Abstract
Regulatory T cells (TREGs) constitutively expressing CD4, CD25, and the transcription factor Foxp3 can prevent a wide range of experimental and spontaneous autoimmune diseases in mice. In humans, CD4+CD25bright T cells, predominantly within the CD45RO+ activated/memory subset in adults and the CD45RA+ naive T-cell subset in infants, are considered to be the equivalent subset. Using novel combinations of monoclonal antibodies (mAbs), we examined expression of CD25 in human infant thymus, cord blood, adult peripheral blood, lymph node, and spleen. In addition to the CD4+CD25bright T cells, subfractionation on the basis of CD45 splice variants indicated that all samples contained a second distinct population of cells expressing a slightly lower level of CD25. In adult peripheral blood, this population expressed a naive CD45RA+ phenotype. The corresponding population in lymph node, spleen, and cord blood showed some evidence of activation, and expressed markers characteristic of TREGs, such as cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). Sorted CD4+CD25+CD45RA+ T cells from both cord and adult blood expressed very high levels of mRNA for Foxp3 and manifested equivalent suppressive activity in vitro, indicating that they are bone fide members of the regulatory T-cell lineage. Targeting naive TREGs in adults may offer new means of preventing and treating autoimmune disease.
Introduction
Regulatory CD4+ T cells (TREGs) with suppressive function play a major role in maintaining self tolerance. One subset of TREGs, called “natural” TREGs, are selected after recognition of self-antigen in the thymus. They are distinct from conventional T cells in that they do not require T-cell receptor (TCR)-mediated signals to maintain constitutive expression of CD25, the α chain of the interleukin-2 (IL-2) receptor. In mice, TREGs constitute a phenotypically distinct population of CD25high cells that comprise 5% to 10% of thymic, peripheral blood, lymph node, and spleen CD4+CD8- T-cell populations.1,2 In humans, however, up to 40% of peripheral-blood CD4+ T cells express CD25 to some extent.3 It has been reported that only a minority of human CD4+ T cells expressing the highest levels of CD25 (called CD25bright cells) have suppressor activity,3 and therefore many recent studies have focused on this population.4-7 However, the proportion of cells in the CD25bright population, estimated at 1% to 2% of peripheral-blood CD4+ T cells, is considerably smaller than in the homologous mouse population, suggesting that some human TREGs may be excluded from analysis by the conventional CD25bright gating strategy. Better markers or combinations of markers are therefore clearly required in order to accurately identify regulatory T cells in humans.
In this study, we assessed the utility of a large number of possible TREG markers, including human leukocyte antigen (HLA)-DR, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor (GITR), and OX40, which are expressed by human TREGs. In addition, we subfractionated CD4+ T cells on the basis of CD45 splice variants. In humans, expression of CD45RO and CD45RA is usually regulated in a reciprocal manner, with the switch from 1 variant to another marking differentiative steps associated with TCR-dependent selection and activation. Positively selected T cells express CD45RO in the thymus,8 convert to CD45RA at the time of emigration to the periphery,9 and then switch back to CD45RO after antigen recognition.10 A small percentage of antigen-experienced T cells, particularly those expressing CD8, revert from expression of CD45RO to CD45RA as they undergo differentiation to terminal effector cells.11 CD25bright cells reside predominantly within the activated/memory CD45RO+ population in adult peripheral blood.3 In contrast, CD25bright cells in cord blood express CD45RA, suggesting that they are naive.12,13
The data presented here show that human thymus, cord and adult blood, lymph nodes, and spleen all contain 2 populations of CD25+CD4+ cells: the well-recognized CD25bright population expressing CD45RO, and a second population of CD45RA+ cells expressing a slightly lower level of CD25. The mean number of CD45RA+ TREGs in adult blood is approximately half the mean number of CD45RO+ TREGs, and declines significantly with age. To confirm that the CD45RA+ cells are bona fide members of the natural TREG lineage, 2 independent assays were used. First, Foxp3, a transcription factor required for the development and function of TREGs,14-16 was measured by quantitative polymerase chain reaction (qPCR). Both CD45RA+ and CD45RO+ populations of CD4+CD25+ cells from thymus and cord and adult blood expressed Foxp3 mRNA at between 100 to 500 times the level of naive CD4+CD25-CD45RA+ T cells. Interestingly, cord and adult blood CD25-CD45RO+ cells expressed intermediate levels of Foxp3 mRNA when assayed directly ex vivo, suggesting that expression of Foxp3 is not restricted to TREGs in humans. Cord and adult blood populations were also tested for in vitro suppressive activity. Both CD45RA+ and CD45RO+ TREGs showed equivalent potency in suppression assays.
Materials and methods
Murine samples
B6.SJLPtprca and syngeneic C57BL/6 RAG-1-deficient mice were obtained from the Animal Resources Centre (Perth, WA, Australia) and maintained under specific pathogen-free (SPF) conditions at the Centenary Institute animal facility. The Animal Ethics Committee of the University of Sydney approved all experimental procedures and housing arrangements. Peripheral-blood and lymph-node cells were prepared as detailed previously.17 For adoptive transfer, 107 lymph node cells were injected into the tail veins of RAG-1-deficient animals and the lymph nodes were harvested 21 days later.
Human samples
Peripheral blood was obtained from healthy adult donors at the Centenary Institute of Cancer Medicine and Cell Biology, the Royal Prince Alfred Hospital (Camperdown, NSW, Australia) and Nepean Hospital (Penrith, NSW, Australia). Buffy coats and spleens from cadaveric organ donors were obtained from the Australian Red Cross Blood Service (Sydney, NSW, Australia). Cord-blood samples were obtained from the placentas of healthy full-term infants delivered at the Nepean Hospital. Thymus fragments were obtained from infants undergoing elective cardiac surgery at the Children's Hospital, Westmead (NSW, Australia). Lymph nodes draining normal colonic tissue were isolated from freshly resected colonic specimens from patients undergoing surgery for cancer or incontinence at the Royal Prince Alfred Hospital. The study was carried out under the supervision of the Ethics Committees of the Central and Western Sydney Area Health Services and the Children's Hospital at Westmead. Informed consent was provided according to the Declaration of Helsinki.
Isolation of mononuclear cells from specimens
Peripheral-blood, buffy-coat, and cord-blood mononuclear cells were prepared by centrifugation over Ficoll-Paque (Lymphoprep; Nycomed, Oslo, Norway). Splenic mononuclear cells were prepared as described previously.18 Lymph nodes within fresh operative colon samples were identified by palpation and dissected away from the bowel wall using fine scissors and forceps. Single-cell suspensions of lymph nodes and thymus fragments were prepared as described previously.18 All mononuclear-cell preparations were cryopreserved in liquid nitrogen until required.
Antibodies and flow cytometry staining
The mouse anti-human monoclonal antibodies (mAbs) used in this study are listed in Table 1. Anti-CD4, anti-CD25, and anti-CD45RO hybridomas (clones OKT4, 7GB6, and UCHTL-1, respectively) were the kind gift of Prof Derek Hart (Mater Medical Research Institute, Brisbane, Australia). Antibodies purified from hybridoma supernatants were labeled with Alexa488 (Molecular Probes, Eugene, Oregon) and FITC (Sigma, St Louis, MO) by standard protocols. Biotin conjugates were developed with streptavidin conjugated with Alexa594 (Molecular Probes) or PerCp (PharMingen, Mississauga, ON, Canada). PE- and FITC-conjugated anti-mouse mAbs (Southern Biotechnology, Birmingham, AL) were used to detect unconjugated anti-CCR7 (PharMingen).
Correlation between peripheral-blood leukocyte expression of CD25, CD4, and 38 surface markers
Staining pattern versus CD25*for cells gated for expression of CD4† . | Antibodies . |
|---|---|
| All cells negative or very low | CD8†, CD21‡, CD23‡, CD40L†, CD54†, CD70§, CD74†, CD84†, CXCR5†, CD148∥ |
| Large negative population, including all CD25+ cells | CD56†, CD95L†, CD244§, CXCR4† |
| All cells positive | CD3† |
| Most cells positive, including all CD25+ cells | CD5†, CD11a¶, CD28#, CD57† |
| CD27‡, CD38†, CD44†, CD45RA†, CD45RO†, CD58†, CD62L†, CD71†, CD95†, | |
| Profiles shown in Figure 2 | CTLA-4 (CD152)†, HLA-DR†, CCR7† |
| Resembles that of GITR (Figure 2A) | OX40 (CD134)†, GITR**, CD30#, CD69†, CD103†, CD122†, CD150∥ |
Staining pattern versus CD25*for cells gated for expression of CD4† . | Antibodies . |
|---|---|
| All cells negative or very low | CD8†, CD21‡, CD23‡, CD40L†, CD54†, CD70§, CD74†, CD84†, CXCR5†, CD148∥ |
| Large negative population, including all CD25+ cells | CD56†, CD95L†, CD244§, CXCR4† |
| All cells positive | CD3† |
| Most cells positive, including all CD25+ cells | CD5†, CD11a¶, CD28#, CD57† |
| CD27‡, CD38†, CD44†, CD45RA†, CD45RO†, CD58†, CD62L†, CD71†, CD95†, | |
| Profiles shown in Figure 2 | CTLA-4 (CD152)†, HLA-DR†, CCR7† |
| Resembles that of GITR (Figure 2A) | OX40 (CD134)†, GITR**, CD30#, CD69†, CD103†, CD122†, CD150∥ |
Obtained from BD Biosciences, San Jose, CA
Obtained from PharMingen
Obtained from Ancell, Bayport, MN
Obtained from Immunotech, Marseille, France
Obtained from DNAX Research Institute, Palo Alto, CA
Obtained from Caltag, Burlingame, CA
Obtained from Miltenyi Biotec, Gladbach, Germany
Obtained from R&D Systems, Minneapolis, MN
The following mAbs reactive with murine cells were used: anti-CD4-FITC and anti-CD25-PE (PharMingen), and anti-CD45.1 (A20.1, conjugated in-house with Alexa594).
Antibody staining was performed as described previously.17 After staining, human cells were fixed in 1% paraformaldehyde before analysis using a FACSCalibur or FACSVantage (Becton Dickinson, Mountain View, CA). Intracellular staining was performed after fixation in 2% paraformaldehyde, followed by permeabilization and staining in buffer containing 0.1% saponin. Analysis of flow data was performed using FlowJo software (Treestar, San Carlos, CA).
Cell sorting for RNA preparation
For sorting, cord-blood and adult buffy-coat or thymus mononuclear cells (5 × 108) were stained with a combination of (1) anti-CD4-PE, anti-CD25-APC, and anti-CD45RA-biotin or (2) anti-CD3-PE, anti-CD4-PECy5, anti-CD8-APC, anti-CD25-Alexa488, and anti-CD45RA-biotin, respectively. In both cases, anti-CD45RA-biotin was detected with streptavidin-Alexa594. In 1 experiment, adult blood cells were stained with anti-CD4-PE, anti-CD25-APC, anti-CD45RO-FITC, and anti-CD27-biotin detected with streptavidin-Alexa594. Positive selection with anti-PE beads (Miltenyi Biotech, Auburn, CA) using an AutoMACS (Miltenyi Biotech) was performed before sorting on a FACSVantage cell sorter.
In vitro suppression assay
For in vitro cultures, CD4+ T cells were first purified by negative selection with the CD4 T cell Isolation kit II (Miltenyi Biotec). For coculture of CD25+ and CD25- cells, CD25+ T cells were positively selected using PE-coupled anti-CD25 mAb and anti-PE-coupled magnetic beads. The purity of the selected populations ranged from 92% to 95%. For purification of CD25brightCD45RA-, CD25intCD45RA-, CD25+CD45RA+, and CD25-CD45RA+ cells, bead-selected CD4+ cells were stained using anti-CD4-PE, anti-CD25-APC, and anti-CD45RA-biotin detected with streptavidin-PerCP, and sorted using sorting a FACSAria cell sorter (Becton Dickinson). Purity of the sorted populations was greater than 95%. Irradiated (3000 rad) allogeneic adult peripheral-blood mononuclear cells (PBMCs) depleted of CD3+ cells were used as antigen-presenting cells in all assays.
In vitro suppression assays were performed in 96-well round bottom well plates in medium consisting of RPMI 1640 supplemented with 5% heat inactivated fetal calf serum (FCS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All wells contained 5 × 104 antigen-presenting cells, 2 × 104 responder cells (bead-selected CD4+ CD25- or sorted CD4+CD25-CD45RA+) and 0.25 μg/mL anti-CD3 (UCHT-1 or Hit3a; PharMingen). The number of putative suppressor cells added to each well was either 2 × 104, 0.5 × 104, 2 × 103, or none, giving final suppressor-to-responder ratios of 1:1, 0.25:1, 0.1:1, or 0:1, respectively. In control cultures, responders were added instead of suppressors. For CFSE assays, responders were labeled with CFSE and cell division was determined after 72 hours using flow cytometry. Percentage of proliferation was calculated relative to the mean number of divided cells in control wells containing only responder cells. For thymidine assays, preliminary experiments in which cultures were pulsed after 3, 4, or 5 days of culture indicated that the degree of suppression was stable over that period, so all subsequent cultures were pulsed with 3HTdR at 72 hours and harvested 16 hours later to allow direct comparison of CFSE and thymidine assays.
Real-time qPCR
Total RNA was extracted from 1 to 5 × 105 sorted cells using Trizol reagent. The total RNA preparation was reverse transcribed using Superscript II reverse-transcriptase and oligo(dT)2-18 primer (Invitrogen, Carlsbad, CA) in a final volume of 20 μL. For real-time quantitative PCR (RT qPCR), the reaction mixture contained 2 μL cDNA, 10 μL Platinum SYBR Green SuperMix UDG (Invitrogen), and 0.25 pM forward and reverse primer in a final volume of 18 μL. qPCR was performed on a Rotor-Gene 3000 system (Corbett Research, Mortlake, NSW, Australia) under the following conditions: stage 1, 94°C for 5 minutes; and stage 2, 94°C for 20 seconds, 58°C for 20 seconds, and 72°C for 20 seconds. An additional step at 75°C for 15 seconds was added to reduce the background due to primer dimers. Amplification (45 cycles) was carried out and relative expression of each gene was determined by normalization to β-actin. The primer sequences were as follows: Foxp3 sense GGCAAATGGTGTCTGCAAGTG and antisense GGATGATGCCACAGATGAAGC; and β-actin sense TCGACAACGGCTCCGGCATGTGCAAG and antisense AGCCACACGCAGCTCATTGTAGAAG. Primer sequences for T-bet and GATA3 were as previously described.19 All primers were supplied by Invitrogen.
Results
Comparison of CD25 expression by human and mouse CD4+ T cells
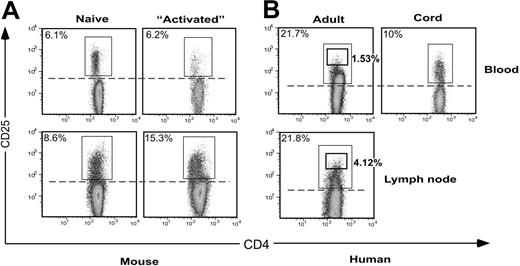
Expression of CD25 by mouse and human CD4+ T cells is compared in Figure 1. In murine peripheral blood, a distinct population of 6% CD25-expressing cells was clearly distinguishable from the negative population (Figure 1A). A similar profile of expression by 10% of CD4+ T cells was seen in human cord blood (Figure 1B). However, the profile for adult blood CD4+ T cells was quite different, with only the 1% to 2% of cells expressing the highest levels of CD25 being distinguishable as a separate subpopulation, while the level of expression on the other 98% of cells ranged from intermediate to truly negative. Setting a gate at the level indicated by the cord-blood profile (dotted line, Figure 1B) transected the major population of CD4+ T cells in adult peripheral blood and in human but not mouse lymph node. The large population of human cells expressing intermediate levels of CD25 is likely to correspond to effector/memory cells, since it was present in adult but not cord blood. Lack of significant numbers of effector/memory cells in mice (usually housed in pathogen-free conditions) could be responsible for the difference between mouse and human profiles. To test this, mouse CD4+ T cells were transferred into lymphopenic RAG-1-deficient animals to allow rapid homeostatic proliferation, differentiation, and acquisition of effector function.20 However, even under these conditions, the divided cells that constituted over 90% of CD4+ T cells in the reconstituted host animals did not express an equivalent level of CD25 to human cells (Figure 1A, “Activated”). Thus, human blood CD4+ effector/memory cells express more CD25 than their murine counterparts. The physiologic significance of such expression remains unclear.
Comparison of expression of CD4 versus CD25 in human and mouse. Peripheral-blood leukocytes were stained with mAbs to CD4 and CD25, and gated for lymphocytes expressing CD4. (A) In mice, the number of conventional activated CD4+ T cells expressing CD25 was low compared with the number of regulatory CD4+CD25+ cells, even when a large number of CD4+ T cells were actively proliferating during reconstitution of lymphopenic host mice (“Activated” plots). (B) In adult blood, up to 20% to 30% of CD4+ T cells expressed CD25, and many of these cells appeared to be effector and memory cells resulting from encounter with foreign antigens. In contrast, the human cord-blood profile resembled that of mice.
Comparison of expression of CD4 versus CD25 in human and mouse. Peripheral-blood leukocytes were stained with mAbs to CD4 and CD25, and gated for lymphocytes expressing CD4. (A) In mice, the number of conventional activated CD4+ T cells expressing CD25 was low compared with the number of regulatory CD4+CD25+ cells, even when a large number of CD4+ T cells were actively proliferating during reconstitution of lymphopenic host mice (“Activated” plots). (B) In adult blood, up to 20% to 30% of CD4+ T cells expressed CD25, and many of these cells appeared to be effector and memory cells resulting from encounter with foreign antigens. In contrast, the human cord-blood profile resembled that of mice.
The data also indicate that murine TREGs express slightly less CD4 than conventional T cells, particularly in blood (Figure 1A and Gavin et al21 ). For human cells, this was also the case, although it appeared that TREGs expressing slightly less CD25 than the CD25bright subset were obscured by the numerous CD25+ antigen-experienced cells. The latter population expressed more CD4 than naive CD25- cells, a finding that is consistent with published data derived from mice.22,23 Unfortunately, the difference in CD4 expression between the 2 populations is not sufficient to allow discrimination on a single-cell basis. Thus there is a need for further markers to provide better discrimination between human TREGs and antigen-experienced conventional T cells.
Testing alternative gating strategies for identification of human TREGs
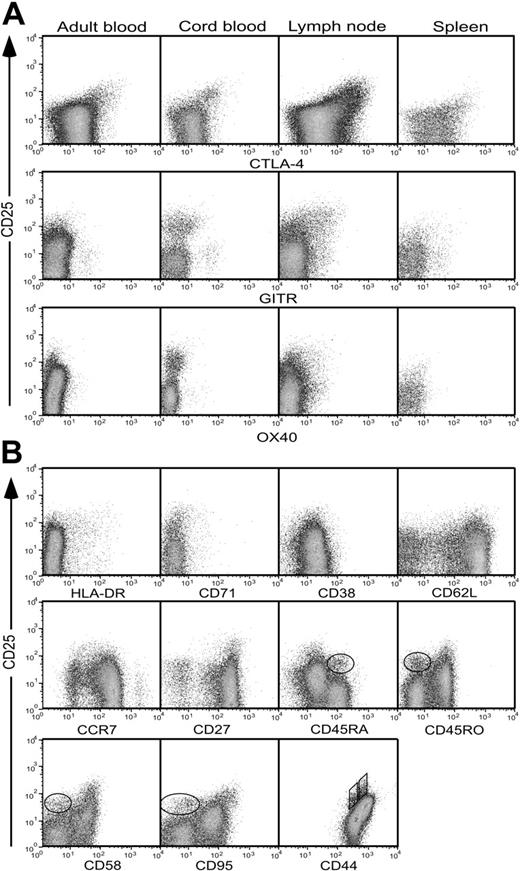
In mice, 2 of the best markers for identifying natural TREGs are the costimulatory molecule CTLA-4 (also known as CD152)24-26 and GITR (now designated TNFRSF18).27 We have found that OX40 (TNFRSF4), another member of the tumor necrosis factor (TNF) receptor superfamily, is also well expressed by murine TREGs (Caroline Higgins and B.F.d.S.G., unpublished data, March 2003; and Gavin et al21 and McHugh et al28 ). We used mAbs directed to the homologous human molecules in an attempt to refine the detection of human TREGs in adult and cord blood, lymph node, and spleen. Only CTLA-4 was expressed at a uniformly high level by CD4+CD25+ T cells from all 4 sites (Figure 2A, top panel). However a significant number of CD25- cells also expressed CTLA-4, especially in lymph nodes and spleen, limiting its usefulness in identifying TREGs in a definitive manner. GITR was expressed at low levels by lymph node, spleen, and cord-blood CD4+CD25+ T cells, but was virtually undetectable on adult blood CD4+CD25+ T cells (Figure 2A, middle panel). Moreover, the cells expressing the highest levels of GITR in cord and adult blood were within the CD4+CD25- subset. Expression of OX40 followed the same pattern as that of GITR, although the signal level was even lower (Figure 2A, bottom panel). Thus, none of these markers allowed a reliable distinction to be made between CD25+ and CD25- cells, particularly in adult blood.
Expression of regulatory T-cell markers by CD4+ lymphocytes. (A) Expression of CTLA-4, OX40, or GITR by CD4+ T cells from adult or cord blood, spleen, or lymph nodes. Cells were stained for intracellular expression of CTLA-4 and surface expression of OX40 and GITR, together with CD4 and CD25. Profiles are gated for lymphocytes expressing CD4. (B) Expression of HLA-DR, CD71, CD38, CD62L, CCR7, CD27, CD45RA, CD45RO, CD58, CD95, and CD44 by adult peripheral-blood cells costained for CD4 and CD25. Profiles are gated for live cells expressing CD4. The circled populations in the CD45RA, CD45RO, CD58, and CD95 plots represent a second population of CD25+ T cells distinguishable from the CD25bright population. In the CD44 profile, 2 distinct populations of CD25+ T cells are identified using parallelograms. Results are representative of between 3 and 9 independent experiments for each sample and stain.
Expression of regulatory T-cell markers by CD4+ lymphocytes. (A) Expression of CTLA-4, OX40, or GITR by CD4+ T cells from adult or cord blood, spleen, or lymph nodes. Cells were stained for intracellular expression of CTLA-4 and surface expression of OX40 and GITR, together with CD4 and CD25. Profiles are gated for lymphocytes expressing CD4. (B) Expression of HLA-DR, CD71, CD38, CD62L, CCR7, CD27, CD45RA, CD45RO, CD58, CD95, and CD44 by adult peripheral-blood cells costained for CD4 and CD25. Profiles are gated for live cells expressing CD4. The circled populations in the CD45RA, CD45RO, CD58, and CD95 plots represent a second population of CD25+ T cells distinguishable from the CD25bright population. In the CD44 profile, 2 distinct populations of CD25+ T cells are identified using parallelograms. Results are representative of between 3 and 9 independent experiments for each sample and stain.
To test for additional markers that would allow more accurate identification of TREGs within adult blood CD4+CD25+ T cells, mAbs against a further 33 surface molecules were screened by flow cytometry (Table 1), of which 11 are illustrated in Figure 2B. The panel included a number of markers whose overexpression by CD25+ versus CD25- cells had been noted previously.3 Expression of HLA-DR showed a disappointingly low correlation with CD25, as did CD71, CD38, CD62L, and CCR7 (Figure 2B). Interestingly, subfractionation on the basis of the CD45 splice variants CD45RA and CD45RO revealed not only the well-described CD45RA- CD45RO+CD25bright population, but also a discrete population of CD45RA+ naive cells expressing CD25 at a slightly lower level (circled in Figure 2B). In addition, it was apparent that most CD45RO+ cells expressed intermediate levels of CD25 (as postulated in “Comparison of CD25 expression by human and mouse CD4+ T cells”), whereas the majority of CD45RA+ cells were truly negative. The pattern of CD58 and CD95 expression was very similar to that of CD45RO. Staining for CD44 revealed 2 distinct CD25+ populations (boxed in Figure 2B) expressing slightly different levels of CD44, as well as the large CD44high population of activated/memory cells.
To test whether discrete CD45RA+ and CD45RO+ populations expressing CD25 at slightly different levels could be identified within CD4+ T cells from other sites, adult blood was compared with cord blood, infant thymus, adult lymph node, and spleen (Figure 3). All samples showed distinct populations of CD25+ T cells subfractionated by expression of CD45RA and its reciprocal marker CD45RO. The highest level of CD25 was consistently expressed by cells with the phenotype CD45RA-RO+. These cells comprised most CD25+ cells in thymus, where expression of CD45RO indicates a population that has passed positive selection.8 In cord blood, most CD25bright cells expressed CD45RA, although a small population expressed low levels of CD45RO. In contrast to reports that the TREG population in cord blood comprises essentially “pure” naive CD45RA+ TREGs,29 it contained a significant population of CD45RO+ cells expressing slightly lower levels of CD25, which appeared to correspond to the adult effector/memory subset (circled in Figure 3).
As expected, expression of the activation/memory marker CD44 was highest on the large population of CD25+ antigen-experienced cells in adult samples (Figure 3). In cord blood, a corresponding population was apparent, consistent with the presence of an antigen-experienced CD45RO+ population (see two paragraphs prior; circled in Figure 3). In all samples, CD25bright cells expressed a slightly lower level of CD44 than the CD25+ antigen-experienced cells, and the minor third population of CD25+ cells a lower level again (boxed in Figure 3). Expression of CD95 was highest on the CD25bright cells in all samples, suggesting that the CD45RA-CD25bright cells are also CD95hi.
Cross correlation of staining using different combinations of 5 mAbs (Figure 4) confirmed that the CD25+CD45RA+CD45RO- population corresponded to the CD95lo and CD44lo populations, whereas the CD25brightCD45RO+CD45RA- population was CD95hiCD44int. Thus, 2 distinct populations of CD25+ cells can be identified within human CD4+ T cells: CD45RA+CD45RO- CD44loCD95lo and CD45RA-CD45RO+CD44intCD95hi. However, the expression of further T-cell activation markers by the 2 populations of CD25+ cells differed according to site. Thus the phenotype of adult CD45RA+CD25+ T cells in peripheral blood was virtually identical to that of most naive adult CD45RA+CD25- T cells, suggesting that they had maintained a “naive” state after leaving the thymus (Figure 4). In lymph nodes, however, they expressed slightly higher levels of CD38, CD95, and CTLA-4, which may indicate some degree of TCR-dependent signaling without conversion to CD45RO. In contrast, the “memory” CD45RO+CD25+ T cells in peripheral blood expressed higher levels of CD95 and CTLA-4 than most CD45RO+ T cells, resembling their CD45RO+CD25+ counterparts in the lymph nodes. Cord-blood CD45RA+RO- cells appeared more activated than their adult counterparts, with increased expression of CD95, CTLA-4, and CD38, and down-regulation of CCR7 and CD62L, whereas cord-blood CD45RA-RObright cells were less activated than their adult counterparts. Indeed, cord-blood CD45RA+CD25+ and CD45RO+CD25bright T cells were phenotypically very similar, probably due to residual expression of activation markers in response to thymic selection signals.30
Expression of CD45RA, CD45RO, CD44, and CD95 markers by human T cells from infant thymus, cord or adult peripheral blood, lymph nodes, and spleen. Profiles are gated for live cells expressing CD4. Two populations of CD25bright and CD25int T cells are identified by the boxes, with the numbers giving the relative percentages in each population. Results are representative of 6 to 9 independent experiments.
Expression of CD45RA, CD45RO, CD44, and CD95 markers by human T cells from infant thymus, cord or adult peripheral blood, lymph nodes, and spleen. Profiles are gated for live cells expressing CD4. Two populations of CD25bright and CD25int T cells are identified by the boxes, with the numbers giving the relative percentages in each population. Results are representative of 6 to 9 independent experiments.
Expression of CD95, CD44, CTLA-4, CD27, CCR7, CD62L, and CD38 by human CD4+ CD45RO-/RA+ and CD45RO+/RA- T cells in cord blood, adult blood and lymph node. Profiles are gated for live cells expressing CD4 and either CD45RA or CD45RO. Results are representative of 2 to 8 independent experiments for each sample.
Expression of CD95, CD44, CTLA-4, CD27, CCR7, CD62L, and CD38 by human CD4+ CD45RO-/RA+ and CD45RO+/RA- T cells in cord blood, adult blood and lymph node. Profiles are gated for live cells expressing CD4 and either CD45RA or CD45RO. Results are representative of 2 to 8 independent experiments for each sample.
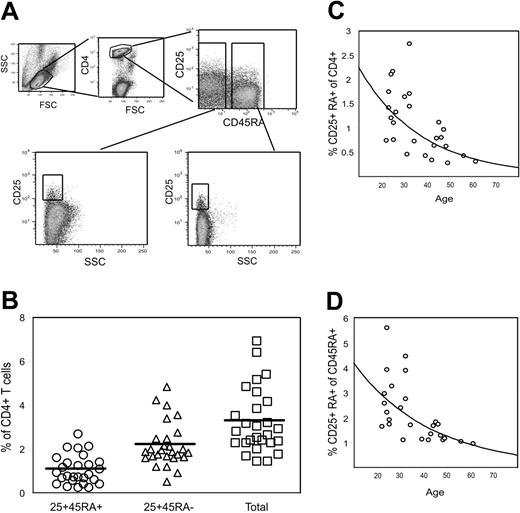
Percentages of naive and memory TREGs in adult peripheral blood
Using peripheral-blood leukocytes from 27 healthy donors, we measured the percentage of CD4+CD25+CD45RA+ and CD4+CD25brightCD45RA- cells using the gating illustrated in Figure 5A. The data indicate that CD4+CD25+CD45RA+ TREGs comprise approximately 30% of the total TREG population in healthy individuals (Figure 5B). Both populations declined as a function of age, although the drop in the number of CD25+ CD45RA+ cells was more marked (about 4-fold between 20 and 60 years [Figure 5C] versus 2-fold for CD25+CD45RA- cells [not shown]). Only one-quarter of the decrease in the number of naive TREGs could be attributed to a decrease in the percentage of CD45RA+ cells within the CD4+ compartment (Figure 5D), indicating complex control of naive TREG production throughout life.
Percentage of CD4+CD25+CD45RA+ and CD4+CD25+CD45RA- T cells in peripheral blood of healthy control subjects. PBMCs from 27 healthy donors were stained with CD4, CD25, and CD45RA mAbs. The percentages of CD4+CD25+CD45RA+ and CD4+CD25+CD45RA- cells in each individual were calculated using the gating strategy illustrated in panel A. (B) percentages of CD25+CD45RA+ and CD25+CD45RA- cells within CD4+ cells. The total for each individual was calculated by adding the 2 values. Horizontal bars represent the group means. (C-D) CD25+CD45RA+ cells are shown as a percentage of CD4+ cells and of CD4+CD45RA+ cells, respectively, as a function of age. Exponential decay curves have been fitted to the data.
Percentage of CD4+CD25+CD45RA+ and CD4+CD25+CD45RA- T cells in peripheral blood of healthy control subjects. PBMCs from 27 healthy donors were stained with CD4, CD25, and CD45RA mAbs. The percentages of CD4+CD25+CD45RA+ and CD4+CD25+CD45RA- cells in each individual were calculated using the gating strategy illustrated in panel A. (B) percentages of CD25+CD45RA+ and CD25+CD45RA- cells within CD4+ cells. The total for each individual was calculated by adding the 2 values. Horizontal bars represent the group means. (C-D) CD25+CD45RA+ cells are shown as a percentage of CD4+ cells and of CD4+CD45RA+ cells, respectively, as a function of age. Exponential decay curves have been fitted to the data.
Identification of regulatory T cells by expression of the transcription factor Foxp3
While a naive CD45RA+ population of CD25+ T cells has been described previously in thymus and cord blood, its status remains controversial, both in terms of phenotype and function.12,31,32 Two complementary strategies were used to provide additional evidence to support the proposition that the CD4+CD45RA+CD25+ population is comprised of regulatory T cells: measurement of Foxp3, a transcription factor required for the development and function of TREGs, and in vitro assays of regulatory function.
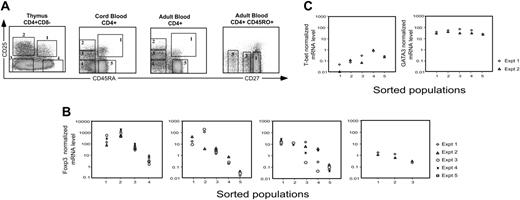
Expression of Foxp3 was measured by RT qPCR in sort-purified subsets of thymus, cord and adult blood, identified by staining with a combination of CD4, CD25, and CD45RA. The sorting strategy, illustrated in Figure 6A, was designed to isolate discrete populations of CD25+CD45RA+ and CD25brightCD45RA- cells (postulated to have regulatory function), CD25intCD45RA- and CD25negCD45RA- cells (believed to comprise effector and memory cells), and the CD25-CD45RA+ naive population (to provide baseline data). β-actin was measured using aliquots of the same RNA and served as an internal standard for the amount of starting mRNA in each sample. In cord and adult blood, the 2 CD25+ populations (populations 1 and 2) expressed at least 500-fold more Foxp3 mRNA than naive cells (Figure 6B). Cord and adult blood population 3, which is believed to comprise antigen-experienced conventional T cells, also expressed a significantly higher level of Foxp3 than naive cells. In the thymus, the highest Foxp3 expression was in the CD25+CD45RA- population (population 2), which is postulated to have passed positive selection but not yet acquired CD45RA. The fold difference between CD25+ and CD25- cells was smaller than in the periphery because thymic CD25- cells expressed intermediate levels of Foxp3 (Figure 6B).
To test whether expression of CD27 could be used to discriminate effector/memory from TREGs in the CD45RA- RO+CD25int fraction,33 adult blood CD4+CD45RO+ cells were sorted into 3 subsets on the basis of CD27 expression (Figure 6A). Cells within these 3 populations were believed to manifest different degrees of antigen-dependent differentiation.34 The level of Foxp3 decreased as the cells down-regulated expression of CD27 (Figure 6B). However, measurement of CD27 expression did not appear to confer an additional advantage in distinguishing CD25intCD45RO+ TREGs from activated/memory cells.
Quantitative analysis of Foxp3, T-bet, and GATA3 mRNA expression in sorted populations of CD4+ T cells. (A) Gating strategy for flow sorting mononuclear cells from the thymus and cord and adult blood. Profiles are gated for lymphocytes expressing CD4. Numbered boxes indicate the sorting gates. (B) RT qPCR for Foxp3 was performed using RNA prepared from sorted cell populations. Normalized Foxp3 mRNA levels were calculated relative to β-actin signals for each sorted population. (C) Expression of mRNA for T-bet and GATA3 in adult peripheral-blood CD4 T-cell populations sorted according to the gating strategy illustrated in panel A. Relative expression was determined by normalization to β-actin. Results are representative of 3 independent experiments.
Quantitative analysis of Foxp3, T-bet, and GATA3 mRNA expression in sorted populations of CD4+ T cells. (A) Gating strategy for flow sorting mononuclear cells from the thymus and cord and adult blood. Profiles are gated for lymphocytes expressing CD4. Numbered boxes indicate the sorting gates. (B) RT qPCR for Foxp3 was performed using RNA prepared from sorted cell populations. Normalized Foxp3 mRNA levels were calculated relative to β-actin signals for each sorted population. (C) Expression of mRNA for T-bet and GATA3 in adult peripheral-blood CD4 T-cell populations sorted according to the gating strategy illustrated in panel A. Relative expression was determined by normalization to β-actin. Results are representative of 3 independent experiments.
Expression of the transcription factors T-bet and GATA3
The intermediate levels of Foxp3 mRNA in populations 3 and 4 (Figure 6B) could be due to TREG contamination, or to expression of Foxp3 by effector/memory cells with no regulatory function, a concept that has received support in the literature.35-37 If the former were true, then we would expect that expression of molecules characteristic of effector/memory cells would increase as Foxp3 expression decreased in subsets 3 and 4 compared with subset 2. Because the transcription factors T-bet and GATA3 have been identified as critical regulators of commitment to the T-helper 1 (Th1) and Th2 effector lineages, respectively,38,39 we measured expression of both by RT qPCR, using the adult blood cDNA previously prepared for Foxp3 analysis (Figure 6B). Expression of T-bet increased progressively between populations 2, 3, and 4. Expression in populations 1 and 2 was even lower than in naive population 5 (Figure 6C), suggesting minimal contamination of the sorted TREGs with Th1 cells. GATA3 was highly expressed by all populations studied, with a less-than 5-fold difference between the highest and lowest populations (Figure 6C).
Both populations of TREGs suppress T-cell proliferation
The second strategy to confirm that sorted CD45RA+ and CD45RO+ TREGs have regulatory function was to perform functional suppression assays in vitro. In an initial set of experiments, we tested whether cord-blood CD4+CD25+ cells, in which the naive TREG subset is prominent, could suppress as potently as adult CD4+CD25+ cells, using division of CFSE-labeled CD4+CD25- cells as the readout (Figure 7A). Overall, adult and cord-blood CD25+ T cells exhibited similar degrees of suppressive activity, and cord and adult blood were capable of suppressing each other in a reciprocal fashion (not shown). Interestingly, substitution of responder for suppressor cells consistently reduced the proliferation of the labeled cohort, presumably by competing with the labeled responder cells for access to anti-CD3 and antigen-presenting cells.
Next, we sorted cord and adult blood and lymph node according to the strategy used previously for preparation of mRNA (Figure 6A), using population 5 (CD4+CD45RA+CD25-) as responder cells. Unfortunately, it was not possible to sort sufficient cells from lymph node population 1 to perform an in vitro test. Suppression was assayed in parallel using CFSE and thymidine readouts (Figure 7B). In CFSE assays, populations 1 and 2 showed equally potent suppressive activity. Populations 3 and 5 also reduced the proliferation at high ratios, but their activity was not maintained at lower cell ratios. In thymidine assays, populations 1 and 2 suppressed with equal potency. In contrast to the CFSE assay, populations 3 and 5 increased the thymidine incorporation, presumably because both dividing “suppressor” and “responder” cells were able to incorporate thymidine.
Discussion
CD4+CD25+ “natural” TREGs are generally believed to express a naive phenotype in human thymus and cord blood, but then convert to an activated/memory phenotype in adults. Here we report that distinct populations of naive and activated/memory CD4+CD25+ TREGs are present throughout neonatal and adult life. Both populations express very high levels of Foxp3 mRNA and exhibit comparable suppressor activity in vitro. CD45RO+ TREGs also express a slightly higher level of CD25 than their CD45RA+ counterparts, which may reflect a response to recent TCR signaling. The level of CD25 expressed by adult CD45RA+ TREGs excludes them from the conventional CD25bright gate that is often used to identify human TREGs.3-7 Thus, the total number of TREGs present in adults is at least 50% higher than has previously been reported.
Suppression of in vitro proliferation by TREGs from cord and adult blood and lymph node. (A) Suppression of proliferation of bead selected CFSE-labeled CD4+CD25- cells by unlabeled CD4+CD25+ TREGs using adult blood or cord blood as the source of both CD4+CD25- and CD4+CD25+ cells. The bars represent the means ± SEM of 4 to 7 independent experiments. (B) Suppression by flow-sorted populations (1, 2, and 3 as defined in Figure 6A) from cord and adult blood and lymph node. Responder cells were sorted autologous CD4+CD45RA+CD25- (population 5), which were also titered in parallel with the other sorted populations. Ratios of suppressor to responder cells are shown on the x-axis. Left panels show thymidine assay; right panels, CFSE assay. Bars represent the mean ± SEM of 2 to 4 replicate cultures from a single experiment. Where no error bars are present, assays were performed in a single culture well. The data for cord and adult blood are representative of 2 to 3 experiments, whereas the lymph node experiment was performed only once.
Suppression of in vitro proliferation by TREGs from cord and adult blood and lymph node. (A) Suppression of proliferation of bead selected CFSE-labeled CD4+CD25- cells by unlabeled CD4+CD25+ TREGs using adult blood or cord blood as the source of both CD4+CD25- and CD4+CD25+ cells. The bars represent the means ± SEM of 4 to 7 independent experiments. (B) Suppression by flow-sorted populations (1, 2, and 3 as defined in Figure 6A) from cord and adult blood and lymph node. Responder cells were sorted autologous CD4+CD45RA+CD25- (population 5), which were also titered in parallel with the other sorted populations. Ratios of suppressor to responder cells are shown on the x-axis. Left panels show thymidine assay; right panels, CFSE assay. Bars represent the mean ± SEM of 2 to 4 replicate cultures from a single experiment. Where no error bars are present, assays were performed in a single culture well. The data for cord and adult blood are representative of 2 to 3 experiments, whereas the lymph node experiment was performed only once.
The presence in human blood of a large population of antigen-experienced conventional CD4+ T cells expressing CD25 interferes with flow gating at the natural division between naive and TREGs that is apparent both in cord blood and in murine lymphocytes (Figure 1). The difficulty in applying consistent gates has contributed to the lack of consensus regarding human TREG phenotype and function.40 To reduce contamination by conventional CD4+ T cells, the gate for human TREGs is usually restricted to a small CD25bright subset that comprises approximately 1% to 2% of normal adult peripheral blood.3 However, the percentage of CD25bright cells in adult peripheral blood is far lower than the number of CD25+ TREGs in either cord blood or murine blood, which led us to postulate that some human TREGs expressing slightly lower amounts of CD25 were excluded from the CD25bright gate. In an effort to reveal the “missing” population of adult TREGs, we screened 36 markers, in combination with CD4 and CD25, with the aim of finding any that could be used to distinguish CD4+CD25+ TREGs from the more numerous activated/memory T cells expressing CD25. Subfractionation on the basis of CD45 splice variants revealed a population of CD45RA+ cells expressing a slightly lower level of CD25 than the CD45RA- cells (Figure 2). Two distinct populations of CD25+ cells expressing CD45RA and CD45RO, respectively, were also present in cord blood, thymus, lymph nodes, and spleen (Figure 3).
When subdivided on the basis of expression of CD45RA versus CD45RO, it was apparent that the 2 CD4+CD25+ populations were distinct not only in expression of CD45 isoforms, but also in a number of other surface markers that are commonly used to indicate activation status (Figure 4). Thus, the CD45RA+/RO- population in adult blood exhibited a fully naive phenotype, whereas the CD25bright population was CD45RA-/RO+, CD44hi, CD95+, and CTLA-4+; retained high expression of CD27; and showed the heterogeneous expression of CD62L, CCR7, and HLA-DR characteristic of antigen-experienced cells. Neither population in adult blood expressed GITR or OX40 at levels that could assist with isolation of TREGs.
In lymph nodes, the CD45RA+/RO- population expressed higher levels of CD95 and CTLA-4 than its peripheral blood counterpart. Lymph node CD45RA-/RO+ TREGs expressed the characteristic TREG markers, CTLA-4, GITR, and OX40, as well as CD95, CD38, and CD27. In cord blood, both subsets of CD25+ cells expressed intermediate levels of CD95, CTLA-4, GITR, OX40, and CD38, and slightly higher CD4 levels than adult TREGs. This expression pattern is likely to result from recent activation during selection in the thymus.30
In the thymus itself, the majority of CD4+CD25+ T cells were present in the CD45RO+CD69+CD95+ population (data not shown) that is believed to comprise positively selected cells.9 A minority of thymic CD4+CD25+ T cells had converted to expression of CD45RA, whereas most cord-blood CD4+CD25+ T cells expressed CD45RA. The small CD45RO+ population in cord blood could represent either recent thymic emigrants that had not yet switched from CD45RO to CD45RA, or cells that had already reacquired CD45RO after antigen contact in the periphery. In addition, comparison of cord and adult blood CD45RO+ cells (Figure 4) indicated that the cord-blood CD25+ population contained significant contamination with effector/memory cells expressing CD25, CD95, CD44, CTLA-4, CCR7 and CD62L at the same level as adult CD45RO+ cells. Thus, cord-blood CD4+CD25+ T cells probably do not represent a pure population of naive TREGs as recently claimed.29
The phenotypes expressed by TREGs in various human organs are of significance because they offer insights into TREG physiology based on the level of expression of TCR-dependent activation markers. After selection in the thymus, CD45RO+ TREGs mature and migrate to the periphery, acquiring expression of CD45RA during the process. Those cells that make contact with appropriate major histocompatibility complex (MHC)-peptide ligands in the periphery continue to express CTLA-4, convert to CD45RO+, and manifest potent regulatory function. Those cells that fail to contact appropriate antigen revert to a quiescent phenotype, which differs from that of naive T cells only in expression of CD25 (required for survival in response to IL-2) and down-regulation of CD4. In the lymph nodes, where all cells receive low level signals via noncognate antigen-MHC interactions,41 even CD45RA+CD25+ cells express markers associated with regulatory function, such as CTLA-4, and CD45RO+CD25+ cells express these markers at a higher level than in the blood. Thus, the physiology of TREGs essentially parallels that of conventional T cells.
Data from 2 complementary strategies support our contention that CD45RA+CD4+CD25+ cells in the thymus and cord and adult blood are bona fide members of the regulatory T-cell compartment. Measurement of Foxp3 mRNA in flow-sorted subsets of cord and adult blood CD25+CD45RA-/RO+ and CD25+CD45RA+/RO- cells indicated that expression in both populations was at least 500-fold higher than in naive cells (Figure 6). In the thymus, the level of Foxp3 expression was generally higher than in cord blood, and CD25+ populations 1 (CD45RA+) and 2 (CD45RA-) expressed at least 100 times more Foxp3 mRNA than CD25- cells.
Interestingly, cord and adult blood CD45RO+ cells expressing CD25 at intermediate to low levels (populations 3 and 4) expressed 10- to 50-fold more Foxp3 mRNA than naive CD25-CD45RA+ cells sorted from the same sample, although the level in CD45RO+ cells was somewhat variable between experiments (Figure 6B). The Foxp3 signal, particularly in population 3, could have resulted from contamination with TREGs, but may also reflect true expression by antigen-experienced conventional T cells, consistent with published reports in which human CD4+CD25- T cells activated in vitro were shown to express Foxp3.35-37 Importantly, we measured Foxp3 directly ex vivo, to remove any possibility of induction during culture under nonphysiologic conditions. We also observed a decrease in Foxp3 mRNA expression as CD45RO+ cells lost expression of CD27 during terminal differentiation (Figure 6). However significant levels of Foxp3 mRNA were still present in the differentiated CD27lo population, once again consistent with a model in which expression of Foxp3 mRNA is not exclusive to natural TREGs in humans. Whether such expression indicates the presence of Foxp3 protein remains to be established. This is an important issue because expression of moderate levels of Foxp3 mRNA in the absence of detectable protein has recently been reported for cord blood lines derived from CD4+CD25- cells.29 Taken together, our data suggest some caution in interpretation of studies in which coexpression of Foxp3 and T-bet mRNA is used to argue for a common origin for human TREGs and Th1 cells,42 as expression of Foxp3 in Th1-derived populations could simply reflect a level of antigen experience in conventional T cells (Figures 6B-C).
The second strategy to test whether both human CD4+CD25+ subpopulations were indeed TREGs was to assess their capacity to suppress proliferation of CD4+CD25- T cells in vitro. Our data indicated that CD4+CD25+ T cells derived from either cord or adult blood suppressed proliferation of an equal number of CD4+CD25- T cells by more than 50% (Figure 7A). Sorted CD45RA+CD25+ and CD45RA-CD25bright populations displayed equivalent potency in suppression assays, while the behavior of CD45RA-CD25int cells mimicked that of CD45RA+CD25- responder cells (Figure 7B).
Our analysis of TREG numbers in a cohort of healthy adults (Figure 5) has indicated a high degree of variability in both naive and memory populations. Some of the variation is age dependent, particularly in the case of naive TREGs, but is not simply due to the decrease in thymic output of naive CD45RA+ cells, as suggested recently.43 Reduced representation of TREGs within the naive CD4 compartment could result either from a selective reduction in thymic production, or from a shorter peripheral lifespan of TREGs compared with conventional T cells. It will be important to test whether naive TREG numbers are also decreased in patients with immunoinflammatory and autoimmune diseases who manifest global deficits in thymic output.44-47
Prepublished online as Blood First Edition Paper, December 6, 2005; DOI 10.1182/blood-2005-06-2403.
Supported by Program and Project Grant funding from the Australian National Health and Medical Research Council (NHMRC), and by a Senior Research Award from the Crohn's and Colitis Foundation of America. S.G.T. is an NHMRC RD Wright Research Fellow. B.F.d.S.G. is an NHMRC Principal Research Fellow.
N.S. designed research, performed research, and wrote the paper; B.S.-N. performed research; S.G.T. contributed clinical samples and reagents, and assisted in writing the paper; S.I.A. contributed clinical samples; M.S. contributed clinical samples; S.L. contributed expertise in pathology; R.N. designed research; and B.F.d.S.G. designed research, performed research, wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Prof Antony Basten for his helpful comments on the manuscript, Caroline Higgins for her provision of murine data, and the staff of the Centenary Institute Flow Cytometry Facility for their assistance with cell sorting.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal