Abstract
There is considerable interest in immunotherapeutic approaches for lymphoma. The expression of proteinase inhibitor 9 (PI-9), a molecule that inactivates granzyme B, is considered an immune escape mechanism in lymphoma. Further, lymphomas frequently overexpress the antiapoptotic molecule bcl-2, which is able to inhibit perforin-dependent cytotoxic pathways. In this study, the impact of PI-9 and bcl-2 expression on the sensitivity of lymphomas to T- and natural killer (NK) cell–mediated cytotoxicity was analyzed. We found PI-9 expression in 10 of 18 lymphoma cell lines and in 9 of 14 primary lymphomas. Overexpression of bcl-2 was found in 8 of 18 cell lines and in 12 of 14 primary lymphomas. All lymphoma cells were sensitive to cytolysis by specific T cells and cytokine-activated NK cells, and no difference in sensitivity was observed with respect to PI-9 or bcl-2 expression. Cytolysis was mediated predominantly through perforin-dependent pathways despite expression of PI-9 and bcl-2. Interestingly, the majority of lymphoma cells were resistant to cytolysis by resting allogeneic NK cells. This was due to the failure of lymphomas to induce degranulation of resting NK cells. These results show that resistance to perforin-dependent pathways is not a relevant immune escape mechanism in lymphoma and therefore is unlikely to impair clinical outcome of immunotherapeutic approaches.
Introduction
T-cell– and natural killer (NK) cell–based therapies have considerable potential in the treatment of B-cell malignancies. Idiotype vaccination is able to elicit T-cell responses and tumor regression in lymphoma patients.1,2 Recently, various common tumor antigens have been identified in lymphoma, facilitating the development of antigen-specific treatment approaches.3,4 Although antilymphoma effects after allogeneic stem cell transplantation have less impact on survival compared with myeloid leukemias,5 clinically relevant graft-versus-lymphoma effects have been observed.6,7 Moreover, recent findings suggest that NK cell–mediated lysis of lymphoma via antibody-dependent cellular cytotoxicity plays an important role for the therapeutic activity of rituximab.8-10
Cytolytic activity of T cells and NK cells is mediated by 2 different pathways: release of perforin together with granzymes from cytotoxic granules and death receptor activation. Resistance to FasL and TRAIL death receptor pathways has been reported to occur in a substantial proportion of human B-cell lymphomas.11-13 Various experimental studies suggest that perforin-dependent cytotoxic pathways are also disturbed in lymphoma.14-19 The expression of proteinase inhibitor 9 (PI-9), which selectively inactivates granzyme B,20,21 is considered an immune escape mechanism in lymphoma.14,15 Transfection of SPI-6, the murine analog of PI-9, into a lymphoma cell line resulted in impaired cytotoxic T-lymphocyte (CTL)–mediated elimination in vivo.15 Similarly, transfection of PI-9 into human breast cancer cells inhibited cytolysis by cytokine-activated killer cells in vitro.21 Overexpression of B-cell leukemia/lymphoma 2 proteins (bcl-2), which is frequently found in both high- and low-grade lymphomas,22 was reported to inhibit cytolysis mediated by CTLs19 and purified perforin/granzyme B.17 The failure of Wilms tumor 1 antigen (WT1)–specific CTLs to kill lymphoma in contrast to myeloma cell lines was attributed to resistance to perforin.23 Expression of PI-9 and bcl-2 were shown to be associated with poor prognosis in anaplastic large-cell lymphoma.18 Resistance to perforin-dependent pathways may thus lead to escape of lymphoma from immunosurveillance and inefficacy of immunotherapeutic approaches. Despite the potential relevance of the expression of PI-9 and bcl-2 for the sensitivity of lymphoma to cell-mediated lysis, no functional studies have been reported in human lymphoma so far.
In this study, we have investigated the sensitivity of various lymphoma cell lines and primary lymphoma cells to CTL- and NK cell–mediated cytotoxicity. We could show that the sensitivity of human lymphoma cells to CTL- and activated NK cell–mediated cytolysis and apoptosis was not impaired despite PI-9 expression or bcl-2 overexpression. In addition, we could demonstrate that the diminished sensitivity of most lymphomas to cytolysis by resting NK cells was caused by the inability of lymphoma cells to induce NK cell degranulation and not by resistance to cytotoxic pathways.
Patients, materials, and methods
Cell lines and culture conditions
The cell lines K562, HL-60, DOHH-2, WSU-NHL, Raji, Daudi, DG-75, SU-DHL-1, SUP-M2, MEC-1, and Jurkat were kept in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS; Biochrom); and Karpas-1106P, SKW-3, JEKO-1, Loucy, SU-DHL-4, and Karpas-422 were kept in RPMI 1640 with 20% FCS. Dulbecco MEM (Biochrom) was culture medium for Granta-519, and basal Iscove MDM (Biochrom) was culture medium for YT, both supplemented with 20% FCS. All cell lines were purchased from DMSZ (Braunschweig, Germany). HLA-A*0201–transfected K562 was kindly provided by Dr Cedrik Britten (University of Mainz, Germany) and grown in RPMI 1640 with 10% FCS.
Primary lymphoma cells
Lymphoma cells from 6 patients with a diagnosis of B-cell chronic lymphocytic leukemia (B-CLL), 2 patients with mantle cell lymphoma (MCL), 1 patient with T-cell granular lymphocytic leukemia (LGL), 1 patient with follicular lymphoma (foll non-Hodgkin lymphoma [NHL]), and 2 patients with acute lymphoblastic leukemia (B-cell ALL, T-cell ALL [T-ALL]) were isolated from peripheral blood. Lymphoma cells from 1 patient with diffuse large B-cell lymphoma (B-DLCL) and one patient with peripheral T-cell lymphoma (T-NHL) were isolated from pleural effusion. Mononuclear cells containing between 60% and 99% lymphoma cells were isolated by density gradient centrifugation using Ficoll-Hypaque 1.077 (Biochrom). All lymphoma samples analyzed in cytolysis assays contained greater than 95% lymphoma cells with the exception of 1 patient with 60% lymphoma cells in which T-cell and NK-cell depletion was performed using CD3 and CD56 Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). All patients have given informed consent for analysis. The approval was obtained from the review board of the Campus Benjamin Franklin, Charité, former Free University of Berlin.
Flow cytometric determination of PI-9, bcl-2, and HLA-A2 expression
Intracellular PI-9 expression was determined using anti–PI-9 antibody clone 7D8 (MBL, Woburn, MA) following fixation and permeabilization of tumor cells. Raji cells were used as positive control. Jurkat cells were used as negative control. Expression of bcl-2 was analyzed using anti–bcl-2 antibody FITC-conjugated clone 124 (DakoCytomation, Glostrup, Denmark). Cells expressing bcl-2 at a level higher than Jurkat cells were classified as bcl-2–overexpressing cells.24 Primary lymphoma cell samples were costained with anti-CD19 antibody (BD Biosciences, San Jose, CA). Expression of HLA-A2 was determined using monoclonal anti–human HLA-A2 (One Lambda, Drefeld, Germany). Data acquisition was performed on FACSCalibur and analyzed using Cellquest Software (BD Biosciences).
Assays for determining sensitivity to death receptor–induced apoptosis
The sensitivity of cell lines to Fas-mediated apoptosis was investigated using agonistic anti-CD95 (IgM clone CH11; Upstate, Lake Placid, NY) and to TRAIL-mediated apoptosis using recombinant human soluble (rhs) TRAIL (Super Killer TRAIL; Alexis Biochemicals, San Diego, CA). “Late” apoptotic changes were determined by propidium iodide (Sigma-Aldrich, Munich, Germany) uptake following 18 hours of incubation of lymphoma cells with anti-CD95 or TRAIL at various concentrations as described by Jayaraman.25 Propidium iodide was added at a final concentration of 1.0 μg/mL to the cells and the proportion of propidium iodide–positive cells was determined by flow cytometry in the FL3 channel. The specific apoptosis of the target cells (and also specific cytolysis for experiments described in “Determination of CTL- and NK cell–mediated cytotoxicity”) was calculated using the following formula: % specific apoptosis = (% apoptosis of target cells – % spontaneous cell apoptosis)/(100% – % spontaneous cell apoptosis).
In addition, a second assay determined “early apoptosis” by intracellular labeling of activated caspases by cell-permeable FITC–VAD-FMK (CaspACE; Promega, Mannheim, Germany).25 Cell lines displaying less than 10% specific apoptosis at the highest ligand concentration were considered to be resistant, between 10% and 30% low sensitive, and between 30% and 70% intermediate sensitive.
Preparation of CTLs and NK effector cells
Heparinized peripheral blood samples were obtained from HLA-A2–positive healthy subjects. Peripheral blooc mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Hypaque. For generation of influenza-specific CTL lines, PBMCs were cultured together with influenza peptide (influenza matrix protein 58-66, GILGFVFTL), IL-2 30 U/mL (R&D Systems, Wiesbaden, Germany), and IL-7 10 ng/mL (R&D Systems) in Iscoves medium supplemented with 10% human AB serum for 10 to 14 days. T-cell lines contained a mean of 7.26% (range, 5.9%-8.28%; n = 4) influenza-specific CD3+CD8+ T cells and no detectable HIV-reactive T cells as determined by intracellular staining of interferon gamma production. The peptides were kindly provided by Prof Dr Stefan Stevanovic (University of Tuebingen, Germany). NK cells were isolated from freshly collected buffy-coat preparations following density gradient centrifugation using CD56 Microbeads (Miltenyi Biotec). Positive magnetic separation of CD56+ cells and depletion of CD56+CD3+ cells by CD3 Microbeads (Miltenyi Biotec) was performed according to guidelines provided by Miltenyi Biotec. For experiments with activated NK cells, NK cells were stimulated with 500 IU/mL IL-2 (Chiron, Emeryville, CA) and with 10 ng/mL IL-12 (R&D Systems) for 24 hours in complete medium with 10% human AB serum.
Determination of CTL- and NK cell–mediated cytotoxicity
Flow-cytometric detection of CTL- and NK cell–mediated cytotoxicity was performed as described by Piriou et al.26 In brief, tumor target cells were labeled with green lipophylic fluorescent dye DIOC18 (3,3′-dioctadecylox-acarbocyanine perchlorate; Sigma-Aldrich, Deisenhofen, Germany). Effector cells and target cells were coincubated at various ratios for 4 hours. Thereafter propidium iodide was added at a final concentration of 5 μg/mL for 5 minutes to determine dead cells. For assessment of CTL-mediated cytolysis, DIOC18-labeled target cells were preloaded with 10 μg/mL influenza peptide or HIV peptide (HIV reverse transcriptase 476-484, ILKEPVHGV) for 2 hours at 37°C and 5% CO2. Thereafter cells were washed 2 times in Iscove medium. For perforin-blocking experiments, effector cells were pretreated with concanamycin A (Sigma Aldrich, Munich, Germany) for 90 minutes at a concentration of 50 nM and kept in the same medium during coincubation with target cells. A sample containing only labeled target cells and propidium iodide was used to determine spontaneous cell death.
Activation of caspases was determined using cell-permeable FITC-labeled pancaspase pseudosubstrate VAD-FMK (CaspACE) as described by Pozarowski et al.27 Target cells were stained with red fluorescence dye PKH-26 (Sigma-Aldrich Chemicals, Steinheim, Germany) and were coincubated with effector cells at various concentrations. After 2 hours, FITC–VAD-FMK was added to a final concentration of 10 μM and cells were kept in an incubator for 50 minutes, washed twice, and analyzed.
Determination of degranulation of NK cells
Degranulation was determined as previously described by Penack et al.28 Briefly, freshly isolated CD56+ cells were coincubated with target cells at different effector-target ratios under identical conditions as during the cytotoxicity assays. Anti-CD107–PE antibody clone H4A3 (BD Biosciences) was added during the entire incubation time. After 1 hour of incubation, monensin (Sigma, Munich, Germany) was added. After 5 hours, cells were washed and resuspended in PBS, and CD107 expression on CD56+ NK cells was analyzed by flow cytometry.
Proteinase inhibitor 9 (PI-9) and bcl-2 expression in lymphoma cells. Proteinase inhibitor 9 (PI-9) expression (A) and bcl-2 expression (B) were determined by flow cytometry. HLA-A2–positive lymphoma cells (▪) were used later for CTL experiments. HLA-A2–negative cells are indicated with □. Background fluorescence intensity was subtracted. Means and standard error of the mean (SEM) of 3 experiments are shown for cell lines (Ai and Bi). One out of 2 experiments is shown for primary lymphoma cells (Aii and Bii). Concordant results were obtained in both experiments. MFI indicates mean fluorescent intensity.
Proteinase inhibitor 9 (PI-9) and bcl-2 expression in lymphoma cells. Proteinase inhibitor 9 (PI-9) expression (A) and bcl-2 expression (B) were determined by flow cytometry. HLA-A2–positive lymphoma cells (▪) were used later for CTL experiments. HLA-A2–negative cells are indicated with □. Background fluorescence intensity was subtracted. Means and standard error of the mean (SEM) of 3 experiments are shown for cell lines (Ai and Bi). One out of 2 experiments is shown for primary lymphoma cells (Aii and Bii). Concordant results were obtained in both experiments. MFI indicates mean fluorescent intensity.
Statistical analysis
Comparison of CTL-induced activation of caspases in influenza-peptide–loaded and control lymphoma cells was performed using nonparametric Wilcoxon test.
Results
Lymphoma cell lines frequently express PI-9 and overexpress bcl-2
Expression of PI-9 and bcl-2 was analyzed in 18 human lymphoma cell lines and in primary lymphoma cells from 14 patients. Lymphoma cells from 10 of 18 cell lines and from 9 of 14 primary lymphomas expressed PI-9 (Figure 1A). Overexpression of bcl-2 was found in 8 of 18 lymphoma cell lines and in 12 of 14 primary lymphomas (Figure 1B). The myeloid cell line K562, which was included as a positive control for T- and NK-cell cytotoxicity, expressed PI-9 but did not overexpress bcl-2.
Sensitivity to Fas receptor– and TRAIL-mediated apoptosis
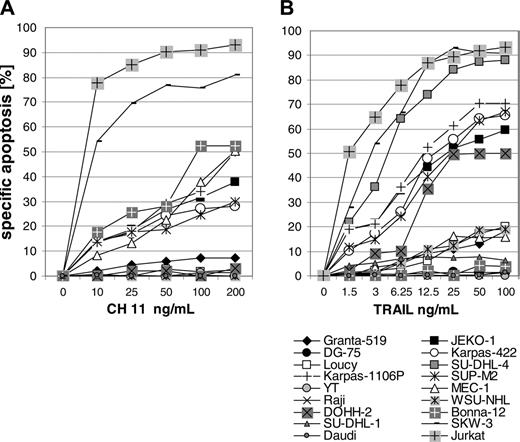
Ten of the 18 lymphoma cell lines were completely resistant to Fas-mediated apoptosis (Figure 2A). Resistance to TRAIL-mediated killing was observed in 6 and low sensitivity in 4 of the 18 lymphoma cell lines (Figure 2B). A second assay detecting activated caspases after 18 hours as an early apoptotic event confirmed the results of the propidium iodide assay (data not shown). Fas- and TRAIL-resistant PI-9–positive cell line DG-75 displayed no detectable specific activation of caspases at the highest concentrations of ligands.
Lymphoma cell lines are sensitive to CTL-mediated cytolysis
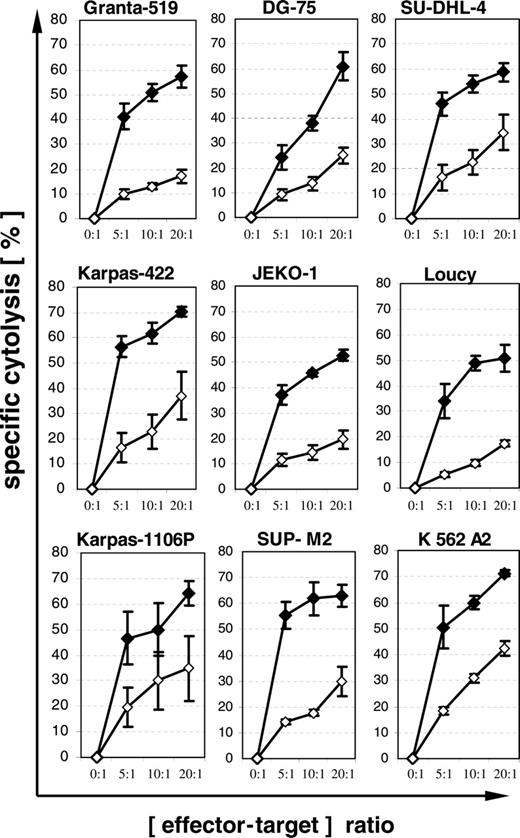
Eight of 18 lymphoma cell lines carried HLA-A2 and thus were suitable for assessment of CTL-mediated cytolysis. The expression of PI-9 and bcl-2 in these 8 lymphoma cell lines, as well as their sensitivity to Fas and TRAIL, are summarized in Table 1. Lymphoma cells were loaded with influenza peptide or HIV peptide as a control for major histocompatibility complex (MHC)–unrestricted cytolysis by cytokine-activated NK and T cells. In our series of 3 different experiments, all lymphoma cell lines were sensitive to cytolysis mediated by influenza-specific CTLs at a level comparable to that of the HLA-A2–transfected K562 line (Figure 3). The different levels of cytolysis of lymphoma cells loaded with HIV peptide are most likely related to the different sensitivity of cell lines to MHC-unrestricted cytolysis mediated by activated natural killers. Importantly, no obvious difference in sensitivity to CTL-mediated cytolysis was observed with respect to expression of PI-9 or bcl-2.
Characteristics of HLA-A2–positive lymphoma cell lines
Cell line . | Histologic subtype . | PI-9 expression . | bcI-2 overexpression . | Sensitivity to Fas . | Sensitivity to TRAIL . |
|---|---|---|---|---|---|
| Granta-519 | Mantle cell lymphoma | + | + | Resistant | Low |
| JEKO-1 | Mantle cell lymphoma | - | - | Intermediate | Intermediate |
| DG-75 | Burkitt lymphoma | + | - | Resistant | Resistant |
| Loucy | T-ALL | - | + | Resistant | Low |
| Karpas-422 | B-DLCL | + | + | Low | Intermediate |
| SU-DHL-4 | B-DLCL | + | + | Resistant | High |
| SUP-M2 | Anaplastic large-cell lymphoma | - | - | Intermediate | Intermediate |
| Karpas-1106P | Mediastinal lymphoblastic B-NHL | - | - | Intermediate | Intermediate |
Cell line . | Histologic subtype . | PI-9 expression . | bcI-2 overexpression . | Sensitivity to Fas . | Sensitivity to TRAIL . |
|---|---|---|---|---|---|
| Granta-519 | Mantle cell lymphoma | + | + | Resistant | Low |
| JEKO-1 | Mantle cell lymphoma | - | - | Intermediate | Intermediate |
| DG-75 | Burkitt lymphoma | + | - | Resistant | Resistant |
| Loucy | T-ALL | - | + | Resistant | Low |
| Karpas-422 | B-DLCL | + | + | Low | Intermediate |
| SU-DHL-4 | B-DLCL | + | + | Resistant | High |
| SUP-M2 | Anaplastic large-cell lymphoma | - | - | Intermediate | Intermediate |
| Karpas-1106P | Mediastinal lymphoblastic B-NHL | - | - | Intermediate | Intermediate |
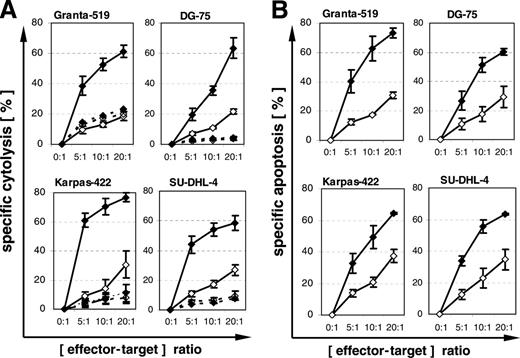
Subsequent experiments with the 4 PI-9–positive lymphoma cell lines Granta-519, Karpas-422, DG-75, and SU-DHL-4 were performed to define pathways relevant for CTL-mediated lysis. Blockade of perforin pathways with concanamycin A led to nearly complete inhibition of CTL-mediated cytolysis (Figure 4A). Staining with the pancaspase inhibitor FITC–VAD-FMK revealed activation of caspases by CTLs in all 4 cell lines, including the Fas- and TRAIL-resistant DG-75 (Figure 4B). These findings suggest that granzyme B is not completely inhibited by PI-9, because in perforin pathway–dependent cytolysis, granzyme B is the only known component of cytotoxic granules able to induce activation of caspases.29
Sensitivity of lymphoma cells to apoptosis mediated by Fas receptor and by TRAIL. Fas-mediated (A) and TRAIL-mediated (B) apoptosis were investigated in 18 lymphoma cell lines. Specific apoptosis was determined in an 18-hour propidium iodide assay. One out of 2 experiments is shown. Concordant results were obtained in both experiments. Jurkat cell line was used as positive control.
Sensitivity of lymphoma cells to apoptosis mediated by Fas receptor and by TRAIL. Fas-mediated (A) and TRAIL-mediated (B) apoptosis were investigated in 18 lymphoma cell lines. Specific apoptosis was determined in an 18-hour propidium iodide assay. One out of 2 experiments is shown. Concordant results were obtained in both experiments. Jurkat cell line was used as positive control.
Lymphoma cells are weakly sensitive or resistant to cytolysis mediated by resting but not by cytokine-activated allogeneic NK cells
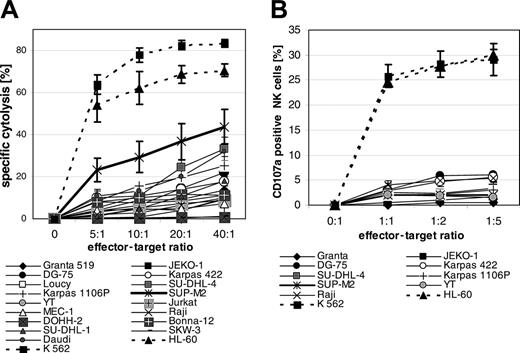
In our series of 4 different experiments with freshly isolated NK cells from 4 different donors, all 18 lymphoma cell lines were resistant to or much less sensitive to NK cell–mediated cytolysis compared with myeloid cell lines K562 and HL-60, with only the exception of SUP-M2 (Figure 5A). In order to elucidate possible mechanisms of lymphoma resistance to resting NK cells, we analyzed whether lymphoma cells would induce degranulation of NK cells by determination of CD107 molecules on the surface of effector cells.30 NK cell cytotoxicity assay and degranulation assays were performed with NK cells from the same donor simultaneously 3 times in 9 cell lines. Lymphoma cell lines induced no or significantly diminished CD107 expression on NK cells compared with K562 and HL-60 cells (Figure 5B), indicating that lymphoma cells fail to induce degranulation of cytotoxic granules rather than being resistant to cytotoxic granule components.
CTL-mediated cytolysis of lymphoma cells. Eight HLA-A2–positive lymphoma cell lines were loaded with influenza peptide (♦) or HIV peptide as negative control (⋄) and coincubated for 4 hours with an influenza-peptide–specific T-cell line. The ninth cell line, K 562 A2, is a myeloid cell line and was used as a positive control. Specific cytolysis was determined by propidium iodide assay. Means and SEM of 3 experiments are shown.
CTL-mediated cytolysis of lymphoma cells. Eight HLA-A2–positive lymphoma cell lines were loaded with influenza peptide (♦) or HIV peptide as negative control (⋄) and coincubated for 4 hours with an influenza-peptide–specific T-cell line. The ninth cell line, K 562 A2, is a myeloid cell line and was used as a positive control. Specific cytolysis was determined by propidium iodide assay. Means and SEM of 3 experiments are shown.
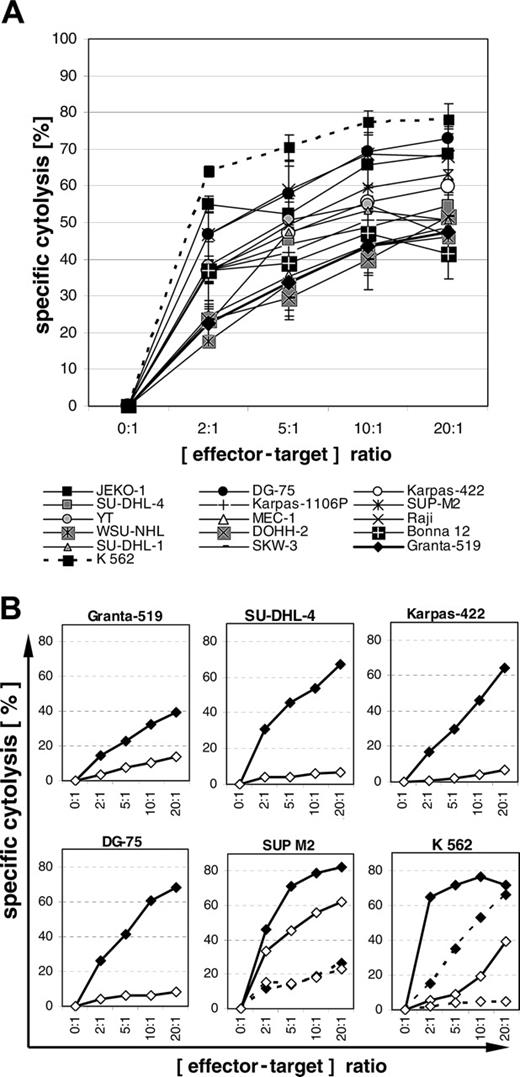
Next the sensitivity of lymphoma cell lines toward cytokine-activated NK cells was assessed. As evident from 3 experiments performed with 3 different donors, all 15 lymphoma cell lines investigated turned out to be sensitive to lysis by cytokine-activated NK cells (Figure 6A). The expression of PI-9, overexpression of bcl-2, and resistance to FasL and TRAIL were not associated with the level of sensitivity. In a fourth experiment, in which activated NK cells were generated from positively separated CD56+ and CD3+-depleted PBMCs, comparable results were observed (data not shown). As shown for CTL-mediated cytolysis, the cytolysis of the 4 PI-9–positive lymphoma cell lines Granta-519, DG-75, Karpas-422, and SU-DHL-4 by NK cells was almost completely blocked by concanamycin A (Figure 6B), indicating a perforin-dependent pathway. Only in the highly NK-sensitive cell line SUP-M2, which was effectively lysed by activated as well as by resting NK cells, did concanamycin A not inhibit cytolysis by either activated or resting NK cells, indicating that the lysis of this cell line was predominantly mediated via the death receptor pathways. This finding also explains the observation that SUP-M2 was sensitive to cytolysis by resting NK cells in the absence of degranulation (Figure 5A-B).
Inhibition of CTL-mediated cytolysis by concanamycin A (CMA) and CTL-mediated activation of caspases. (A) Four PI-9–positive and HLA-A2–positive lymphoma cell lines were preloaded with influenza peptide (♦) or HIV peptide (⋄) and coincubated with an influenza-peptide–specific T-cell line for 4 hours without (solid lines) or with (broken lines) 50 nM CMA. Specific cytolysis was detected by propidium iodide assay. Means and SEM of 3 experiments are shown. (B) Lymphoma cells were preloaded with influenza peptide (♦) or HIV peptide (⋄) and coincubated with an influenza-peptide–specific T-cell line for 2 hours. Specific apoptosis was detected by FITC–VAD-FMK. Means and SEM of 3 experiments are shown. The difference between specific (influenza peptide) and unspecific (HIV peptide) apoptosis for all cell lines is statistically significant (P ≤ .01, Wilcoxon test).
Inhibition of CTL-mediated cytolysis by concanamycin A (CMA) and CTL-mediated activation of caspases. (A) Four PI-9–positive and HLA-A2–positive lymphoma cell lines were preloaded with influenza peptide (♦) or HIV peptide (⋄) and coincubated with an influenza-peptide–specific T-cell line for 4 hours without (solid lines) or with (broken lines) 50 nM CMA. Specific cytolysis was detected by propidium iodide assay. Means and SEM of 3 experiments are shown. (B) Lymphoma cells were preloaded with influenza peptide (♦) or HIV peptide (⋄) and coincubated with an influenza-peptide–specific T-cell line for 2 hours. Specific apoptosis was detected by FITC–VAD-FMK. Means and SEM of 3 experiments are shown. The difference between specific (influenza peptide) and unspecific (HIV peptide) apoptosis for all cell lines is statistically significant (P ≤ .01, Wilcoxon test).
Cytolysis mediated by resting allogeneic NK cells and degranulation of NK cells. (A) Lymphoma cells were coincubated with resting NK cells for 4 hours. Specific cytolysis was determined by propidium iodide assay. Means and SEM of 3 experiments are shown. (B) Degranulation of resting NK cells was determined by CD107a staining after coincubation with lymphoma cells for 5 hours. The degranulation of NK cells was performed with NK cells of the same donor as in the cytolysis assays shown in panel A. Means and SEM for 3 experiments are shown.
Cytolysis mediated by resting allogeneic NK cells and degranulation of NK cells. (A) Lymphoma cells were coincubated with resting NK cells for 4 hours. Specific cytolysis was determined by propidium iodide assay. Means and SEM of 3 experiments are shown. (B) Degranulation of resting NK cells was determined by CD107a staining after coincubation with lymphoma cells for 5 hours. The degranulation of NK cells was performed with NK cells of the same donor as in the cytolysis assays shown in panel A. Means and SEM for 3 experiments are shown.
Primary lymphoma cells are sensitive to cytolysis mediated by specific CTLs
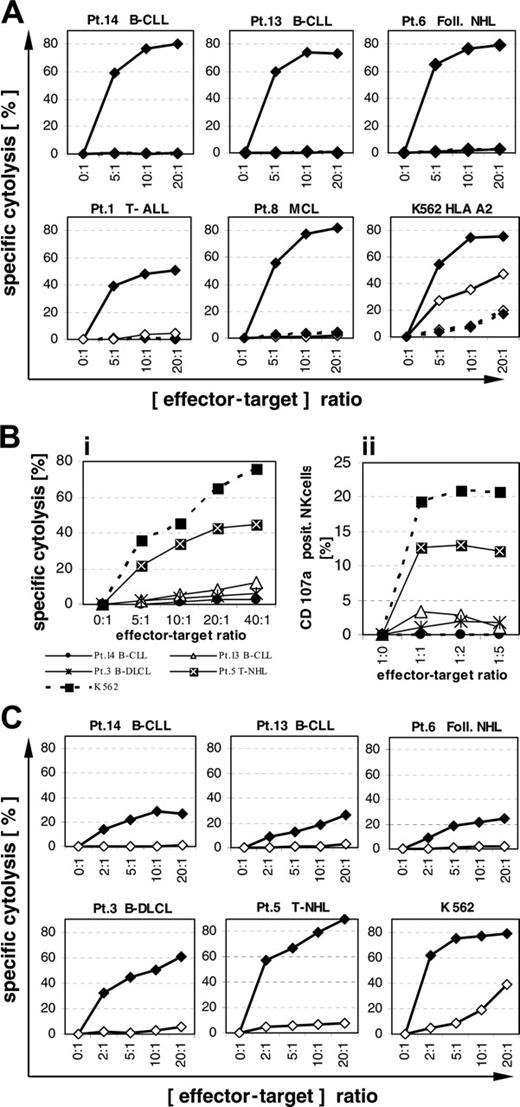
Lymphoma cells from 5 patients expressing PI-9, overexpressing bcl-2, and carrying HLA-A2 (patients 1, 6, 8, 13, 14) were examined for their sensitivity to cytolysis by influenza-specific CTLs. Primary lymphoma cells loaded with influenza peptide from all 5 patients showed good sensitivity to cytolysis mediated by specific CTLs at a level similar to that of HLA-A2–transfected K562 (Figure 7A). No or very little cytolysis was observed in lymphoma cells loaded with the control HIV peptide, suggesting low sensitivity to killing by activated NK cells. Blockade of perforin pathways with concanamycin A led to nearly complete inhibition of CTL-mediated cytolysis (Figure 7A).
Primary lymphoma cells are weakly sensitive or resistant to cytolysis mediated by resting but not by cytokine-activated allogeneic NK cells
Lymphoma cells from 4 patients expressing PI-9 (patients 3, 5, 13, 14) were examined for their sensitivity to cytolysis by resting allogeneic NK cells. Except for patient 5 with peripheral T-NHL, all lymphomas overexpressed bcl-2 and all expressed PI-9, with patient 3 at a level similar to the cell line YT with the highest expression level. Only lymphoma cells from patient 5 were sensitive to cytolysis by resting NK cells (Figure 7Bi). Resistance of the 3 other lymphoma cell samples to cytolysis by resting NK cells correlated with their failure to induce degranulation of NK cells (Figure 7Bii).
Lymphoma cells from the same 4 patients (patients 3, 5, 13, 14) and from patient 6 were examined for their sensitivity to cytolysis by cytokine-activated NK cells (Figure 7C). Lymphoma cells from patient 3 with diffuse large-cell lymphoma and from patient 5 with T-NHL displayed high sensitivity to cytolysis mediated by activated NK cells similar to K562. Lymphoma cells from 2 other patients with B-CLL and from 1 patient with follicular lymphoma were much less sensitive to cytolysis mediated by activated NK cells. Lymphoma cells of all 3 patients (6, 13, 14) were, however, highly sensitive to CTL-mediated lysis as shown in Figure 7A, suggesting no resistance to cytolysis by perforin-dependent pathways. Probably the low sensitivity was due to the failure of these lymphoma cells to efficiently induce degranulation of activated NK cells, which, however, could not be directly assessed due to a high unspecific binding of anti-CD107 antibodies by cytokine-activated NK cells.
Blockade of perforin pathways with concanamycin A led to nearly complete inhibition of cytolysis mediated by activated NK cells in all 5 primary lymphoma samples (Figure 7C).
Discussion
The investigations presented here confirm and extend previous findings of frequent expression of PI-9, overexpression of bcl-2, and resistance to Fas- and TRAIL-mediated apoptosis in lymphoma cells.11-14,22 The assumption that expression of PI-9 may confer resistance to cytolysis was, however, not confirmed by our experiments. In contrast, we provide evidence that human lymphoma cell lines and primary lymphoma cells were all sensitive to cytolysis by both CTLs and activated NK cells, independent of their PI-9 expression. Further, the overexpression of bcl-2, previously shown to inhibit cytolysis mediated by purified perforin/granzyme B17,31 and CTLs,19 did not diminish the sensitivity of lymphoma to cytolysis. The nearly complete blocking of cytolysis by concanamycin A32 showed that both CTLs and NK cells lysed the PI-9–expressing and bcl-2–overexpressing lymphoma cells via perforin-dependent pathways. One possible explanation for the sensitivity of PI-9–expressing and bcl-2–overexpressing lymphoma cells to cytolysis mediated by perforin-dependent pathways is the diversification of components of cytotoxic granules. This notion is supported by in vitro and in vivo experimental studies by the group of Trapani (Davis et al33 and Smyth et al34 ) with perforin (pfp–/–) and granzyme (grzA–/– and grzB–/–) knockout mice. Granzyme B–deficient (grzB–/–) murine lymphocytes retain potent cytolytic activity in vitro in contrast to perforin-deficient (pfp–/–) lymphocytes that display nearly no cytolytic activity.33 These in vitro experiments were confirmed to be relevant in vivo,34 as perforin-deficient mice were unable to eradicate tumors, whereas both granzyme A and B were dispensable in NK/CTL-mediated tumor eradication and might be substituted by other granzymes.
Cytolysis mediated by cytokine-activated NK cells and inhibition by concanamycin A. (A) Lymphoma cells were coincubated with cytokine-activated NK cells at various effector-target ratios for 4 hours. Means and SEM of 3 experiments are shown. (B) Lymphoma cells were coincubated with cytokine-activated NK cells (solid lines) and resting NK cells (broken lines; experiments with SUP M2 and K562 only) for 4 hours without (♦) or with (⋄) 50 nM concanamycin A. One out of 2 experiments is shown. Concordant results were obtained in both experiments. Specific cytolysis was determined by propidium iodide.
Cytolysis mediated by cytokine-activated NK cells and inhibition by concanamycin A. (A) Lymphoma cells were coincubated with cytokine-activated NK cells at various effector-target ratios for 4 hours. Means and SEM of 3 experiments are shown. (B) Lymphoma cells were coincubated with cytokine-activated NK cells (solid lines) and resting NK cells (broken lines; experiments with SUP M2 and K562 only) for 4 hours without (♦) or with (⋄) 50 nM concanamycin A. One out of 2 experiments is shown. Concordant results were obtained in both experiments. Specific cytolysis was determined by propidium iodide.
Cytolysis of primary PI-9–positive lymphoma cells mediated by CTLs and NK cells. (A) PI-9–positive and HLA-A2–positive lymphoma cells from 5 patients were preloaded with influenza peptide (♦) or HIV peptide (⋄) and coincubated with an influenza-peptide–specific T-cell line for 4 hours without (solid lines) or with (broken lines) 50 nM concanamycin A. Specific cytolysis was detected by propidium iodide assay. One out of 2 experiments is shown. Concordant results were obtained in both experiments. (Bi) Lymphoma cells were coincubated with resting NK cells for 4 hours. Specific cytolysis was detected by propidium iodide assay. (Bii) Degranulation of resting NK cells was determined by CD107a staining after coincubation with lymphoma cells for 5 hours. The degranulation of NK cells was assessed in NK cells of the same donor as in the cytolysis assays shown in subpanel Bi. One out of 2 experiments is shown. Concordant results were obtained in both experiments. (C) Lymphoma cells were coincubated with cytokine-activated NK cells for 4 hours without (♦) or with (⋄) 50 nM concanamycin A. Specific cytolysis was determined by propidium iodide. One out of 2 experiments is shown. Concordant results were obtained in both experiments.
Cytolysis of primary PI-9–positive lymphoma cells mediated by CTLs and NK cells. (A) PI-9–positive and HLA-A2–positive lymphoma cells from 5 patients were preloaded with influenza peptide (♦) or HIV peptide (⋄) and coincubated with an influenza-peptide–specific T-cell line for 4 hours without (solid lines) or with (broken lines) 50 nM concanamycin A. Specific cytolysis was detected by propidium iodide assay. One out of 2 experiments is shown. Concordant results were obtained in both experiments. (Bi) Lymphoma cells were coincubated with resting NK cells for 4 hours. Specific cytolysis was detected by propidium iodide assay. (Bii) Degranulation of resting NK cells was determined by CD107a staining after coincubation with lymphoma cells for 5 hours. The degranulation of NK cells was assessed in NK cells of the same donor as in the cytolysis assays shown in subpanel Bi. One out of 2 experiments is shown. Concordant results were obtained in both experiments. (C) Lymphoma cells were coincubated with cytokine-activated NK cells for 4 hours without (♦) or with (⋄) 50 nM concanamycin A. Specific cytolysis was determined by propidium iodide. One out of 2 experiments is shown. Concordant results were obtained in both experiments.
Our finding of CTL-induced activation of caspases in the PI-9–positive and death receptor–resistant lymphoma cell line DG-75 suggests, however, that the level of PI-9 expression in lymphomas is not sufficient to completely block granzyme B. In perforin-dependent pathways, granzyme B is the only known human granzyme that triggers cell death via the activation of caspases.29,35,36 Alternatively, recently published data suggest that PI-9 may become inactivated by components of the cytotoxic granules, as it was shown that granzyme M is able to inactivate PI-9.37 Finally, in a very recent study the group of Medema (Bots et al38 ) showed that they could further clarify the mechanisms of PI-9–mediated inhibition of perforin pathways by identifying a second anticytotoxic molecule, namely SPI-CI, an inhibitor of granzyme M. Expression of both molecules was necessary for prevention of CTL-mediated lysis of murine colorectal cancer cell lines.38 Although expression of a human analog of SPI-CI has not been studied in human lymphoma cell lines yet, their sensitivity to T-cell cytolysis observed in our study makes it unlikely that an SPI-CI analog would be functional in lymphoma.
In contrast to the sensitivity of lymphomas to the cytolytic activity of activated NK cells, 17 of the 18 cell lines and 3 of the 4 investigated primary lymphoma cells were either resistant to or only weakly sensitive to resting NK cells. The assessment of CD107, which is a membrane molecule of cytotoxic granules, on the cell surface has been shown to be an elegant way to detect cytotoxic degranulation.30 In a recent study, we could show that CD107 expression correlates well with NK cell cytotoxicity.28 By means of CD107 surface expression we have demonstrated that the low sensitivity of lymphoma cells to resting allogeneic NK cells was not due to resistance to cytotoxic pathways but due to their failure to induce degranulation of NK cells. In contrast, the 2 NK-sensitive AML cell lines K562 and HL-60 rapidly induced degranulation of resting NK cells. A potential explanation for the failure of lymphoma to induce degranulation of resting allogeneic NK cells may be a lack of ligands able to activate resting NK cells.39
In conclusion, our findings indicate that lymphoma cells in general are sensitive to lysis by cytotoxic effector cells. Neither expression of PI-9 nor overexpression of bcl-2 result in inhibition of perforin-dependent cytotoxic pathways. These observations encourage the further development of T-cell–based immunotherapeutic approaches to human lymphoma.
Prepublished online as Blood First Edition Paper, December 22, 2005; DOI 10.1182/blood-2005-07-2880.
Supported by a scholarship from the European Society for Medical Oncology (ESMO) (R.G.).
R.G. designed research, performed research, analyzed data, and wrote the paper; C.S. designed research, analyzed data, and wrote the paper; A.M.A., A.L., A.B., and I.-K.N. performed research; L.U. and E.T. designed research and provided material; and U.K. designed research and wrote the paper.
Presented in abstract form at the 19th annual meeting of the International Society for Biological Therapy of Cancer, San Francisco, CA, November 4-7, 2004.40
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal