Abstract
Small GTPases play critical roles in hemostasis, though the roster of such molecules in platelets is not complete. In this study, we report the presence of Ras-related GTPases of the ADP-ribosylation factor (Arf) family. Platelets contain Arf1 or 3 and Arf6, with the latter being predominantly membrane associated. Using effector domain pull-down assays, we show, counter to other GTPases, that Arf6-GTP is present in resting platelets and decreases rapidly upon activation with collagen or convulxin. This decrease does not completely rely on secondary agonists (ADP and thromboxane A2) or require integrin signaling. The decrease in free Arf6-GTP temporally precedes activation of Rho family GTPases (RhoA, Cdc42, and Rac1). Using a membrane-permeant, myristoylated peptide, which mimics the N-terminus of Arf6, we show that the Arf6-GTP decrease is essential for collagen- and convulxin-induced aggregation, platelet adherence, and spreading on collagen-coated glass. Treatment with this peptide also affects the activation of Rho family GTPases, but has little effect on RalA and Rap1 or on agonist-induced calcium mobilization. These data show that Arf6 is a key element in activation through GPVI, and is required for activation of the Rho family GTPases and the subsequent cytoskeletal rearrangements needed for full platelet function.
Introduction
Platelets circulate in the bloodstream, where they sense vascular damage and initiate hemostasis. At the lesion, platelets are activated by various agonists, such as exposed collagen,1 locally generated thrombin,2 or factors released by the platelets themselves (eg, ADP and thromboxane A2 [TxA2]).3 Activation is initiated through several classes of membrane receptors that bind to these agonists at a vascular lesion.1-3 These receptors initiate intracellular signaling cascades to induce platelet responses (eg, adhesion, spreading, secretion, and cytoskeletal rearrangement). The roles of small GTPases of the Ras superfamily have gained much attention, since they appear to link signaling events from various platelet receptors to defined outcomes, such as shape change,4-6 aggregation,7,8 and secretion.9-11 Members of the Rab family play roles in granule secretion, with Rab4 and Rab6 being involved in alpha granule release9,10 and Rab27 in dense core granule release.11 Rap1 appears to play a role in integrin αIIbβ3 activation.7,8 RalA is shown to be activated in response to various stimuli,12-14 but its role is still unclear. Rho family GTPases (Rho, Rac, and Cdc42) play roles in platelet phosphoinositide signaling and in the regulation of cytoskeleton rearrangements.4-6
The ADP-ribosylation factor (Arf) family consists of Ras-related, small GTPases that control both vesicular transport and cytoskeletal dynamics.15,16 Arfs contain an N-terminal myristoyl group, which enables them to interact with membranes. This modification and the adjacent N-terminal amino acids are critical for function,17 and myristoylated peptides, mimicking the N-terminus, are effective, isoform-specific inhibitors of Arf activity.17,18 The family is divided into 3 classes. Class I Arfs (1-3) play roles in vesicular trafficking by recruiting coat proteins onto membranes at the site of transport-vesicle budding. Class II Arfs (4-5) are less well understood. Arf6 is the only class III Arf and is involved in secretion and in actin dynamics.16 Uniquely, Arf6 is predominantly membrane bound due to its basic nature (pI > 9) and does not cycle between cytosol and membrane compartments, as do other Arfs.
As for other small GTPases, Arf6-GTP is considered the active state and can interact with downstream effectors, such as phospholipase D (PLD),19 phosphatidylinositol-4-phosphate 5-kinase type I (PI5KI),20 and arfaptin 2,21,22 resulting in the recruitment of these effectors to the plasma membrane. The GTP/GDP cycle of Arf6 is mediated by interactions with its regulators, guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). The importance of these GEF and GAP proteins was discussed in recent reviews where it was noted that unlike other small GTPases, Arf functions are not mediated just by the GTP-bound state but through its cycling between states.15,16,23,24
Collagen binds to and signals through 2 different platelet glycoproteins, GPVI and integrin α2β1, though GPVI appears to be the major collagen receptor.1 Signaling through GPVI has been studied in great mechanistic detail.25 Upon binding to collagen (or convulxin), Fc receptor (FcR) γ-chains form a complex with GPVI and are phosphorylated. Syk, a tyrosine kinase, is then recruited to the plasma membrane via an interaction with FcR γ-chains and becomes activated. Syk in turn phosphorylates other kinases and phospholipases promoting an increase in intracellular Ca2+ and in phosphoinositide production. Binding to GPVI also leads to the activation of several small GTPases, Rap1,26 Rap2,27 Rac1, and Cdc42,28 reaching a maximum within 30 seconds. Rap1, Rap2, and Rac1 activation through GPVI requires phosphatidylinositol 3-kinase (PI3K) activity and an increase in intracellular Ca2+.26-28
Little is known about Arfs in platelets. Proteomic studies show that either Arf1 or 3 is present,29 and an Arf GAP called ASAP1 was purified from platelet extracts.30 Here, we provide the first report that Arf6 is predominantly present on platelet membranes. Of interest, resting platelets have significant levels of free Arf6-GTP, which rapidly decreases upon platelet activation by collagen or convulxin. The decrease in Arf6-GTP does not require secondary agonists such as ADP or TxA2. To investigate the role of Arf6 in platelets, we used a synthetic, N-terminal-myristoylated Arf6 (myr-Arf6) peptide, which specifically blocks PLD activation in PC12 cells.19 When myr-Arf6 was incubated with platelets, it blocked collagen- and convulxin-induced aggregation, F-actin dynamics, and the activation of Rho family GTPases (Rho, Rac, and Cdc42) but not the activation of other Ras family members (Ral and Rap). The myr-Arf6 peptide also blocked the agonist-stimulated decrease in Arf6-GTP. Taken together, these data suggest that stimulation through GPVI leads to changes in Arf6-GTP, which is upstream of the Rho family of GTPases.
Materials and methods
Antibodies and reagents
An anti-Arf6 peptide antibody was produced by Bethyl Laboratory (Montgomery, TX), as described in Song et al.31 The anti-RabGDI and anti-SNAP23 were previously reported.32,33 The following antibodies were from commercial sources: anti-Arf1/3, anti–α-tubulin (clone B-5-1-2), and alkaline phosphatase–conjugated secondary antibodies (Sigma, St Louis, MO); anti-panArf (clone 1D9; Affinity Bioreagents, Golden, CO); anti-Rac1, anti-Cdc42, anti-RalA, and anti-Rap1 (BD Bioscience, San Jose, CA); and anti-Rho (Cytoskeleton, Denver, CO); FITC-conjugated anti-rabbit antibody was from Vector Laboratories (Burlingame, CA). Fura-2/AM was from Molecular Probes (Eugene, OR). TRITC-conjugated phalloidin was from Sigma, and Brefeldin A (BFA), Latrunculin A, Jasplakinolide, and A23187 were from Calbiochem (San Diego, CA). U46619 was from Cayman Chemical (Ann Arbor, MI). Apyrase, acetylsalicyclic acid (aspirin), and prostaglandin I2 (PGI2) were from Sigma. Myristoylated N-terminal Arf6 or Arf1 peptide and nonmyristoylated Arf6 peptide (see Figure 4 for sequences) were custom synthesized by Anaspec (San Jose, CA) and shown to be 95% pure by high-performance liquid chromatography (HPLC) and mass spectroscopy analysis. RGDS and TRAP peptides were obtained from Anaspec. Type I collagen, ADP, and thrombin were from Chrono-Log (Havertown, PA). Convulxin was from Centerchem (Norwalk, CT). Complete, EDTA-free protease inhibitor cocktail was from Roche (Indianapolis, IN).
Washed platelet preparation
Freshly banked platelets were obtained as units from the Central Kentucky Blood Center (Lexington, KY). Platelet-rich plasma (PRP) was isolated in the presence of 0.37 units/mL apyrase and 10 ng/mL PGI2 by centrifugation at 150g for 10 minutes at 20°C. PRP was centrifuged at 900g for 10 minutes and pelleted platelets were resuspended in HEPES-Tyrode buffer (20 mM HEPES/KOH [pH 6.5], 128 mM NaCl, 2.8 mM KCl, 1 mM MgCl2, 5 mM d-glucose, 12 mM NaHCO3, 0.4 mM NaH2PO4) with apyrase and PGI2. Washed platelets were prepared by centrifugation at 900g for 7 minutes followed by resuspension in HEPES-Tyrode buffer (pH 7.4). Platelets were adjusted to 2 × 108 platelets/mL, unless otherwise indicated. To block the secondary stimulation by ADP and TxA2, 0.37 units/mL apyrase and/or 2 mM aspirin were added.
Platelet aggregation
Platelet suspension (500 μL) was warmed in a cuvette (37°C) for 5 minutes with stirring (800 rpm) using a Model 460Vs Lumi-Dual aggregometer (Chrono-Log), followed by incubation with DMSO (vehicle) or Arf peptides for 2 minutes. Agonists (10 μg/mL collagen or 0.1 μg/mL convulxin) were added and aggregation curves were acquired using a Model 810 Aggro/Link computer interface and Aggro/Link software (Chrono-Log).
Immunofluorescence microscopy
Sterile glass coverslips were coated with 0.2 mg/mL type I collagen for 2 hours at room temperature. Washed platelets were incubated with DMSO or Arf peptides for 5 minutes at 37°C and placed onto collagen-coated coverslips for 5 minutes. Platelets were fixed with 3.7% (vol/vol) formaldehyde for 15 minutes and quenched with 50 mM NH4Cl. Cells were rinsed with 10% fetal bovine serum (FBS)/PBS and incubated with anti-Arf6 IgG (1:100 dilution) in 10% FBS/PBS containing 0.2% saponin. After washes with 10% FBS/PBS, cells were incubated with FITC-conjugated anti–rabbit IgG (1:200 dilution) and TRITC-conjugated phalloidin (1:500 dilution), in 10% FBS/PBS with 0.2% saponin. Coverslips were mounted and examined with an E-600 epifluorescence microscope (Nikon, Melville, NY). Objective lenses used were a 40 ×/0.75 numeric aperture (NA) DIC M and 100 ×/1.40 NA oil DIC H (Nikon). Type A immersion oil (Fryer, Huntley, IL) was used for the 100 × objective.
Small GTPase pull-down assay
Washed platelets (4 × 108/mL) were incubated with stirring for 2 minutes (37°C) prior to 2-minute incubation with DMSO (vehicle) or Arf peptides. The platelets were then stimulated with indicated agonists. Reactions were stopped by adding 2 × ice-cold HEPES-lysis buffer (20 mM HEPES [pH 7.4], 128 mM NaCl, 3 mM MgCl2, 2% Triton X-100, 0.2% SDS, 1% deoxycholic acid, 20% glycerol, 2 × EDTA-free protease inhibitor cocktail), and the sample was flash frozen until all samples were collected. The lysates were cleared by centrifugation at 15 000g for 4 minutes, and the supernatants were incubated with 10 μL glutathione-agarose beads bound to 20 μg of a GST-fusion protein containing a specific GTPase interaction domain for 20 minutes at 4°C. The GST-fusion proteins for each of the small GTPases examined were as follows: GST-humanGGA3VHS-GAT(1-313) for Arf,34,35 GST-mousePAK3RBD(65-137) for Rac and Cdc42,36 GST-mouseRhotekinRBD(7-89) for Rho,37 GST-humanRalGDSRBD(788-885) for Rap,38 GST-humanRLIP76RBD(397-518) for Ral.12 The bead-bound complexes were washed with 1 × HEPES-wash buffer (20 mM HEPES [pH 7.4], 128 mM NaCl, 2 mM MgCl2, 1% Triton X-100, 10% glycerol), and proteins were eluted with 2 × SDS sample buffer. Eluents were separated by SDS-PAGE and the small GTPases were detected by Western blot. Vistra ECF (Amersham Biosciences, Piscataway, NJ) was used for visualization and images were obtained using Typhoon 9400 (Amersham Biosciences). Quantification was performed with ImageQuant 5.2 software (Amersham Biosciences).
Platelet membrane isolation
Washed platelets (4 × 108/mL) were suspended in sonication buffer (50 mM Tris [pH 7.4], 250 mM sucrose, protease inhibitor cocktail) and disrupted by sonication using a Fisher sonic dismembrator (Fisher Scientific, Hampton, NJ). Membrane and cytosolic fractions were separated by ultracentrifugation at 186 000g for 1.5 hours.
Intracellular Ca2+ measurement in platelets
Intraplatelet Ca2+ concentration was measured using Fura-2/AM, as outlined in Ohlmann et al.39 Fluorescence resulting from the alternate excitations at 340 nm and 380 nm was measured with a 509-nm filter using a model LS55 Luminescence Spectrometer (Perkin Elmer, Boston, MA), and the ratio of 340:380 nm was calculated simultaneously using FL WinLab4.0 software (Perkin Elmer).
Results
Arfs are present in platelets
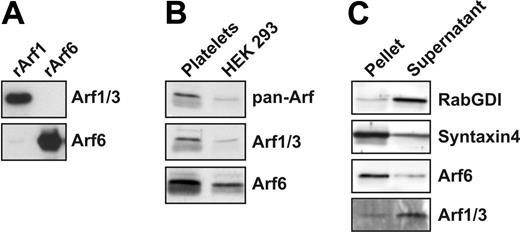
Given the limited data regarding the presence and/or role of Arf proteins in platelets, initial experiments sought to determine which Arf proteins were present. For this, commercial antibodies recognizing both Arf1 and 3, as well as an antipeptide antibody specific for Arf6, were used. Figure 1A demonstrates the specificity of these antibodies against recombinant Arfs. Extracts from HEK-293 cells serve as a control since they contain at least Arf1 and 6.40,41 Figure 1B shows that Arf1 or 3 and Arf6 are present in platelet extracts. Arf family members often show different cellular localizations; Arf1 cycles between the cytosol and the membrane fraction, while Arf6 is predominantly membrane bound.40 The localization of the platelet Arfs is shown in Figure 1C. The membrane protein syntaxin 4 and the cytosolic protein Rab GDI were used as markers to evaluate the quality of the fractionation. Arf6 is largely present in the membrane fraction, while most of Arf1/3 is present in the cytosol. Further analysis of purified lipid rafts42 and Triton X-100–insoluble, cytoskeleton fractions shows that Arf6 is not significantly associated with either structure (Figure S1A, available on the Blood website; see the Supplemental Figures link at the top of the online article). Immunofluorescence microscopy shows a punctate distribution of Arf6, which does not align with alpha granules or dense core granules (Figure S1B and data not shown). Taken together, these data demonstrate that Arf1/3 and Arf6 are present in platelets.
Arf guanine nucleotide states change upon platelet activation
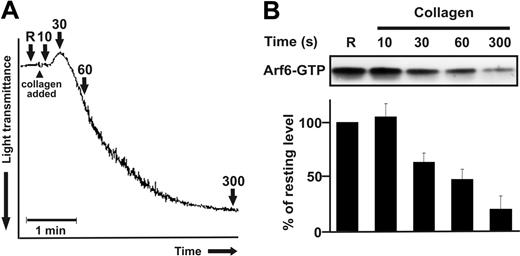
Like other small GTPases, the guanine nucleotide–bound state is important for Arf function.43,44 Arf-GTP is thought to be the “active” form because it interacts with downstream effectors to promote various cellular outcomes. To examine whether Arf-GTP changes upon platelet activation, a common pull-down approach was used.34,35 The adaptor protein GGA3, which contains an effector domain (GAT) that specifically binds to Arf-GTP (either Arf1/3 or Arf6), was used to monitor the GTP/GDP-bound state at different stages of collagen-stimulated aggregation. Platelet suspensions were stimulated with collagen, and the activation process was monitored using an aggregometer (Figure 2A). At specific stages, platelets were lysed and prepared for the GST-GGA3 pull-down and Western blot (Figure 2B). Of interest, resting platelets and platelets at the initial time point (15 seconds), before shape change, have the highest levels of detectable Arf6-GTP (approximately 8% of the total Arf6). As aggregation continues, there is a rapid decrease in Arf6-GTP, reaching a minimum when the full aggregate is formed (n = 5). At this stage, there is an 80% decrease in Arf6-GTP relative to resting cells. This decrease is not due to a loss or degradation of Arf6 protein since there is no change in the total levels detected in the extracts (Figure S2A). No Arf6 is recovered when either GST alone or GST-GGA3VHS, which lacks the Arf6-GTP-binding domain,34 is used (Figure S2B). The snake venom toxin, convulxin (a GPVI agonist), also induces a decrease in Arf6-GTP that is time and dose dependent (Figure S2C and Figure 3).
Arfs are present in platelets. (A) Recombinant HA-Arf1 and HA-Arf6 proteins were immunoblotted with anti-Arf1/3 and anti-Arf6 antibodies. (B) Platelet extract (50 μg) and HEK 293 cell extract (10 μg) were immunoblotted with anti-panArf, anti-Arf1/3, and anti-Arf6 antibodies. (C) Washed platelets (4 × 108/mL) were sonicated and fractionated by centrifugation. The pellet and the supernatant fractions were immunoblotted with anti-RabGDI, anti–syntaxin 4, anti-Arf6, and anti-Arf1/3 antibodies.
Arfs are present in platelets. (A) Recombinant HA-Arf1 and HA-Arf6 proteins were immunoblotted with anti-Arf1/3 and anti-Arf6 antibodies. (B) Platelet extract (50 μg) and HEK 293 cell extract (10 μg) were immunoblotted with anti-panArf, anti-Arf1/3, and anti-Arf6 antibodies. (C) Washed platelets (4 × 108/mL) were sonicated and fractionated by centrifugation. The pellet and the supernatant fractions were immunoblotted with anti-RabGDI, anti–syntaxin 4, anti-Arf6, and anti-Arf1/3 antibodies.
Arf6-GTP decreases upon collagen stimulation. Washed platelets (4 × 108/mL) were stimulated with 10 μg/mL collagen for the indicated times and lysed by adding 2 × HEPES-lysis buffer. After clarification, the supernatants were analyzed for Arf6-GTP. Arf6 was detected by Western blot using anti-Arf6 antibody and quantified using ImageQuant 5.2 (Molecular Dynamics, Sunnyvale, CA) software. (A) A standard collagen-induced aggregation curve is depicted and the points at which samples were taken are indicated with arrows. (B) The Western blot is representative of 5 independent experiments, and intensities of Arf6 were normalized to that of resting platelets. Error bars indicate SD.
Arf6-GTP decreases upon collagen stimulation. Washed platelets (4 × 108/mL) were stimulated with 10 μg/mL collagen for the indicated times and lysed by adding 2 × HEPES-lysis buffer. After clarification, the supernatants were analyzed for Arf6-GTP. Arf6 was detected by Western blot using anti-Arf6 antibody and quantified using ImageQuant 5.2 (Molecular Dynamics, Sunnyvale, CA) software. (A) A standard collagen-induced aggregation curve is depicted and the points at which samples were taken are indicated with arrows. (B) The Western blot is representative of 5 independent experiments, and intensities of Arf6 were normalized to that of resting platelets. Error bars indicate SD.
Arf6-GTP decreases upon GPVI activation. (A) Washed platelets were incubated in the presence or absence of 2 mM aspirin with stirring for 30 minutes at 37°C, or in the presence or absence of 0.37 units/mL apyrase for 1 minute prior to the stimulation with 0.1 μg/mL convulxin. Platelets were lysed at the designated time points, and the clarified supernatants were used to pull down Arf6-GTP. Arf6 was detected by Western blot. (B-C) Washed platelets were incubated in the presence or absence of 500 μM RGDS peptide for 1 minute at 37°C, and stimulated with 0.1 μg/mL convulxin. Platelets were lysed at the designated time points and the clarified supernatants were used to pull down Arf6-GTP, followed by Western blot using an anti-Arf6 antibody. (B) Aggregation traces are shown with arrows where time points were taken. (D) Washed platelets were stimulated with the following agonists for 3 minutes and extracts were used to analyze Arf6-GTP levels: thrombin (0.3 U/mL), TRAP (20 μM), U46619 (1 μM), A23187 (1 μM), convulxin (0.1 μg/mL), ADP (10 μM).
Arf6-GTP decreases upon GPVI activation. (A) Washed platelets were incubated in the presence or absence of 2 mM aspirin with stirring for 30 minutes at 37°C, or in the presence or absence of 0.37 units/mL apyrase for 1 minute prior to the stimulation with 0.1 μg/mL convulxin. Platelets were lysed at the designated time points, and the clarified supernatants were used to pull down Arf6-GTP. Arf6 was detected by Western blot. (B-C) Washed platelets were incubated in the presence or absence of 500 μM RGDS peptide for 1 minute at 37°C, and stimulated with 0.1 μg/mL convulxin. Platelets were lysed at the designated time points and the clarified supernatants were used to pull down Arf6-GTP, followed by Western blot using an anti-Arf6 antibody. (B) Aggregation traces are shown with arrows where time points were taken. (D) Washed platelets were stimulated with the following agonists for 3 minutes and extracts were used to analyze Arf6-GTP levels: thrombin (0.3 U/mL), TRAP (20 μM), U46619 (1 μM), A23187 (1 μM), convulxin (0.1 μg/mL), ADP (10 μM).
The state of Arf1/3 was also examined in resting and stimulated platelets. Arf1/3-GTP is not detectable in resting platelets and does not change upon the platelet activation (Figure S2D). Consistently, BFA, an Arf1 GEF inhibitor,45 does not affect collagen-induced aggregation at 10 μg/mL (Figure S2E). Taken together, these data do not support a role for Arf1 or 3 in platelet activation.
Arf6 is downstream of GPVI in activated platelets
Since both collagen and convulxin induce a decrease in Arf6-GTP, the next question was whether this was truly due to stimulation through GPVI or whether it was caused by secondary agonists (eg, ADP and TxA2) released from activated platelets. To test this, the secondary agonists were blocked by the addition of apyrase and/or aspirin. In Figure 3A, convulxin induces a time-dependent decrease (relative to resting cells) in Arf6-GTP. Inclusion of apyrase or aspirin alone does not significantly affect the convulxin-stimulated decrease in Arf6-GTP levels. However, apyrase and aspirin in combination results in a partial restoration of Arf6-GTP (< 50% of the resting platelets), suggesting that the 2 secondary agonists together may play some role in the convulxin-initiated decrease in Arf6-GTP. Nonetheless, these data suggest that convulxin is primarily responsible for the change in Arf6-GTP. We also examined the role of integrin outside-in signaling in Arf6-GTP decrease. As shown in Figure 3C, RGDS peptide, a selective blocker for integrins,46 does not affect the Arf6-GTP decrease. Under these same conditions, platelet aggregation is completely inhibited (Figure 3B). These data suggest that integrin outside-in signaling is not required for the convulxin-mediated Arf6-GTP decrease.
Other platelet agonists were tested to determine if a decrease in Arf6-GTP was downstream of their signaling cascades (Figure 3D). Thrombin and TRAP both stimulate a decrease in Arf6-GTP, suggesting that activation of Arf6′s GTPase activity is downstream of PAR receptors. The calcium ionophore A23187 has a similar effect, suggesting a role for increased intraplatelet calcium. Of interest, weaker agonists such as ADP and the thromboxane analog U46619 have only modest effects on Arf6-GTP levels. This lack of effect is consistent with the results in Figure 3A and confirms the conclusion that ADP and thromboxane-induced signaling are not critical for controlling Arf6-GTP levels.
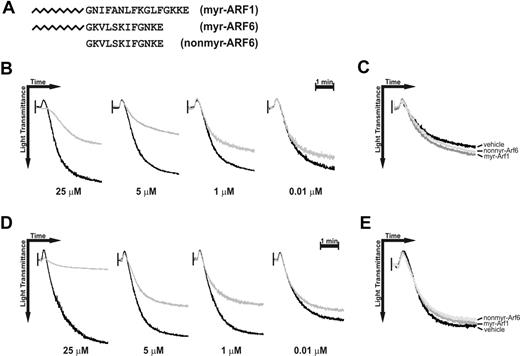
Myr-Arf6 peptide affects platelet aggregation. Washed platelets were incubated with DMSO (vehicle) or Arf peptides for 2 minutes with stirring at 37°C, followed by stimulation with 10 μg/mL collagen (B-C) or 0.1 μg/mL convulxin (D-E). (A) Sequences of the Arf peptides used. (B,D) Different doses of myr-Arf6 (gray line) were preincubated for 2 minutes prior to stimulation, and the aggregation traces were aligned with those from reactions containing an equal amount (1%) of DMSO (black line). (C,E) Myr-Arf1 (25 μM), nonmyr-Arf6 (25 μM), and DMSO were preincubated for 2 minutes prior to the stimulation.
Myr-Arf6 peptide affects platelet aggregation. Washed platelets were incubated with DMSO (vehicle) or Arf peptides for 2 minutes with stirring at 37°C, followed by stimulation with 10 μg/mL collagen (B-C) or 0.1 μg/mL convulxin (D-E). (A) Sequences of the Arf peptides used. (B,D) Different doses of myr-Arf6 (gray line) were preincubated for 2 minutes prior to stimulation, and the aggregation traces were aligned with those from reactions containing an equal amount (1%) of DMSO (black line). (C,E) Myr-Arf1 (25 μM), nonmyr-Arf6 (25 μM), and DMSO were preincubated for 2 minutes prior to the stimulation.
Myr-Arf6 peptide affects platelet aggregation and Arf6-GTP/GDP cycling
Analysis of the roles of small-GTPases is usually accomplished by transfection of mutant proteins into cells. Since this is not possible with platelets, we chose to use cell-permeant, lipid-modified peptides to disrupt protein-protein interactions between Arf6 and its potential effectors. Arf function is dependent on its myristoylated N-terminus, a fact that was initially exploited in the study of Arf1 function in inter-Golgi transport.17 Kahn and colleagues designed myristoylated peptides, based on Arf1′s N-terminus, that proved to be effective inhibitors of Golgi transport (Kahn et al17 ) and endosome fusion (Lenhard et al47 ). Subsequently, Caumont et al designed a similar set of Arf6 peptides that blocked PLD activation in PC12 cells.19 To apply this approach to platelets, 3 peptides were synthesized (see Figure 4A for sequences): a myristoylated Arf1 (myr-Arf1), a nonmyristoylated Arf6 (nonmyr-Arf6), and a myristoylated Arf6 (myr-Arf6). The nonmyr-Arf6 peptide serves as a nonpermeant peptide control, and, since Arf1 does not play a role in platelet function (Figure S2), the myr-Arf1 peptide serves as a permeant, sequence-specificity control for the myr-Arf6 peptide.
Figure 4B-C show the aggregation curves of collagen-stimulated platelets that were preincubated with each peptide. The myr-Arf6 peptide shows dose-dependent inhibition of collagen-mediated platelet aggregation as well as shape change (Figure 4B). Neither the myr-Arf1 nor the nonmyr-Arf6 peptide affects aggregation when used at 25 μM (Figure 4C). Convulxin-stimulated platelets are also affected by myr-Arf6 peptide pretreatment, but not by myr-Arf1 or nonmyr-Arf6. The effect is dose dependent and parallels the effects seen with collagen (Figure 4D-E).
We assessed the possibility of a detergent effect by measuring platelet intracellular Ca2+ using Fura-2/AM-loaded platelets in the presence of myr-Arf6. We observed no significant increase in intracellular Ca2+ concentration when myr-Arf6 peptide was added in the presence of extracellular Ca2+ (not shown). In addition, myr-Arf6 peptide shows no effect on the rate or the extent of increase in intracellular Ca2+ in convulxin-stimulated platelets (not shown). Furthermore, the subsequent decrease in the intracellular Ca2+, after stimulation, is unaffected by myr-Arf6 (not shown). Taken together, we conclude that myr-Arf6 shows no detergent effect on the resting or stimulated platelets, nor does it affect Ca2+ mobilization in response to convulxin.
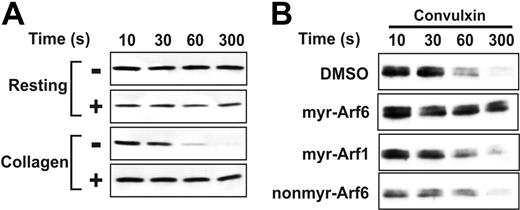
Myr-Arf6 peptide affects Arf6-GTP hydrolysis. (A) Washed platelets (4 × 108/mL) were incubated with DMSO (–) or myr-Arf6 peptide (+, 25 μM) for 2 minutes with stirring at 37°C, followed by stimulation with 10 μg/mL collagen. After incubation for the indicated times, platelets were lysed and the supernatants were analyzed for Arf6-GTP. (B) Washed platelets were incubated with DMSO or indicated Arf peptides (25 μM) for 2 minutes with stirring at 37°C, followed by stimulation with 0.1 μg/mL convulxin. After incubation for the indicated times, platelets were lysed and the supernatants were analyzed for Arf6-GTP.
Myr-Arf6 peptide affects Arf6-GTP hydrolysis. (A) Washed platelets (4 × 108/mL) were incubated with DMSO (–) or myr-Arf6 peptide (+, 25 μM) for 2 minutes with stirring at 37°C, followed by stimulation with 10 μg/mL collagen. After incubation for the indicated times, platelets were lysed and the supernatants were analyzed for Arf6-GTP. (B) Washed platelets were incubated with DMSO or indicated Arf peptides (25 μM) for 2 minutes with stirring at 37°C, followed by stimulation with 0.1 μg/mL convulxin. After incubation for the indicated times, platelets were lysed and the supernatants were analyzed for Arf6-GTP.
To further characterize the effect of the myr-Arf6 peptide on platelets, we examined its effects on Arf6-GTP. In Figure 5A, myr-Arf6 does not affect Arf6-GTP in resting platelets, but has a significant effect on the decrease of Arf6-GTP in collagen-stimulated cells. Myr-Arf6 peptide completely blocks the loss of Arf6-GTP. A similar effect is seen when convulxin is used as agonist. Figure 5B shows that only myr-Arf6 blocked Arf6-GTP decrease in convulxin-stimulated platelets, implying that the effect of myr-Arf6 is specific. While further analysis will be required, Figures 4, 5 suggest that loss of Arf6-GTP may be an important step in platelet activation and aggregation in response to GPVI-mediated signaling.
Myr-Arf6 affects F-actin dynamics in collagen-stimulated platelets
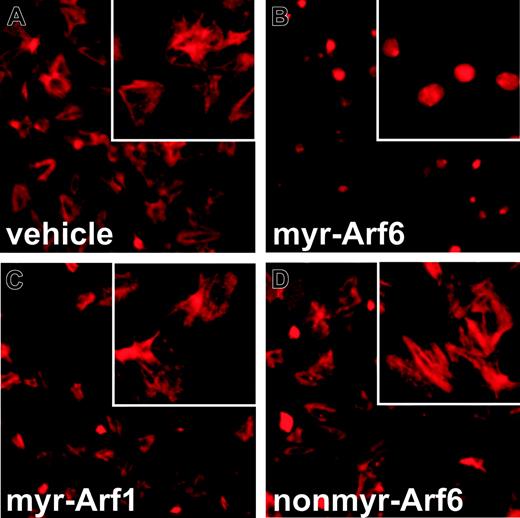
Platelets contain vast amounts of actin and undergo extensive cytoskeletal changes upon stimulation.48 In addition to its roles in endocytosis, exocytosis, and membrane trafficking, Arf6 also controls actin dynamics, cell adhesion, and spreading.31,49,50 Since the myr-Arf6 peptide affects collagen-stimulated shape change and aggregation (Figure 4), we examined its effects on platelet adherence to and spreading on collagen-coated coverslips. Platelets, allowed to bind to collagen-coated coverslips, show a characteristically spread morphology. Actin cables are clearly seen when the cells are stained with TRITC-conjugated phalloidin (Figure 6A). Cortical actin, at the platelet periphery, is also evident in some cells. Platelets pretreated with the myr-Arf6 peptide show a starkly different morphology (Figure 6B). Fewer cells are bound to the surface and the adherent cells are rounded. The phalloidin staining pattern is highly concentrated in the middle of the rounded cells and no actin cables are visible. This staining pattern and general lack of spreading is very similar to the morphology of platelets treated with the actin depolymerizing drug,51 Latrunculin A (data not shown). The 2 control peptides, myr-Arf1 (Figure 6C) and nonmyr-Arf6 (Figure 6D), are without effect, and the platelets, after peptide treatment, have the same morphology as control cells. From these data, it appears that the myr-Arf6 peptide disrupts platelet adherence to and spreading on collagen-coated surfaces. The distinctive differences in phalloidin staining patterns also suggest that disrupting Arf6 function disrupts actin dynamics in the platelet.
Myr-Arf6 affects the activation of Rho family GTPases
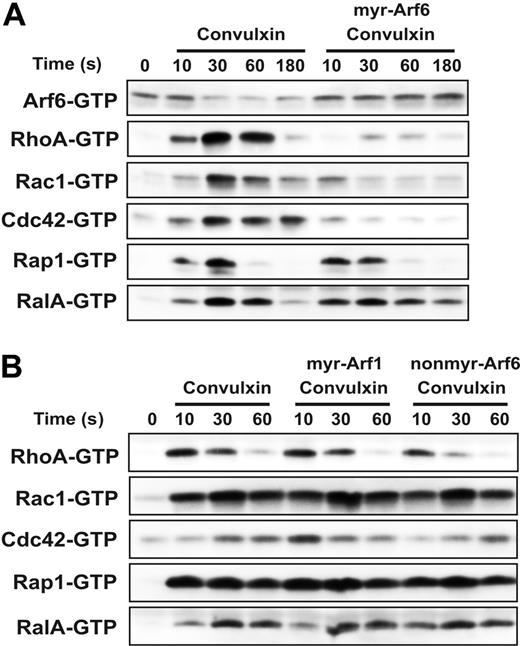
Given the effects of the mry-Arf6 peptide on platelet spreading and actin dynamics, and the cross talk between Arf6 and Rac signaling seen in epithelial cells,21,49,52 it seemed possible that Arf6 and Rho family members could be interrelated in platelets. Platelets contain Rho, Rac, and Cdc42 and each is activated to its GTP-bound state in response to agonists.4-6 Two other small GTPases, RalA and Rap1, were examined since they too are activated in response to platelet stimulation.12,38 The effect of the Arf6 peptide on these 5 small GTPases was examined using a GST-effector domain pull-down strategy with fusion proteins specific to each of the 5 GTPases. Platelets were pretreated with peptide and then stimulated with convulxin. Samples were taken at the indicated time points and the GTP-bound forms of each protein were analyzed. In Figure 7A, each of the proteins shows a biphasic activation profile that peaks at 30 seconds, consistent with previous reports.12,26,28 It is interesting to note that the decrease in Arf6-GTP appears to slightly precede activation of the other small GTPases. The myr-Arf6 peptide blocks the decrease in Arf6-GTP and has an inhibitory effect on the activation of the Rho family GTPases: Rac1, Cdc42, and RhoA. Myr-Arf6 peptide treatment repressed the activation of the Rho family for the whole incubation period correlating with the sustained Arf6-GTP levels. Rap1 and RalA activation is hardly affected by myr-Arf6 peptide treatment though the activation of RalA seems to be prolonged slightly. Myr-Arf1 and nonmyr-Arf6 peptide do not affect the activation profile of any of these small GTPases, supporting the specificity of myr-Arf6′s effect on Rho family GTPases (Figure 7B). These data demonstrate that failure to convert Arf6-GTP to Arf6-GDP blocks activation of the Rho family but not of Rap1 or RalA. From the data presented in Figure 7 as well as in Figure 5, we suggest that stimulation through GPVI leads to a conversion of Arf6-GTP to Arf6-GDP, which is important for the subsequent activation of the Rho family GTPases.
Myr-Arf6 peptide inhibits F-actin formation. Washed platelets were treated with DMSO (A) or 25 μM myr-Arf6 (B), myr-Arf1 (C), or nonmyr-Arf6 (D) peptides for 5 minutes at 37°C. After washing, the platelets were allowed to bind to collagen-coated coverslips for 10 minutes at 37°C, fixed with 3.7% formaldehyde, and quenched with 50 mM NH4Cl. TRITC-conjugated phalloidin in 10% FBS/PBS with 0.2% saponin was used to stain for F-actin. Images were taken using a SPOT CCD camera, model 9.0 monochrome-6 (Diagnostic Instruments, Sterling Heights, MI) and processed using SPOT Advanced software, version 4.1.1 (Diagnostic Instruments). Only contrast and brightness were adjusted with Photoshop 7.0 software (Adobe, San Jose, CA).
Myr-Arf6 peptide inhibits F-actin formation. Washed platelets were treated with DMSO (A) or 25 μM myr-Arf6 (B), myr-Arf1 (C), or nonmyr-Arf6 (D) peptides for 5 minutes at 37°C. After washing, the platelets were allowed to bind to collagen-coated coverslips for 10 minutes at 37°C, fixed with 3.7% formaldehyde, and quenched with 50 mM NH4Cl. TRITC-conjugated phalloidin in 10% FBS/PBS with 0.2% saponin was used to stain for F-actin. Images were taken using a SPOT CCD camera, model 9.0 monochrome-6 (Diagnostic Instruments, Sterling Heights, MI) and processed using SPOT Advanced software, version 4.1.1 (Diagnostic Instruments). Only contrast and brightness were adjusted with Photoshop 7.0 software (Adobe, San Jose, CA).
Myr-Arf6 peptide blocks the activation of Rho family GTPases. Washed platelets (4 × 108/mL) were incubated with DMSO or indicated Arf peptides (25 μM) for 2 minutes with stirring at 37°C, followed by stimulation with 0.1 μg/mL convulxin for the indicated times (A-B). The platelet suspensions were lysed and the supernatants were used to pull down the indicated small GTPases, as described in “Materials and methods.” Western blots were performed using anti-Arf6, anti-Rap1, anti-RalA, anti-Rac1, anti-Cdc42, and anti-RhoA antibodies.
Myr-Arf6 peptide blocks the activation of Rho family GTPases. Washed platelets (4 × 108/mL) were incubated with DMSO or indicated Arf peptides (25 μM) for 2 minutes with stirring at 37°C, followed by stimulation with 0.1 μg/mL convulxin for the indicated times (A-B). The platelet suspensions were lysed and the supernatants were used to pull down the indicated small GTPases, as described in “Materials and methods.” Western blots were performed using anti-Arf6, anti-Rap1, anti-RalA, anti-Rac1, anti-Cdc42, and anti-RhoA antibodies.
Discussion
In nucleated cells, Arf family GTPases control membrane transport, exocytosis, and the actin cytoskeleton (eg, filopodia and lamellipodia formation).16 All of these processes are essential aspects of a platelet's role in hemostasis. Yet, this report marks the first analysis of the role of Arf proteins in platelets. In this paper, we show that Arf family GTPases, Arf1/3 and Arf6, are present in platelets and that they localize to cytosol and membranes, respectively (Figure 1). Arf6 is predominantly in the GTP-bound state in resting cells and Arf6-GTP rapidly decreases upon stimulation with a number of strong agonists (Figures 2, 3). The decrease in Arf6-GTP temporally precedes the activation of Rho family members: Rho, Rac, and Cdc42 (Figure 7). Disrupting this transition (Arf6-GTP to Arf6GDP) inhibits agonist-induced shape change, adhesion, spreading, aggregation, and the activation of the Rho family GTPases (Figures 4,6). Taken together, these data suggest that Arf6 is a pivotal component of the platelet signaling cascade downstream of GPVI- and PAR-mediated signaling but potentially upstream of Rho family members.
Generally, small GTPases exist in inactive GDP-bound states that are activated to the GTP-bound state. This transition is mediated by GEFs and is often in response to specific signals. Our observations of Arf6 in platelets run counter to that general scheme since Arf6-GTP is highest in resting cells and decreases upon activation. This decrease was not due to a loss of total Arf6 and was reproducible in more than 7 distinct preparations of platelets from different donors. It is coincident with the initial phases of activation but is independent of integrin engagement and not significantly dependent on secondary agonists such as ADP and TxA2. The decrease in Arf6-GTP could be due to a signaling cascade that either activates an Arf6 GAP or inactivates an Arf6 GEF. Consistently, Arf GAPs are multidomain proteins with pleckstrin homology (PH), src homology (SH2 and SH3), and other interaction domains that could easily be affected by platelet signaling cascades.23,24 It is also formally possible that the observed decrease of Arf6-GTP is due to sequestration by an effector protein that makes Arf6-GTP inaccessible to GST-GGA3 binding. Such an interaction would be expected to have high affinity since it is not outcompeted by the addition of approximately 0.4 μM GST-GGA3. Regardless of mechanism, the net result of platelet activation is a loss of free Arf6-GTP, suggesting that Arf6-GTP could play a passivating role and must be converted to the inactive GDP form before full platelet activation can proceed. This could occur via an Arf6-GTP–mediated localization of, or competition with, a Rho family GEF. Both mechanisms have been proposed for nucleated cells.15,53,54 Alternatively, Arf6-GDP could play a positive role in platelet activation. At present, 16 Arf GAPs and 14 Arf GEFs have been identified in mammalian genomes44 ; therefore, the potential for Arfs' involvements in multiple pathways is quite possible. Unfortunately, only one Arf regulator, DEF1/ASAP1 (an Arf1 GAP55 ), has been identified in platelets, so further mechanistic speculation is tenuous at best.
The potential roles for class I Arfs, 1 and/or 3, were also examined. The antibodies used cannot distinguish between the 2 isoforms and the mass-spectroscopy data available cannot either,29 therefore it is uncertain which isoforms are present. While at least one, Arf1 or 3, is present in platelets, no GTP-bound forms were detected in either resting or activated cells. Consistently, pretreatment of platelets with BFA had no effect on platelet aggregation or secretion (Figure S2E and data not shown). These data suggest that though the class I Arfs are present in platelets, they are not affected by platelet activation and do not play a detectible role in collagen- or convulxin-induced aggregation. At present, our data suggest a role for only Arf6.
Expression of Arf GTPase mutants, Arf GEFs, and Arf GAPs has assisted in the functional study of Arfs in nucleated cells. Such methods are not readily possible for platelets. Acylated peptides have proved to be useful tools since they cross the lipid bilayer and can be used as inhibitors of specific protein-protein interactions. Acylated peptides have been used in platelets to probe the roles of the cytoplasmic tails of αIIb/β3,56 the cytoplasmic region of the PARs,57 and the roles of cytosolic protein kinase A.58 This approach has also been used to determine the role of Arf6 in exocytosis from PC12 cells.19 In these studies, Caumont et al suggested that the peptide disrupted activation of PLD.19 It is important to remember that the Arf peptides are predicted to disrupt interactions with Arf-binding proteins. That list could include effectors, GEFs, GAPs, or potential targeting receptors.
To probe the role of Arf6 in platelets, we used myr-Arf6 and 2 control peptides, nonmyr-Arf6 and myr-Arf1 (Figures 4, 5, 6, 7). Pretreatment with myr-Arf6 blocked collagen- and convulxin-induced aggregation and shape change. The peptide did not permeabilize the platelets nor did it affect Ca2+ mobilization in response to convulxin (not shown). Myr-Arf6 showed a dose-dependent effect on platelet adherence to and spreading on collagen-coated coverslips. Myr-Arf6 pretreatment also inhibited the agonist-induced reduction in Arf6-GTP. The simplest explanation for these effects is that the myr-Arf6 peptide inhibits an Arf6 GAP, prolonging the lifetime of Arf6-GTP in activated cells. It is equally likely that the peptide disrupts some key protein-protein interaction required for signaling immediately downstream of Arf6. Further understanding of the myr-Arf6 peptide's effects awaits the identification of potential Arf6 effector proteins in platelets. However, despite the mechanistic questions, these data are consistent with a critical role for Arf6 in collagen- and convulxin-mediated platelet activation.
Rho family members, Rac, Rho, and Cdc42, are characterized by the mode through which they affect the actin cytoskeleton. Rho promotes stress fiber formation, while Rac and Cdc42 promote lamellipodia and filopodia formation, respectively.59 This family of GTPases clearly plays a number of key roles in platelet function.4-6,28 Arf6 can also affect the actin cytoskeleton in nucleated cells, and in some cases the effects are coordinated with the Rho family. Expression of an Arf6 GTPase mutant (Arf6-Q67L) promotes membrane ruffling and filopodia formation.31,60,61 Cells expressing a GTP-binding–defective Arf6 (Arf6-T27N) fail to spread or form adhesive plaques.31,61 Arf6 and Rac colocalize to a perinuclear area in cells and expression of an Arf6-Q67L relocalizes Rac to the plasma membrane,62 suggesting that Arf6 may control Rac activity by changing its localization. Arf6-Q67L also causes disassembly of actin stress fibers,50 which may indicate some indirect effect of Arf6 on Rho, though none has been demonstrated. These data are consistent with our observations in platelets. Treatments that affect Arf6-GTP have a drastic effect on the platelet cytoskeleton (Figure 6). These treatments also affect the activation of Rho family GTPases (Figure 7), suggesting a functional relationship between Arf6 and the Rho family in platelets. Mechanistically, these interactions could be facilitated through effector proteins such as Arfptin 2/POR1, which bind both Arf6 and Rac.21,63 Future studies will be required to identify such proteins in platelets and to determine their effects on activation-induced cytoskeletal changes.
In summary, our data provide the first evidence that Arf6 plays a role in platelet activation and is involved in actin remodeling via Rho family GTPase activation. However, these conclusions lead to many questions for future studies. What is upstream of Arf6 and what is downstream? What platelet signaling events affect Arf6-GTP and what events are disrupted by the prolonged presence of Arf6-GTP? Furthermore, it will be necessary to identify and characterize potential Arf6 regulators in platelets. Arf function in nucleated cells is tied to many of the same processes that are essential to platelet function in hemostasis. From that same literature, several possible scenarios could explain the data reported here. Arf6 has been linked to PLD activation,64,65 PI5KI localization,20,66 as well as control of Rho family GTPase.49,50,62 Each or all of these Arf6-mediated processes could be important for platelet function. In this report, we show that Arf6 plays a role in platelet function; now, further analysis will be required to understand how Arf6 fits into the various signaling cascades that lead to thrombosis.
Prepublished online as Blood First Edition Paper, December 13, 2005; DOI 10.1182/blood-2005-09-3563.
Supported by a grant from National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), HL-56652. The Imaging Facility is supported by NIH grant number P20 RR20171 from the National Center for Research Resources.
W.C. designed the research, performed the research, analyzed the data, and wrote the paper. Z.A.K. designed the research, performed the research, and analyzed the data. S.W.W. designed the research, analyzed the data, wrote the paper, and reviewed the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Richard A. Kahn (Emory University, Atlanta, GA), Dr Juan S. Bonifacino (NIH, Bethesda, MD), Dr James E. Casanova (University of Virginia, Charlottesville, VA), Drs Johannes L. Bos and Kris A. Reedquist (Utrecht University, Utrecht, the Netherlands), and Dr Douglas A. Andres (University of Kentucky, Lexington, KY) for their generous gift of DNA constructs and antibodies. The authors wish to especially thank Dr Julie G. Donaldson for her generous gift of reagents and for her productive discussions and comments. We also thank Donaldson lab members, including Dr Fraser D. Brown, for DNA construct and antibodies and their helpful scientific and technical advice. We thank Dr Whiteheart's lab members, Dr Elena A. Matveeva, Garland L. Crawford, Qiansheng Ren, and Chunxia Zhao, for their helpful comments on the paper. We especially thank Garland L. Crawford for his scientific and technical help with this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal