbβ3, we have analyzed platelets from mice lacking CalDAG-GEFI, a Ca2+- and DAG-sensitive nucleotide exchange factor for Rap1 expressed in circulating platelets and responsible for Rap1b activation in response to many soluble agonists.24 CalDAG-GEFI–/– platelets showed a significantly reduced ability to adhere to monomeric collagen as well as a reduced cell spreading, measured as mean platelet area of adherent platelets (Figure 5A-B). However, when the same number of control and CalDAG-GEFI–/– platelets were analyzed, a comparable tyrosine phosphorylation of PLCγ2 was observed (Figure 5C), demonstrating that early events in the outside-in signaling through integrin α2β1 occurred normally. Similarly, analysis of pleckstrin phosphorylation by immunoblotting with antiphosphoserine PKC-substrates antibody revealed that in adherent CalDAG-GEFI–/– platelet activation of PLC was comparable to that measured in control murine platelets (Figure 5D). Nevertheless, we observed that adhesion of CalDAG-GEFI–/– to monomeric collagen totally failed to promote Rap1b activation, indicating that CalDAG-GEFI mediates GTP binding to Rap1b downstream of integrin α2β1 (Figure 5E). Finally, measurement of fibrinogen binding to adherent platelets showed a near total reduction of integrin αIIbβ3 activation in CalDAG-GEFI–deficient platelets (Figure 5F). These results indicate that CalDAG-GEFI mediates integrin α2β1-dependent stimulation of Rap1b and is required for subsequent activation of integrin αIIbβ3.
Analysis of platelet adhesion and integrin α2β1-dependent outside-in signaling in CalDAG-GEFI–deficient murine platelets. Platelets from wild-type (+/+) and CalDAG-GEFI–deficient (–/–) mice were incubated with immobilized monomeric collagen. Platelet adhesion (A) and spreading (B) were measured using collagen-coated glass coverslips and determined by fluorescence microscopy analysis upon staining of adherent platelets with TRITC-phalloidin. The reported results are the mean ± SD of 3 independent experiments. The statistical significance of the observed differences, calculated by the Student t test, is also shown. PLCγ2 tyrosine phosphorylation, pleckstrin phosphorylation, and Rap1b activation were analyzed in samples containing identical amount of proteins from platelets adherent to collagen-coated 60 mm–diameter dishes as well as from nonadherent platelets, indicated as A (adherent) and NA (nonadherent) on the top of panels C, D, and E. (C) PLCγ2 was immunoprecipitated with specific antibody and then analyzed by immunoblotting with antiphosphotyrosine antibody (P-Tyr), followed by immunoblotting with anti-PLCγ2 antibody, as indicated on the right. (D) Activation of PKC was analyzed on total platelet proteins from adherent and nonadherent platelets by immunoblotting with antiphosphoserine PKC substrates. The position of phosphorylated pleckstrin (47 kDa) detected in adherent but not in nonadherent platelets is indicated by the arrow on the left. On the right, the position of molecular mass markers is reported. (E) Analysis of Rap1b activation (Rap1-GTP) in the same number of adherent and nonadherent platelets, as well as the total amount of Rap1 in the cell lysate used (Rap1b-TOT), are reported. Results from panels C, D, and E are representative of 2 independent experiments. (F) The specific binding of biotinylated fibrinogen to the same number of wild-type (+/+) and CalDAG-GEFI–deficient (–/–) murine platelets adherent to monomeric collagen through integrin α2β1. Fibrinogen binding measured in adherent wild-type platelets is reported as 100%, and the data are the means ± SD of 3 separate experiments.
Analysis of platelet adhesion and integrin α2β1-dependent outside-in signaling in CalDAG-GEFI–deficient murine platelets. Platelets from wild-type (+/+) and CalDAG-GEFI–deficient (–/–) mice were incubated with immobilized monomeric collagen. Platelet adhesion (A) and spreading (B) were measured using collagen-coated glass coverslips and determined by fluorescence microscopy analysis upon staining of adherent platelets with TRITC-phalloidin. The reported results are the mean ± SD of 3 independent experiments. The statistical significance of the observed differences, calculated by the Student t test, is also shown. PLCγ2 tyrosine phosphorylation, pleckstrin phosphorylation, and Rap1b activation were analyzed in samples containing identical amount of proteins from platelets adherent to collagen-coated 60 mm–diameter dishes as well as from nonadherent platelets, indicated as A (adherent) and NA (nonadherent) on the top of panels C, D, and E. (C) PLCγ2 was immunoprecipitated with specific antibody and then analyzed by immunoblotting with antiphosphotyrosine antibody (P-Tyr), followed by immunoblotting with anti-PLCγ2 antibody, as indicated on the right. (D) Activation of PKC was analyzed on total platelet proteins from adherent and nonadherent platelets by immunoblotting with antiphosphoserine PKC substrates. The position of phosphorylated pleckstrin (47 kDa) detected in adherent but not in nonadherent platelets is indicated by the arrow on the left. On the right, the position of molecular mass markers is reported. (E) Analysis of Rap1b activation (Rap1-GTP) in the same number of adherent and nonadherent platelets, as well as the total amount of Rap1 in the cell lysate used (Rap1b-TOT), are reported. Results from panels C, D, and E are representative of 2 independent experiments. (F) The specific binding of biotinylated fibrinogen to the same number of wild-type (+/+) and CalDAG-GEFI–deficient (–/–) murine platelets adherent to monomeric collagen through integrin α2β1. Fibrinogen binding measured in adherent wild-type platelets is reported as 100%, and the data are the means ± SD of 3 separate experiments.
Discussion
Our findings demonstrate that the small GTPase Rap1b is activated upon platelet adhesion through integrin α2β1 by the action of the Ca2+- and DAG-dependent exchange factor CalDAG-GEFI. Moreover, we provide evidence that activated Rap1b is required for the cross talk between the platelet collagen receptor, integrin α2β1, and the fibrinogen receptor, integrin αIIbβ3.
Rap1b has been implicated in the regulation of different aspects of cell adhesion and migration, and its function in the inside-out activation of several integrins has been widely documented.4-7,23-25 Transfection with a constitutively active mutant of Rap1 generally results in an increased level of integrin-mediated adhesion, which, by contrast, is prevented by the expression of dominant-negative mutants of Rap1 or by the expression of RapGAP. In human platelets, Rap1b has been recognized as participating in the inside-out activation of integrin αIIbβ3 and, as documented by direct and indirect evidence, the regulation of platelet aggregation.23-29 In this study, by using 4 structurally different specific ligands for the platelet integrin α2β1, we have demonstrated for the first time that Rap1b is also involved in integrin outside-in signaling.
The activation of Rap1b upon integrin-mediated cell adhesion has been suggested previously but never directly demonstrated, and conflicting results have been reported.10-12 Early studies with platelets in suspension showed that direct stimulation of integrin αIIbβ3 by the activating monoclonal antibody LIBS-6 is associated with increased GTP binding to Rap1b.33 Here we provide definitive evidence that platelet adhesion through integrin α2β1 induces a robust activation of Rap1. Together with GPVI, integrin α2β1 mediates platelet interaction with collagen.14 The relative spatiotemporal contribution of these 2 different receptors to collagen-induced platelet activation and thrombus formation is still a matter of controversy, but several findings suggest that, upon platelet interaction with collagen, outside-in signaling through integrin α2β1 may contribute significantly to thrombus stability.34-37 The engagement of integrin α2β1 initiates a kinase-based signaling pathway leading to tyrosine phosphorylation of Syk and PLCγ2, an increase of intracellular Ca2+ concentration, and stimulation of Rac and Cdc42 GTPases, eventually resulting in platelet spreading.15,20-22 Here we have demonstrated that activation of PLC downstream of integrin α2β1 is essential for subsequent Rap1b activation. Moreover, using platelets from knockout mice, we have identified CalDAG-GEFI as the nucleotide exchange factor responsible for integrin α2β1-promoted activation of Rap1b.
CalDAG-GEFI is highly expressed in the brain and in hematopoietic cells and in cell culture is activated by the PLC-generated second messengers Ca2+ and diacylglycerol.38 In platelets, it is responsible for Rap1b activation and integrin αIIbβ3-mediated aggregation induced by many soluble agonists, including thrombin and ADP.24 We show in this study that CalDAG-GEFI is centrally important not only for this agonist-induced regulation of platelet Rap1b but also for Rap1b activation downstream of integrin engagement. Our findings thus extend the importance of this exchange factor for Rap GTPase function in platelet physiology.
Having demonstrated that Rap1b is a signaling element stimulated downstream of integrin α2β1 engagement in platelets, we reasoned that this GTPase could play a role in the cross talk among different integrin receptors by linking integrin-generated outside-in signals to integrin inside-out activation. We demonstrate here the existence of such cross talk between integrin α2β1 and integrin αIIbβ3, as we detected specific fibrinogen binding to platelets adhering to monomeric collagen and other integrin α2β1-specific ligands. A partial activation of integrin αIIbβ3 induced by platelet adhesion to monomeric collagen has been previously reported.39 We have extended these findings by investigating the intracellular signaling pathways regulating integrin α2β1-mediated activation of integrin αIIbβ3. We found that similarly to what was observed for Rap1b, platelet adhesion to monomeric collagen induced the activation of integrin αIIbβ3 through a PLC-dependent mechanism. Moreover, in CalDAG-GEFI–deficient platelets, integrin αIIbβ3 activation induced by engagement of integrin α2β1 was almost completely suppressed. Notably, in adherent CalDAG-GEFI–deficient platelets, activation of PLC occurred normally, but stimulation of Rap1b was completely abolished. These results suggest an essential role for CalDAG-GEFI in integrin cross talk and demonstrate that prevention of integrin α2β1-mediated Rap1b stimulation results in an impaired activation of integrin αIIbβ3 despite normal PLC activity. We thus propose that binding of integrin α2β1 to specific ligands during platelet adhesion activates PLC (most likely PLCγ2, which has been shown to be phosphorylated under these conditions),15,20,21 producing second messengers able to stimulate CalDAG-GEFI and promote Rap1b activation, which, in turn, is responsible for inside-out activation of integrin αIIbβ3. This conclusion is supported by the finding that when Rap1b activation is blocked (ie, in CalDAG-GEFI–deficient platelets), activation of PLC is able to support only a minimal stimulation of integrin αIIbβ3. To confirm this observation, we sought to obtain Rap1b activation by alternative platelet stimulation after neutralization of PLC-derived second messengers, but any attempts have been unsuccessful. Such a failure, however, could be predicted based on the previously demonstrated crucial role of Ca2+ and PKC in regulating Rap protein activation in platelets24,26,28 and based on the lack of expression of other known Rap1GEFs, such as EPAC or C3G, in these cells (B.B., G.F.G, and M.T., unpublished results, 2005).
In our experiments, we constantly observed a residual 20% to 25% activation of integrin αIIbβ3 under conditions of complete inhibition of Rap1b. These results indicate that Rap1b is certainly a main regulator of platelet integrin αIIbβ3 activation but that additional Rap1b-independent mechanisms promoting fibrinogen binding operate in platelets adherent through integrin α2β1. This is neither surprising nor unexpected, because a similar scenario has been documented in platelets and megakaryocytes stimulated in suspension with soluble agonists. For instance, a residual integrin αIIbβ3-triggered platelet aggregation in response to many soluble agonists has been found in CalDAG-GEFI–deficient platelets and in Rap1b knockout mice.24,25 Moreover, expression of exogenous Rap1GAP or of a dominant-negative mutant of Rap1b inhibited but did not abolish integrin αIIbβ3 activation in mouse megakaryocytes.23 In this context, our data confirm that also in adherent platelets Rap1b is an important albeit not the sole integrin regulator.
Previous studies in different cell lines have shown that Rap1b can regulate the activity of a broad range of integrins, including β2 and β1 integrins.4-7 Although much of the attention has been focused on integrin αIIbβ3 activation of platelets, it is clear that other integrins also undergo inside-out regulation. In particular, it has been found that stimulation of platelets with different soluble agonists results in the activation of integrin α2β1, as evaluated by increased adhesion to specific ligands, increased binding of soluble monomeric collagen, and analysis with activation-dependent antibodies.15-19 Our work favors, in addition, a function for Rap1b in the inside-out regulation of platelet integrin α2β1. ADP-induced activation of the collagen receptor is predominantly mediated by binding to the Gi-coupled P2Y12 receptor, which is also responsible for Rap1b activation.17,27,28 In this work, we observed a marked reduction of platelet adhesion to monomeric collagen in CalDAG-GEFI–deficient platelets compared with adhesion in normal platelets and found a significant reduction of platelet spreading. These findings suggest that the CalDAG-GEFI–Rap1b axis is active in the autoregulation of integrin α2β1 through a positive feedback mechanism aimed to reinforce and consolidate platelet adhesion. Our analysis with pharmacologic inhibitors of specific intracellular signaling pathways demonstrated a reduction of integrin α2β1-mediated platelet adhesion upon inhibition of PLC or upon neutralization of PLC-derived second messengers when Rap1b activation was also prevented. A recent report has demonstrated that integrin α2β1-induced intracellular calcium increase is necessary for efficient platelet adhesion and spreading on a collagen-derived peptide.21 Moreover, outside-in positive autoregulation of integrin function and consolidation of cell adhesion has been described for β2 integrins in human polymorphonuclear cells.40 The existence and relevance of such a positive feedback mechanism for platelet integrin α2β1 regulation and the possible role of Rap1b in this process is an intriguing possibility that deserves further investigation. We propose that Rap1b could integrate different intracellular signaling pathways generated by both soluble agonists and adhesion receptors.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-07-3023.
Supported by grants from the Ministero dell'Istruzione, Università e Ricerca Scientifica (PRIN 2003), Consorzio Interuniversitario Biotecnologie (M.T.; CIB); and the National Institute of Child Health and Human Development (A.M.G.; NIH-NICHD HD28341—Novel Second Messenger Signaling in the Stratum).
B.B. and G.F.G. designed and performed experiments and analyzed data; F.C. performed experiments and analyzed data; J.R.C. and A.M.G. provided vital reagents, analyzed data, and edited the manuscript; C.B. analyzed data and provided overall direction; and M.T. designed research, analyzed data, wrote the paper, and provided overall direction.
B.B. and G.F.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal