Comment on Mueller et al, page 3330
All-trans retinoic acid (ATRA) has helped commute the death sentence formerly imposed by acute promyelocytic leukemia (APL) to a 70% chance of long-term life. To free the remaining 30% of patients, we have to know how ATRA works.
In this issue, Mueller and colleagues present evidence that inhibition of PU.1 expression is a critical event leading to acute promyelocytic leukemia (APL), and accordingly that restoration of PU.1 expression is the dominant mechanism of therapy for this disease by all-trans retinoic acid (ATRA).
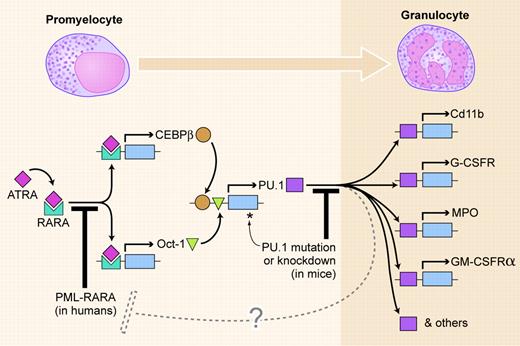
The figure shows a simplified version of a myeloid differentiation pathway revealed by Mueller et al. In normal promyelocytes, the ligand-bound retinoic acid receptor α (RARA) induces transcription of CEBPB and Oct-1, the products of which in turn up-regulate the PU.1 gene. PU.1 then induces transcription of a bank of genes, leading to differentiation, in this case to a granulocyte.
The PML-RARA fusion gene occurs in 98% of human APL. Its product acts as a dominant-negative inhibitor of RARA (the first leukemogenic block in the figure). It is proposed that the crucial effect of PML-RARA is indirect inhibition of transcription of PU.1. The inhibition is not complete, so low levels of PU.1 mRNA and protein are produced, insufficient to induce differentiation.
Considerable evidence is presented for the pivotal role ascribed to PU.1. PML-RARA down-regulates PU.1 expression, and, even in vivo, ATRA up-regulates it. ATRA up-regulation of CEBPβ and Oct-1 precedes and enhances up-regulation of PU.1, which is in turn essential for the differentiation effect. And overexpression of PU.1 is as effective as ATRA treatment in inducing differentiation and cell cycle arrest.
One stone is left unturned; namely, apoptosis: ATRA treatment induces degradation of the PML-RARA protein, reportedly via the action of caspase-3.1 Although Mueller and colleagues confirm this observation in ATRA-treated cells, they do not report whether PU.1 overexpression also causes PML-RARA degradation (this is indicated in the figure by the dashed block.) But related to this is the bottom line of cell numbers: Figure S3 of Mueller et al's article shows that PU.1 treatment actually reduces the leukemia cell load as efficiently as ATRA.FIG1
Leukemogenic blocks to PU.1-mediated myeloid differentiation. Two blocks to the pathway are shown: by the PML-RARA fusion gene product in human APL and by reduced function of PU.1 itself in knockdown or radiation-induced mouse acute myeloid leukemia (AML). The dashed line indicates degradation of PML-RARA, possibly induced by PU.1. Illustration by Kenneth Xavier Probst.
Leukemogenic blocks to PU.1-mediated myeloid differentiation. Two blocks to the pathway are shown: by the PML-RARA fusion gene product in human APL and by reduced function of PU.1 itself in knockdown or radiation-induced mouse acute myeloid leukemia (AML). The dashed line indicates degradation of PML-RARA, possibly induced by PU.1. Illustration by Kenneth Xavier Probst.
There are 2 reasons for turning to mouse models at this point. The first is that Mueller and colleagues have resolved a conundrum over a leukemia-suppressor function for PU.1. Mouse models had demonstrated recently that reduction of PU.1 activity, either by genetic manipulation2 or by radiation-induced deletion and mutation,3,4 is highly leukemogenic (the second block to the differentiation path in the figure). Yet as discussed by Mueller et al, human AML samples showed an extremely low frequency (1.5% overall) of mutation of the gene (Mueller et al5 and the references in the paper by Mueller et al in this issue). It is evident now that the mouse disease most closely models APL.
Second, several of the questions mentioned here have been addressed. In mouse radiation-induced leukemia, overexpression of Pu.1 induces apoptosis and reduces clonogenicity.3
So why is the leukemogenic mechanism in humans so indirect? What is protecting the human PU.1 gene from the mutation, deletion, and translocation events suffered by other tumor-suppressor genes? More importantly, will these observations have therapeutic implications? Directly stimulating PU.1 expression could circumvent the problems associated with ATRA therapy, namely toxicity of the drug and acquired resistance of the leukemic cells to differentiation. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal