Abstract
B-lymphocyte homeostasis and function are regulated by complementary actions of the TNFR family members TACI, BCMA, and BAFF-R, which are expressed by mature B cells. How these receptors are differentially activated is not entirely understood, because the primary ligand BAFF binds to all three. We searched for alternative ligands for TACI using recombinant TACI-Fc fusion protein as a probe and identified syndecan-2 as a new binding partner. TACI binding appears to require heparan sulfate posttranslational modifications of syndecan-2, because free heparin or pretreatment with heparitinase blocked the interaction. Syndecan-2 bound TACI but bound neither BAFF-R nor BCMA. Transfected cells expressing syndecan-2 activated signaling through TACI, as indicated by an NFAT-specific reporter. Syndecan-1 and syndecan-4 were also able to induce TACI signaling in a similar manner. This is the first identification of ligands that selectively activate TACI without simultaneously triggering BCMA or BAFF-R. This finding may help explain the alternative outcomes of signaling from this family of receptors in B cells.
Introduction
Members of the tumor necrosis factor receptor (TNFR) superfamily control numerous processes related to cell survival, death, or effector functions. The 3 TNFR members TACI, BCMA, and BAFF-R constitute a subfamily that controls the development and survival of B lymphocytes as well as antibody production (see Mackay et al1 and Bodmer et al2 for reviews). BAFF-R is a potent regulator of mature B-cell survival, as mutant mice lacking the gene display substantially reduced numbers of transitional-2 (T2) and later-stage B lymphocytes due to accelerated apoptosis.3-7 Conversely, TACI has been postulated to suppress B-cell proliferation or survival, because TACI knockout mice were found to have elevated numbers of mature B lymphocytes in spleen and blood.8,9 TACI was also shown to be essential for efficient TI-2 antibody production. The third receptor, BCMA, does not appear to be important for overall B-cell homeostasis10 but is required for optimal survival of plasma cells in mice.11
Two TNF homologs able to bind and activate these receptors have been described. The primary ligand, BAFF (also known as BLyS, zTNF4, TALL1, and THANK), exists in both soluble and cell-associated forms and interacts with all 3 receptors.12-23 BAFF is inducibly expressed by myeloid cells, most notably macrophages and dendritic cells.24,25 BAFF enhances B-cell proliferation and survival and increases antibody secretion. The importance of this ligand was suggested by overexpression studies in BAFF-transgenic mice, which develop autoimmune antibodies and symptoms of systemic lupus erythematosus apparently as a result of overstimulation of autoreactive B cells.18,26-28 Conversely, the complete knockout of the BAFF gene in mice leads to severe loss of mature B cells and deficiency of antibody production.29,30 BAFF is important in human diseases as well, because it is frequently elevated in certain autoimmune states and has been shown to function as an autocrine growth stimulator in several types of B-cell malignancies.31-36
The second known ligand, APRIL, is less abundantly expressed, except by tumor cells, and has been postulated to participate in autocrine growth of some cancers.37 APRIL can bind to both BCMA and TACI and appears to contribute to the process of IgA antibody production but has not been shown to regulate B-cell homeostasis.38
It has been puzzling that no unique ligand exists for TACI, especially given its effects on B-cell homeostasis that are opposite to those of BAFF-R and BCMA. Previous studies identifying BAFF and APRIL as potential ligands for TACI were done using the ligands as affinity probes. We hypothesized, therefore, that the reverse approach of using TACI itself as a probe might lead to the discovery of other ligands able to selectively initiate signaling through this receptor. In this study, using an expression cloning method, we identify the heparan sulfate proteoglycan syndecan-2 as a new binding partner for TACI.
Materials and methods
Cell culture and transfections
Jurkat and TAg Jurkat cells39 were maintained in RPMI 1640 supplemented with 10% FCS. 293T and 293 cells were propogated in DMEM containing 10% FCS. For transfections 107 TAg Jurkat cells were electroporated with 5 μg plasmid DNA, while 293T cells were transfected using SuperFect reagent (Qiagen, Valencia, CA), as per the manufacturer's instructions.
Synd2/Jurkat stable cell lines
Jurkat cells were electroporated with 5 μg Synd2/pEAK12 cDNA. Twenty-four hours after transfection, cells were split into pools and selected with 0.5 μg/mL puromycin. Positive pools were identified by their ability to bind TACI-Fc by flow cytometry.
Antibodies
The TACI antibody used is an IgG-purified rabbit polyclonal.40 Heparan sulfate-specific (10E4 epitope) antibodies were obtained from Seikagaku (Tokyo, Japan).
TACI-Fc and 0-Fc fusion proteins
A fusion gene encoding residues 1 to 165 of the extracellular domain of human TACI and the Fc portion of human IgG1 gene was constructed in the signal pIg-plus vector (Novagen, Madison, WI). Stable cell lines expressing either TACI-Fc or only the Fc portion of the construct (0-Fc) were produced in 293 cells. Cells were grown in serum-free medium supplemented with ITS-A (Invitrogen, Carlsbad, CA) for 4 to 5 days prior to supernatant collection. Proteins were purified from supernatants on protein A-Sepharose beads using standard methods. TACI-Fc and 0-Fc were dialyzed against PBS/10% glycerol. Preparations were judged to be at least 95% pure by Coomassie staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Figure 1A).
Flow cytometry
Adherent 293T cells were rapidly lifted from tissue culture flasks with 1 mM EDTA/PBS and immediately rinsed with complete medium. TAg Jurkat or 293T cells were incubated with either TACI-Fc or 0-Fc at 1 μg per 106 cells in complete medium for 30 minutes on ice. Cells were washed and incubated with a FITC-conjugated anti-Fc (1:200) (Jackson ImmunoResearch Labs, West Grove, PA) for an additional 30 minutes. To allow for the exclusion of dead cells from flow analyses, cells were stained with propidium iodide. Similarly, cells were stained with 1 μg heparan sulfate-specific antibodies per 106 cells, followed by FITC-conjugated anti-IgM (1:200) (Jackson ImmunoResearch Labs).
NFAT reporter assays
TAg Jurkat cells were electroporated with the NFAT-SEAP (secreted alkaline phosphatase) reporter together with expression plasmids encoding either full-length TACI, TACI C-107 (lacking the intracellular domain), or empty vector. Cells were permitted to recover for 24 hours prior to coculture with either 293T cells, Jurkat/Synd2 stable cell lines, untransfected Jurkat cells, or drug treatments, after which cells were incubated an additional 24 hours before assaying for SEAP as described by Bram et al.41
Library screening
A cDNA library from 293T cells was prepared in the mammalian expression vector pEAK12 by Edge Biosystems (Gaithersburg, MD). For the initial screen 109 TAg Jurkat cells were electroporated with the 293T cDNA library and permitted to recover for 48 hours, at which point the cells were incubated with 1 μg TACI-Fc per 106 cells. Cells were incubated with FITC-conjugated anti-Fc antibodies, and the top 2% of TACI-Fc-binding cells were collected by fluorescence-activated cell sorting (FACS). The cDNAs were extracted from these cells by Hirt preparation,42 amplified in Escherichia coli, and used for the following round of screening. After 4 rounds, the remaining TACI-Fc-binding cells were divided into several pools and the amplified cDNAs further screened to identify positive cDNA clones.
RT-PCR
Reverse transcription (RT) reactions were conducted using a SuperScriptII kit (Life Technologies, Gaithersburg, MD). RNA complementary to the cDNA was removed by the addition of RNase H. Quality of the cDNA was checked by amplifying a 450-bp fragment of G3PDH. The presence of BAFF was determined using primers BLYS-5′-3 (5′-GGCCCCAACCTTCAAAGTTCAAGTAGTGAT-3′) and BLYS-3′-2 (5′-GTAAGTAGGTCACAGCAGTTTCAATGCACC-3′) to amplify an 897-bp fragment, whereas APRIL transcripts were examined using april-S full (5′-AACCACAGCTGTTGGCAGGGTCCCCAGCTC-3′) and april-AS full (5′-ACCCACCCTGGTCTTCCAAGCTGGGAGCCA-3′) primers to amplify an 828-bp product. Reaction mixtures for polymerase chain reactions (PCRs) contained 3 μL RT mix, 2 mM MgCl2, 200 μM of each of the 4 dNTPs, 20 μM of each primer, and 2.5 units of Taq polymerase in 50-μL volumes. Amplification was performed under standard conditions with an annealing temperature of 60°C.
Coimmunoprecipitations
Tranfected 293T cells were rinsed in PBS and lysed with 1% NP-40 in PBS containing 0.1 U/mL aprotinin, 1 mM AEBSF, and 2 μg/mL leupeptin. Immunoprecipitates were incubated with either anti-FLAG M2-agarose (Sigma, St Louis, MO) or antidigoxin-agarose (an antibody isotype-matched control) (Sigma) for 2 hours at 4°C. Alternatively, immunoprecipitates were incubated with our TACI rabbit polyclonal antibody40 or rabbit IgG and subsequently with protein A-Sepharose. The beads were washed with fresh lysis buffer, resuspended in 1 × Laemmli sample buffer, and the extracts subjected to reducing SDS-PAGE. For immunoblotting the proteins were transferred to nitrocellulose membranes. These membranes were blocked with 5% milk in PBS and 0.1% Tween and incubated with either the TACI polyclonal antibody or FLAG antibody in blocking buffer. The membranes were subsequently incubated with peroxidase-linked anti-rabbit IgG (1:2000) or anti-mouse IgG (1:3000) (Zymed, South San Fransisco, CA). Immunoreactive proteins were detected by enhanced chemiluminescence (ECL) (Amersham, Arlington Heights, IL).
Heparin- and heparan-carbozone binding assay
To determine the ability of TACI to bind to heparan sulfate groups in the absence of the syndecan-2 core protein, we incubated human TACI-Fc or 0-Fc (5 μg) with 50 μL of either heparin-carbozone or heparan-carbozone agarose beads (Celsus Laboratories, Cincinnati, OH) at room temperature for 30 minutes. The beads were washed several times with PBS, resuspended in Laemmli sample buffer to elute bound proteins, and the eluate fractionated by reducing SDS-PAGE.
Proliferation assays
Mouse B cells. Splenic B cells were isolated from wild-type and TACI knockout mice8 using magnetic-activated cell separation (MACS) columns with CD45 (B220) beads (Miltenyi Biotec, Auburn, CA) and cultured in 96-well round-bottom microtiter plates at a density of 105 cells per well. Jurkat and Synd2/Jurkat cells were treated with 100 μg/mL mitomycin C (Sigma) for 45 minutes, rinsed, and permitted to recover in fresh medium for an additional 45 minutes. The B220 selected cells were cocultured with 3 × 105 Jurkat cells in the presence of 10 ng/mL IL-4 (PeproTech, Rocky Hill, NJ), 1 μg/mL anti-IgM F(ab′)2 (Jackson ImmunoResearch Labs), 100 ng/mL BAFF (PeproTech), 1 μg/mL neutralizing anti-CD40L (R&D Systems, Minneapolis, MN), alone or in combination.
Human B cells. Positively selected CD19 cells from the buffy coats of volunteer donors were cocultured with pEAK12/Jurkat or Synd2/Jurkat cells that had been irradiated with 30 Gy (3000 rad). Cell ratios were 1:3 (Jurkat to CD9). Cocultures were performed in the presence of 100 ng/mL BAFF (RDI, Concord, MA), 2 μg/mL anti-IgM F(ab′)2 (Biosource, Camarillo, CA), 100 ng/mL IL-4 (PeproTech), alone or in combination.
For all proliferation assays cells were incubated at 37°C in the presence of 5% CO2 for 5 days. Cultures were pulsed with 1 μCi (0.037 MBq) tritiated thymidine (3H-TdR; 5.0 Ci/mmol [185 GBq/mmol]; Amersham, Piscataway, NJ) for 18 hours prior to harvesting, and 3H-TdR incorporation levels were determined by liquid scintillation counting.
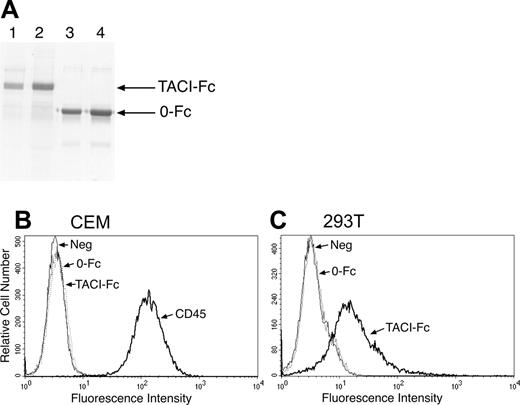
TACI-Fc binds specifically to 293T cells. (A) Recombinant proteins containing (TACI-Fc; lanes 1 and 2) or lacking (0-Fc; lanes 3 and 4) the extracellular domain of TACI fused to the Fc region of human IgG1 were purified as described in “Materials and methods.” Proteins were analyzed by SDS-PAGE and detected by Coomassie blue staining. (B) Cells were examined for expression of putative TACI ligands by incubation with TACI-Fc, the control protein 0-Fc, or an antibody to the CD45 molecule as a positive control. Antibody and Fc binding were detected by flow cytometry. Numerous cell lines tested, including CEM cells, did not bind TACI-Fc, whereas (C) 293T cells showed significant binding to TACI-Fc but not to 0-Fc.
TACI-Fc binds specifically to 293T cells. (A) Recombinant proteins containing (TACI-Fc; lanes 1 and 2) or lacking (0-Fc; lanes 3 and 4) the extracellular domain of TACI fused to the Fc region of human IgG1 were purified as described in “Materials and methods.” Proteins were analyzed by SDS-PAGE and detected by Coomassie blue staining. (B) Cells were examined for expression of putative TACI ligands by incubation with TACI-Fc, the control protein 0-Fc, or an antibody to the CD45 molecule as a positive control. Antibody and Fc binding were detected by flow cytometry. Numerous cell lines tested, including CEM cells, did not bind TACI-Fc, whereas (C) 293T cells showed significant binding to TACI-Fc but not to 0-Fc.
Results
TACI-Fc specifically binds to 293T cells
To identify selective ligands for TACI, we examined the ability of cells to specifically bind to a fusion protein consisting of the extracellular domain of the receptor fused to the Fc domain of human IgG1 (Figure 1A). Screening of various hematopoietic and epithelial cultured cell lines by flow cytometry indicated minimal amounts of binding by TACI-Fc (for example, CEM cells shown in Figure 1B). However, we did detect specific binding of the affinity probe to the transformed human kidney epithelial cell line 293T (Figure 1C). There was no binding of a control (0-Fc) protein, suggesting a specific interaction with the extracellular TACI portion of the fusion protein rather than to its Fc domain.
293T cells activate TACI-specific NFAT reporter
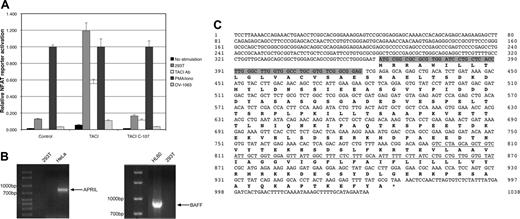
As an independent test for specificity of binding, we asked whether 293T cells could activate TACI-mediated signaling in a coculture assay.40 TAg Jurkat T cells were used as the assay system because they minimally bound TACI-Fc. TAg Jurkat cells were transfected with a full-length TACI expression plasmid and an NFAT-SEAP reporter and were then cultured on monolayers of 293T cells or the negative-control cell line OV-1063. Following overnight coculture, NFAT-specific activation was determined by SEAP quantitation. 293T cells induced a strong activation of NFAT in TACI-expressing Jurkat cells, while OV-1063 had no significant effect (Figure 2A). To further demonstrate that signaling was indeed transmitted through cross-linking of TACI, we tested a mutant expression construct of TACI (“C-107”) lacking the cytoplasmic tail of the receptor. Although expressed at levels equivalent to the full-length TACI (data not shown), this “tailless” TACI was unable to activate NFAT when stimulated either by antibody to the extracellular domain or by coculture with 293T cells (Figure 2A).
Expression cloning of syndecan-2
The 293T cells used did not express detectable levels of mRNA for the known TACI ligands BAFF or APRIL, as assessed by RT-PCR (Figure 2B). Thus, it became of interest to isolate from them a cDNA that might encode a TACI-binding cell surface protein. A cDNA library was prepared from 293T mRNA and inserted into the mammalian expression plasmid pEAK12 (Edge Biosystems), which includes the SV40 origin of replication to facilitate recovery of transiently transfected plasmids.43 For screening, library plasmids were transfected into SV40 large T-antigen-expressing (TAg) Jurkat cells and the resulting cells stained for ligand expression by incubation with TACI-Fc, followed by FITC-conjugated antibody to the Fc domain. Mock-transfected TAg Jurkat cells do not bind TACI-Fc (data not shown and Figure 3A) The brightest 2% of cells were purified by FACS and plasmid DNAs recovered using the Hirt preparation technique, followed by amplification in E coli. After 7 rounds of enrichment, several instances of a single cDNA clone were obtained. Sequence analysis revealed that the syndecan-2 open reading frame was expressed by the enriched plasmid (Figure 2C).
293T cells activate TACI signaling in a coculture assay. (A) To detect functional activation by a ligand on 293T cells, TAg Jurkat T cells were transiently transfected with an NFAT-SEAP reporter plasmid and a plasmid directing expression of full-length TACI (TACI), a truncated version lacking the cytoplasmic tail (TACI C-107), or empty vector (control). Equal aliquots of the transfected cells were treated as indicated, with PMA and ionomycin, providing an indication of the maximal activity of the NFAT reporter. For coculture, transfected TAg Jurkat cells were layered onto previously seeded and washed wells containing growing 293T or OV-1063 cells. NFAT-specific SEAP activity was determined and expressed as a fraction of PMA- and ionomycin-induced activity. 293T cells and TACI antibody induced significant activation of NFAT only in cells transfected with full-length TACI expression plasmid. (B) 293T cells do not express either BAFF or APRIL. mRNA was prepared from 293T cells and from known positive cell lines (HeLa for APRIL and HL-60 for BAFF), and RT-PCR was performed as described in “Materials and methods.” 293T cells do not contain detectable mRNA levels of either APRIL (left panel) or BAFF (right panel). (C) A 293T expression library was screened for binding partners with TACI-Fc and revealed syndecan-2 as a candidate. The extent of our syndecan-2 clone is shown here, where the region encoding the signal sequence is highlighted and the area coding for the transmembrane domain is underlined.
293T cells activate TACI signaling in a coculture assay. (A) To detect functional activation by a ligand on 293T cells, TAg Jurkat T cells were transiently transfected with an NFAT-SEAP reporter plasmid and a plasmid directing expression of full-length TACI (TACI), a truncated version lacking the cytoplasmic tail (TACI C-107), or empty vector (control). Equal aliquots of the transfected cells were treated as indicated, with PMA and ionomycin, providing an indication of the maximal activity of the NFAT reporter. For coculture, transfected TAg Jurkat cells were layered onto previously seeded and washed wells containing growing 293T or OV-1063 cells. NFAT-specific SEAP activity was determined and expressed as a fraction of PMA- and ionomycin-induced activity. 293T cells and TACI antibody induced significant activation of NFAT only in cells transfected with full-length TACI expression plasmid. (B) 293T cells do not express either BAFF or APRIL. mRNA was prepared from 293T cells and from known positive cell lines (HeLa for APRIL and HL-60 for BAFF), and RT-PCR was performed as described in “Materials and methods.” 293T cells do not contain detectable mRNA levels of either APRIL (left panel) or BAFF (right panel). (C) A 293T expression library was screened for binding partners with TACI-Fc and revealed syndecan-2 as a candidate. The extent of our syndecan-2 clone is shown here, where the region encoding the signal sequence is highlighted and the area coding for the transmembrane domain is underlined.
Syndecans are a family of transmembrane heparan sulfate proteoglycans, which have been implicated in diverse biologic processes, including motility, adhesion, proliferation, differentiation, and morphogenesis.44-46 They have been shown to interact functionally with ligands and with receptors; thus, a possible role for syndecan-2 as a TACI ligand was not without precedent. To verify that the isolated plasmid was the correct one, we tested transiently transfected TAg Jurkat cells for TACI-Fc binding activity. Unlike mock-transfected cells, syndecan-2-expressing Jurkat cells bind to TACI-Fc specifically (Figure 3A). There was no binding to the control protein 0-Fc or to the TNFR family members BCMA or BAFF-R (Figure 3A-B).
This experiment suggested that TACI can physically associate with syndecan-2 expressed on the surface of cells. An alternative interpretation, however, could have been that expression of syndecan-2 initiates (within the resident cell) a signal leading to synthesis and surface accumulation of an unrelated ligand able to bind to TACI. Our attempts to express recombinant syndecan-2 from mammalian cells (to direct appropriate posttranslational modifications) were unsuccessful. Therefore, we instead asked whether TACI and syndecan-2 could be coimmunoprecipitated. 293T cells were transfected with plasmid DNAs expressing TACI and FLAG-tagged syndecan-2, and lysates were immunoprecipitated using a monoclonal antibody to the FLAG epitope or an isotype-matched control antibody. Western blotting revealed specific association of TACI with syndecan-2, which was not evident in control lanes (Figure 3C). We conclude that TACI is able to specifically interact with syndecan-2.
TACI-Fc binds to syndecan-2. (A-B) To verify that syndecan-2 was the interacting protein from the 293T library, TAg Jurkat cells were transiently transfected with an expression plasmid containing the syndecan-2 cDNA. Cells were stained with TACI-Fc, 0-Fc, BCMA-Fc (A), or BAFF-R-Fc (B), as indicated, and analyzed by flow cytometry. Some transfected cells bound to TACI-Fc, as shown by the right-shifted peak. Transient transfection does not deliver DNA to all cells in the culture; thus, some of the curve overlaps that of mock-transfected cells. (C) TACI coprecipitates with FLAG-tagged syndecan-2. 293T cells were transiently transfected with plasmids expressing TACI and FLAG epitope-tagged syndecan-2 (top and bottom panels, lanes 1 and 2), or TACI and the parent plasmid pFLEX (top, lane 3), or control plasmid pBJ5 and FLAG-tagged syndecan-2 (bottom, lane 3). Immunoprecipitations were carried out with antibodies specific for the FLAG epitope on syndecan-2 or for TACI, or control antibodies as indicated. Coprecipitated proteins were detected by Western blotting with TACI (top panel) or FLAG (bottom panel) antibodies.
TACI-Fc binds to syndecan-2. (A-B) To verify that syndecan-2 was the interacting protein from the 293T library, TAg Jurkat cells were transiently transfected with an expression plasmid containing the syndecan-2 cDNA. Cells were stained with TACI-Fc, 0-Fc, BCMA-Fc (A), or BAFF-R-Fc (B), as indicated, and analyzed by flow cytometry. Some transfected cells bound to TACI-Fc, as shown by the right-shifted peak. Transient transfection does not deliver DNA to all cells in the culture; thus, some of the curve overlaps that of mock-transfected cells. (C) TACI coprecipitates with FLAG-tagged syndecan-2. 293T cells were transiently transfected with plasmids expressing TACI and FLAG epitope-tagged syndecan-2 (top and bottom panels, lanes 1 and 2), or TACI and the parent plasmid pFLEX (top, lane 3), or control plasmid pBJ5 and FLAG-tagged syndecan-2 (bottom, lane 3). Immunoprecipitations were carried out with antibodies specific for the FLAG epitope on syndecan-2 or for TACI, or control antibodies as indicated. Coprecipitated proteins were detected by Western blotting with TACI (top panel) or FLAG (bottom panel) antibodies.
Syndecan-2 does not bind to BAFF-R
It was important to determine whether or not syndecan-2 could also interact with the BAFF-R receptor, because the latter is thought to direct B-cell function and survival in a fashion opposite that of TACI. BAFF-R-Fc was prepared by subcloning the extracellular domain of the receptor (residues 1 to 71) into the pIg-plus plasmid and recombinant fusion protein prepared as was done for TACI-Fc. BAFF-R-Fc protein had the expected molecular weight, as judged by migration on SDS-PAGE (data not shown). BAFF-R-Fc bound to recombinant BAFF with similar affinity as TACI-Fc (data not shown). There was no detectable binding of BAFF-R-Fc to stably transfected syndecan-2-expressing Jurkat (referred to as Synd2/Jurkat) cells, however, indicating that TACI is the only receptor in this TNFR subfamily able to bind to syndecan-2 (Figure 3B).
TACI binding requires heparan sulfate chains of syndecan-2
Typically, interaction of proteins with syndecans requires the latter to be posttranslationally modified by addition of multiple heparan sulfate side chains.46 To test this requirement for TACI binding, we examined the ability of TACI-Fc to interact with Synd2/Jurkat cells in the absence or presence of heparin as competitor. Although addition of heparin did not interfere with an antibody binding to CD3 on the cell surface (data not shown), there was a complete loss of TACI-Fc binding (Figure 4A), suggesting the importance of heparan sulfate side chains in the interaction. Further consistent with this idea, preincubation of Synd2/Jurkat cells with heparinase or heparitinase to cleave heparan sulfate chains substantially reduced TACI-Fc binding (Figure 4B).
In the FGF system, syndecans are known to bind to both ligand and receptor and to be required for productive interaction of the two.47,48 Recent studies found that heparan sulfate proteoglycans, of which the syndecans are members, bound to the TNF homolog APRIL.49 We therefore asked if syndecan-2 might actually be binding and “presenting” a different protein as the true TACI ligand in this system. To rule out this possibility, we took advantage of the ability of heparin to abrogate TACI-Fc binding to Synd2/Jurkat cells. We reasoned that if an unknown ligand were actually contacting the heparan sulfate side chains of syndecan-2, washing Synd2/Jurkat cells with heparin would elute such an intermediary protein from the surface and decrease the amount of binding to TACI-Fc. However, we found that heparin-washed Synd2/Jurkats immediately recovered TACI-Fc binding activity after excess heparin was removed (Figure 4C). In addition, we excluded the possibility of any detectable APRIL remaining bound to our TACI-Fc by subjecting TACI-Fc to SDS-PAGE and performing a Western blot using APRIL antibody; no APRIL was detected (data not shown). Furthermore, TACI-Fc protein was efficiently retained on immobilized heparan sulfate and heparin columns, while 0-Fc control protein was not (Figure 4D). Together, these results argue that TACI interacts with heparan sulfate chains of syndecan-2.
TACI-Fc binding requires the heparan sulfate side chains of syndecan-2. (A) Parental Jurkat cells and stable Synd2/Jurkat cells were assayed for their ability to bind TACI-Fc in the presence or absence of heparin (as indicated). Addition of heparin completely blocked binding to syndecan-2-expressing Jurkat cells. (B) Treatment of Synd2/Jurkat cells with the heparan sulfate-cleaving enzymes heparinase or heparitinase reduced the ability of cells to bind TACI-Fc. Curves shown in panels A and B were analyzed by flow cytometry in the same experiment but are shown in separate panels for clarity. Negative and positive control curves (Jurkat and Synd2/Jurkat) in panels A and B are the same data. (C) Syndecan-2 does not bind a TACI ligand noncovalently. Synd2/Jurkats were incubated with heparin and then washed extensively to remove heparin and any putative ligands that might have been bound to cell surface syndecan-2. Washed Synd2/Jurkat cells recover their ability to bind TACI-Fc. (D) TACI-Fc binds directly to immobilized heparin or heparan sulfate. 0-Fc (lanes 3 and 4) or TACI-Fc (lanes 6 and 7) were mixed with carbozone resin conjugated to heparin (Hi) or heparan sulfate (Ha). Unbound fusion proteins were removed by extensive washing. Immobilized recombinant protein was subsequently eluted with SDS and analyzed by SDS-PAGE followed by Coomassie blue staining. Lanes 2 and 5 (labeled “S”) indicate the input amount of 0-Fc or TACI-Fc.
TACI-Fc binding requires the heparan sulfate side chains of syndecan-2. (A) Parental Jurkat cells and stable Synd2/Jurkat cells were assayed for their ability to bind TACI-Fc in the presence or absence of heparin (as indicated). Addition of heparin completely blocked binding to syndecan-2-expressing Jurkat cells. (B) Treatment of Synd2/Jurkat cells with the heparan sulfate-cleaving enzymes heparinase or heparitinase reduced the ability of cells to bind TACI-Fc. Curves shown in panels A and B were analyzed by flow cytometry in the same experiment but are shown in separate panels for clarity. Negative and positive control curves (Jurkat and Synd2/Jurkat) in panels A and B are the same data. (C) Syndecan-2 does not bind a TACI ligand noncovalently. Synd2/Jurkats were incubated with heparin and then washed extensively to remove heparin and any putative ligands that might have been bound to cell surface syndecan-2. Washed Synd2/Jurkat cells recover their ability to bind TACI-Fc. (D) TACI-Fc binds directly to immobilized heparin or heparan sulfate. 0-Fc (lanes 3 and 4) or TACI-Fc (lanes 6 and 7) were mixed with carbozone resin conjugated to heparin (Hi) or heparan sulfate (Ha). Unbound fusion proteins were removed by extensive washing. Immobilized recombinant protein was subsequently eluted with SDS and analyzed by SDS-PAGE followed by Coomassie blue staining. Lanes 2 and 5 (labeled “S”) indicate the input amount of 0-Fc or TACI-Fc.
Syndecan-2 activates TACI-mediated signaling
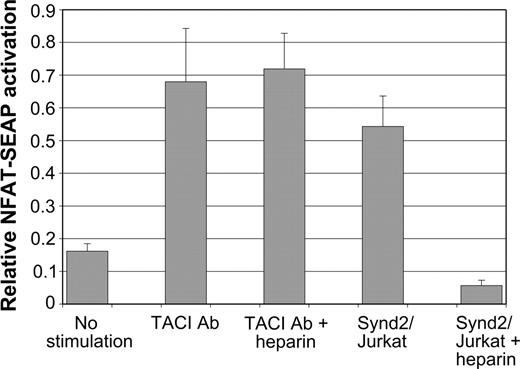
We next asked whether syndecan-2 triggers signaling through TACI. Synd2/Jurkat cells specifically stimulated TACI-expressing Jurkat cells transfected with an NFAT-SEAP reporter plasmid, while control Jurkat cells did not induce activation (Figure 5). NFAT activation appears to proceed through the usual calcineurin-dependent signaling pathway, because low levels (50 ng/mL) of cyclosporin A completely blocked activation (data not shown). Activation also depended upon the presence of syndecan-2 posttranslational modifications, because inclusion of competitor heparin within the coculture abrogated syndecan-2-induced activation (Figure 5). This property of heparin was not due to a nonspecific T-cell inhibition effect, because it did not block TACI antibody-induced NFAT activation (Figure 5).
TACI binding to other members of the syndecan family
Syndecan-2 is a member of the heparan sulfate proteoglycans, which also include syndecan-1 and syndecan-4. We wanted to determine whether or not these other members of the syndecan family could activate TACI. Consequently, the cDNAs for syndecan-1 and -4 were subcloned into the pEAK12 vector. Expression of these proteins on the surface of transiently transfected cells was determined by flow cytometry using heparan sulfate-specific antibodies as described in “Materials and methods.” Syndecan-1- and syndecan-4-expressing cells could bind to TACI-Fc, comparable to syndecan-2-expressing cells (Figure 6A). As holds true for syndecan-2, the other 2 syndecans had the ability to activate TACI-expressing TAg Jurkat cells transfected with an NFAT-SEAP reporter (Figure 6B).
Heparin does not interfere with BAFF binding to TACI
TNF homologs usually bind as homotrimers to the cysteine-rich ligand-binding domains of their cognate receptors.2 BAFF may be slightly atypical, because a recent study suggests that it forms a much larger viruslike supramolecular complex.50 It was thus of interest to determine whether or not syndecan-2 binding to TACI might interfere with BAFF binding. To test this, TACI-Fc was incubated with recombinant BAFF in the absence or presence of saturating levels of heparin (a condition sufficient to block TACI-Fc binding to syndecan-2-expressing Jurkat cells; Figure 4A). The fusion protein was purified by protein A-Sepharose chromatography, and copurified BAFF was detected by Western blotting. Addition of excess heparin did not diminish the degree of BAFF binding to TACI-Fc, suggesting there was not a significant degree of competition between the 2 ligands (Figure 6C).
Proliferation of B cells in response to syndecan-2
We next investigated the physiologic effect of syndecan-2 on primary B cells. We first compared the effects of syndecan-2 on the proliferation of wild-type versus TACI knockout B cells. Purified splenic B cells were cocultured with mitomycin C-treated Jurkat or Synd2/Jurkat cells. Coculture conditions included the addition of IL-4, anti-IgM, BAFF, anti-CD40L, alone or in combination, as indicated in Figure 7A. Wild-type or TACI knockout B cells, stimulated with IL-4 plus αIgM with or without αCD40L, together with either parental Jurkat or Synd2/Jurkat cells, demonstrated only nominal proliferation (Figure 7A). However, with the addition of BAFF, which optimizes B-cell growth conditions, the wild-type B cells demonstrated a considerable increase in proliferation in response to Synd2/Jurkat stimulation. Although TACI knockout B cells also proliferated at a higher rate in response to Synd2/Jurkat cells, this was considerably less than that seen for wild-type cells.
Consistent with these results, we also observed increased proliferation of purified human B cells when cocultured with irradiated Synd2/Jurkat cells compared with coculture with vector-transfected parental cells, in the presence of IL-4, BAFF, and/or antibody to surface IgM (Figure 7B).
Syndecan-2 activates NFAT signaling through TACI. TACI-mediated activation of an NFAT-SEAP reporter plasmid was assayed. TAg Jurkat cells were transfected with a TACI expression plasmid together with NFAT reporter. These cells were cultured in the absence of any stimulation (no stimulation), control Jurkat cells, TACI antibody, or Synd2/Jurkat cells in the presence or absence of competing heparin as indicated. Experiments demonstrate that NFAT activation by syndecan-2-expressing cells is blocked by the competitor heparin. TACI antibody-mediated activation is, however, not blocked by heparin.
Syndecan-2 activates NFAT signaling through TACI. TACI-mediated activation of an NFAT-SEAP reporter plasmid was assayed. TAg Jurkat cells were transfected with a TACI expression plasmid together with NFAT reporter. These cells were cultured in the absence of any stimulation (no stimulation), control Jurkat cells, TACI antibody, or Synd2/Jurkat cells in the presence or absence of competing heparin as indicated. Experiments demonstrate that NFAT activation by syndecan-2-expressing cells is blocked by the competitor heparin. TACI antibody-mediated activation is, however, not blocked by heparin.
Syndecan-1 and syndecan-4 can also bind to and activate TACI. (A) TAg Jurkat cells were electroporated with either empty vector (broken line) or one of the indicated syndecan expression plasmids (solid line). Binding of TACI-Fc to syndecan-expressing cells was determined by flow cytometry. TACI-Fc binds to syndecans-1, -2, and -4 similarly. (B) TAg Jurkat cells were transiently transfected with empty vector plus NFAT reporter plasmid or a TACI cDNA with reporter plasmid, or with syndecan cDNAs. The TACI/NFAT cells were cocultured with either no stimulants, empty vector, or syndecan-expressing cells. Transfection of Jurkat cells with TACI/NFAT alone results in basal activation.40 Syndecan-1 and syndecan-4 activated NFAT-SEAP reporter similarly to syndecan-2. Expression of the syndecans in TAg Jurkat cells was verified by flow cytometry using heparan sulfate antibodies (bottom panel). (C) Heparin does not interfere with BAFF binding to TACI. TACI-Fc was incubated alone or with BAFF in the absence or presence of saturating levels of heparin (10 μg/mL). TACI-Fc was purified from solution by passage over protein A-Sepharose, and adsorbed BAFF eluted with SDS, and then detected by Western blotting. Recombinant BAFF migrates as 2 bands on SDS-PAGE, as indicated by arrows.
Syndecan-1 and syndecan-4 can also bind to and activate TACI. (A) TAg Jurkat cells were electroporated with either empty vector (broken line) or one of the indicated syndecan expression plasmids (solid line). Binding of TACI-Fc to syndecan-expressing cells was determined by flow cytometry. TACI-Fc binds to syndecans-1, -2, and -4 similarly. (B) TAg Jurkat cells were transiently transfected with empty vector plus NFAT reporter plasmid or a TACI cDNA with reporter plasmid, or with syndecan cDNAs. The TACI/NFAT cells were cocultured with either no stimulants, empty vector, or syndecan-expressing cells. Transfection of Jurkat cells with TACI/NFAT alone results in basal activation.40 Syndecan-1 and syndecan-4 activated NFAT-SEAP reporter similarly to syndecan-2. Expression of the syndecans in TAg Jurkat cells was verified by flow cytometry using heparan sulfate antibodies (bottom panel). (C) Heparin does not interfere with BAFF binding to TACI. TACI-Fc was incubated alone or with BAFF in the absence or presence of saturating levels of heparin (10 μg/mL). TACI-Fc was purified from solution by passage over protein A-Sepharose, and adsorbed BAFF eluted with SDS, and then detected by Western blotting. Recombinant BAFF migrates as 2 bands on SDS-PAGE, as indicated by arrows.
Discussion
Using expression cloning, we have identified syndecan-2 as a new ligand for the TNFR family member TACI. Specificity of interaction was indicated by the lack of binding of control proteins (0-Fc, BCMA-Fc, and BAFF-R-Fc) to syndecan-2-expressing cells and by coimmunoprecipitation of syndecan-2 with full-length TACI. The conclusion is further supported by our finding that syndecan-2 appears able to initiate specific signaling in cells expressing the full-length wild-type TACI receptor and not in cells expressing a truncated TACI protein lacking the cytoplasmic signaling domain, nor in cells completely lacking TACI.
Typically, ligands of TNFRs have homology to the prototypic TNF itself; thus, this new relationship is somewhat unusual. There are, however, other examples of non-TNF ligands interacting with and inducing signaling through TNFR family members, such as the nerve growth factor and its receptor p75.51 Members of the syndecan family are expressed in many tissue types, with complex patterns of alternative or overlapping expression during tissue or cell development.52-55 Syndecan-2 is highly expressed in cells of mesenchymal origin, including fibroblasts and endothelial cells, whereas syndecan-1 and syndecan-3 are characteristic of neuronal and epithelial cells; syndecan-4 expression is widespread.56 Syndecans are inducibly expressed as the major cell surface heparan sulfate proteoglycan by macrophages following their activation by certain cytokines, including IL-1α or TNF-α, or by LPS.47 Macrophages are likely to be a highly relevant source of syndecan-2 in vivo with respect to TACI. The primary known ligand for TACI, BAFF, is likewise induced by activation of macrophages24 or dendritic cells.25 The particular types of cytokines that were shown to up-regulate syndecan-2 and BAFF in macrophages are overlapping but not precisely the same. Thus, it may be that fine-tuning of the lymphocyte response to infectious agents is in part mediated by differential or cooperative activation of TACI signaling through macrophage-synthesized ligands.
Syndecan-2 induces proliferation of B cells. (A) B220+ B cells from either wild-type (WT) or TACI knockout mice (KO) were cocultured with mitomycin C-treated Jurkat and Synd2/Jurkat cells at a ratio of 1:3 (105:3 × 105). Coculture conditions included 10 ng/mL IL-4, 1 μg/mL anti-IgM F(ab′)2, 100 ng/mL BAFF, 1 μg/mL anti-CD40L, alone or in combination. Cells were incubated for 5 days, at which time cultures were pulsed with 1 μCi (0.037 MBq) 3H-TdR for 18 hours before harvesting. Stimulation of both WT and TACI-KO B cells with IL-4 and αIgM with or without αCD40L, together with either parental Jurkat or Synd2/Jurkat cells, resulted in minimal proliferation. With the addition of BAFF, B-cell growth conditions became more optimal, and a significant increase in proliferation of WT B cells was seen in response to Synd2/Jurkat. TACI-KO B cells also proliferated at a higher rate in response to Synd2/Jurkat cells but considerably less than did WT B cells. Parental Jurkat and Synd2/Jurkat cells showed minimal proliferation after mitomycin C treatment (far right). (B) CD19+ cells from human donors were cocultured with irradiated pEAK12/Jurkat or Synd2/Jurkat cells. Coculture was performed in the presence of 100 ng/mL BAFF, 2 μg/mL anti-IgM F(ab′)2, 100 ng/mL IL-4, alone or in combination. Cells were pulsed with tritiated thymidine as in Panel A. In the absence of stimulation, or presence of BAFF, αIgM, or BAFF plus αIgM, B-cell growth is minimal in response to both pEAK12/Jurkat and Synd2/Jurkat cells. In the presence of IL-4, BAFF plus IL-4, or αIgM plus IL-4, B-cell growth increased significantly, particularly in response to Synd2/Jurkat cells.
Syndecan-2 induces proliferation of B cells. (A) B220+ B cells from either wild-type (WT) or TACI knockout mice (KO) were cocultured with mitomycin C-treated Jurkat and Synd2/Jurkat cells at a ratio of 1:3 (105:3 × 105). Coculture conditions included 10 ng/mL IL-4, 1 μg/mL anti-IgM F(ab′)2, 100 ng/mL BAFF, 1 μg/mL anti-CD40L, alone or in combination. Cells were incubated for 5 days, at which time cultures were pulsed with 1 μCi (0.037 MBq) 3H-TdR for 18 hours before harvesting. Stimulation of both WT and TACI-KO B cells with IL-4 and αIgM with or without αCD40L, together with either parental Jurkat or Synd2/Jurkat cells, resulted in minimal proliferation. With the addition of BAFF, B-cell growth conditions became more optimal, and a significant increase in proliferation of WT B cells was seen in response to Synd2/Jurkat. TACI-KO B cells also proliferated at a higher rate in response to Synd2/Jurkat cells but considerably less than did WT B cells. Parental Jurkat and Synd2/Jurkat cells showed minimal proliferation after mitomycin C treatment (far right). (B) CD19+ cells from human donors were cocultured with irradiated pEAK12/Jurkat or Synd2/Jurkat cells. Coculture was performed in the presence of 100 ng/mL BAFF, 2 μg/mL anti-IgM F(ab′)2, 100 ng/mL IL-4, alone or in combination. Cells were pulsed with tritiated thymidine as in Panel A. In the absence of stimulation, or presence of BAFF, αIgM, or BAFF plus αIgM, B-cell growth is minimal in response to both pEAK12/Jurkat and Synd2/Jurkat cells. In the presence of IL-4, BAFF plus IL-4, or αIgM plus IL-4, B-cell growth increased significantly, particularly in response to Synd2/Jurkat cells.
Our finding of a new ligand for TACI that is unable to bind to the other TNFR family members BAFF-R and BCMA may help explain the differential outcomes of signaling mediated by the 3 receptors. It has been somewhat puzzling that all bind to the most abundant ligand (BAFF) with equal affinities but with directly opposite functional outcomes in terms of antibody responsiveness and B-cell homeostasis. Syndecan-2 or related heparan sulfate molecules may therefore tip the balance of signaling toward or away from TACI depending upon the appropriate cellular context.
Studies recently performed demonstrate that heparan sulfate proteoglycans, of which the syndecans are members, are important for APRIL-induced tumor growth by mediating the binding of APRIL to tumor cells.57 In addition, the binding of APRIL to heparan sulfate proteoglycans results in the oligomerization of APRIL, which is required for the activation of TACI signaling.49 Other work has shown that heparan sulfate is a potent endogenous activator of the immune system.58,59 Numerous stimuli, including infection, tissue damage, general inflammation, and tumor invasion, induce the rapid cleavage and release of heparan sulfate fragments from the surfaces of cell. These heparan sulfate molecules can bind to Toll-like receptor 4 (TLR4) on dendritic cells and induce their maturation to more effectively present antigen. Although speculative at this point, we would propose the possibility that another downstream effector of heparan sulfate is TACI on B cells. This would fit well with the demonstrated importance of TACI in the TI-2 antibody response,8,9 which is one of the first lines of defense against infection with encapsulated bacteria.
Lastly, the possibility of “reverse signaling” is raised by the findings in this work. Although ligands traditionally are thought to activate signaling pathways via binding to receptors in a unidirectional mode, there are many examples of reverse-directional or bidirectional signaling, in which a known receptor triggers second messengers through interaction with the cognate ligand. The TNF family members TNF-α,60 and TRANCE61 are examples in which cross-linking of the ligand initiates signaling cascades within the ligand-expressing cell. Syndecan-2 has itself been implicated in signaling events, including regulation of the actin cytoskeleton.62 Thus, TACI may induce signaling events in syndecan-2-presenting cells as part of a reciprocal interaction between B cells and macrophages during the process of cytokine-induced antigen presentation.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-01-0256.
Supported by National Institutes of Health (NIH) grant CA76274, the Mayo Foundation, Joseph Bloom Children's Disease Research, and NIH training grant T32 HL-07910 (D.B.).
Two of the authors (G.v.B., R.J.B.) have declared a financial interest in a company (Zymogenetics) whose potential product (TACI-Ig) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal