CD4+ T-cell entry to the intestinal mucosa is central to the generation of mucosal immunity as well as chronic intestinal inflammation, yet the mechanisms regulating this process remain poorly defined. Here we show that murine small intestinal CD4+ lamina propria lymphocytes express a heterogeneous but restricted array of chemokine receptors including CCR5, CCR6, CCR9, CXCR3, and CXCR6. CD4+ T-cell receptor transgenic OT-II cells activated in mesenteric lymph nodes acquired a distinct chemokine receptor profile, including expression of CCR6, CCR9, and CXCR3 that was only partially reproduced in vitro after priming with mesenteric lymph node dendritic cells. A subset of these effector CD4+ T cells, expressing CD69 and α4β7, entered the intestinal lamina propria and the majority of these cells expressed CCR9. CCR9–/– OT-II cells were disadvantaged in their ability to localize to the intestinal lamina propria; however, they were readily detected at this site and expressed α4β7, but little CCR2, CCR5, CCR6, CCR8, CCR10, CXCR3, or CXCR6. Thus, whereas CD4+ T cells activated in gut-associated lymphoid tissue express a restricted chemokine receptor profile, including CCR9, targeting both CCR9-dependent and CCR9-independent entry mechanisms is likely to be important to maximally inhibit accumulation of these cells within the small intestinal mucosa.
Introduction
The intestinal lamina propria (LP) contains a large number of previously activated/memory CD4+ T cells that play a central role in intestinal immunity and in the induction and maintenance of chronic intestinal inflammation.1 T-cell entry into the intestinal mucosa is mediated by distinct sets of cell adhesion molecules expressed on the T cell and intestinal microvascular endothelial surface. Interaction between the gut-associated integrin α4β7, on the T-cell surface, with its ligand mucosal addresin cell adhesion molecule 1 (MAdCAM-1) on intestinal microvascular endothelium cells is important for T-cell entry into the LP.2-5 In addition, antibody neutralization studies have suggested a role for P-selectin and P-selectin glycoprotein ligand 1 (PSGL-1) in effector CD4+ T-cell entry to this site.5 T-cell entry into nonlymphoid tissues is also regulated by chemokine/chemokine receptors and the chemokine receptor CCR9 is required for efficient effector CD8+ T-cell localization to the small intestinal epithelium.6,7 However, since CCR9–/– mice have normal numbers of LP T cells,8,9 the particular role of CCR9 or additional chemokine receptors in CD4+ T-cell localization to the intestinal LP remains unclear.
The ability of T cells to enter nonlymphoid effector tissues is acquired following T-cell priming in secondary lymphoid organs and is mediated, in part, through the de novo expression of chemokine receptors.10,11 In vitro, the chemokine receptor profile induced following CD4+ T-cell priming is highly dependent on the culture conditions and the nature of antigen-presenting cells.12-14 In vivo, distinct subsets of B-helper T cells, characterized by their expression of CXCR5, and tissue inflammatory T cells, characterized by their expression of CXCR3, are rapidly and concurrently generated from naive CD4+ T cells in the lymph nodes (LNs).11 Moreover, recent studies have demonstrated a critical role for the LN environment for the induction of certain chemokine receptors. Thus CD8+ and CD4+ T cells are induced to express CCR9 or respond to the CCR9 ligand CCL25, respectively, after activation in mesenteric lymph nodes (MLNs) but not peripheral lymph nodes (PLNs).6,7,15 The induction of CCR9 and additional chemokine receptors during CD4+ T-cell priming in MLNs and the role of the LN environment in regulating induction of these receptors remains largely uncharacterized.
In the current study we have examined chemokine receptor induction during CD4+ T-cell activation in MLNs and their subsequent role during effector CD4+ T-cell entry to the intestinal LP. Our results demonstrate that CD4+ T cells primed in MLNs acquire a distinct and restricted set of chemokine receptors and that these cells use both CCR9-dependent and CCR9-independent mechanisms of entry into the small intestinal LP.
Materials and methods
Animals
C57BL/6J, C57BL/6J.Ly5.1 (Jackson Laboratory, Bar Harbor, ME, USA), OT-II, and CCR9–/–16 mice were bred and maintained at the BMC animal facility, Lund University, Sweden. CCR9–/– OT-II mice were generated by crossing OT-II × CCR9–/–C57BL/6J(F7) with CCR9–/–C57BL/6J(F7) mice. Ly5.2+Ly5.1+OT-II mice were obtained by crossing OT-II with C57BL/6J.Ly5.1 mice. All animals were used between 6 and 14 weeks of age. All experiments were performed with the permission of the local animal ethics committee.
Antibodies and reagents
Anti-CD4 (RM4-4, RM4-5, GK1.5), anti-Ly5.1 (A20), anti-α4β7 (DATK32), anti-CD62L (Mel-14), anti-CD44 (IM7), anti-CD69 (H1.2F3), isotype control antibodies, biotinylated mouse anti–rat IgG2a (RG7/1.30), mouse anti–rat IgG2b (G15-337), mouse anti–rat IgM (G53-238), and streptavidin-allophycocyanin (SA-APC) were from PharMingen (San Diego, CA). Anti-Ly5.2 (104) was from eBioscience (San Diego, CA), and anti-FcRII/III (2.4G2) was from American Type Culture Collection (Rockville, MD). Biotinylated goat anti–rat IgG, goat anti–rat IgM, and goat anti–rabbit IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA). The following antichemokine receptor antibodies were used during the course of this study: anti–mouse CCR2 (MC2117 ), anti–mouse CCR5 (MC6817 ), anti–mouse CCR6 (1CI218 ), anti–mouse CCR8 (8F419 ), anti–mouse CCR9 (5F220 ), anti–mouse CCR10 (6E10; Millennium Pharmaceuticals, Cambridge, MA), anti–mouse CXCR3 (4C47 ), anti–mouse CXCR6 (5C5; Millennium Pharmaceuticals), and polyclonal anti–mouse CCR9 (K62921 ). 7-amino-actinomycin D (7-AAD) was from Sigma-Aldrich (Steinheim, Germany). Both CCR9 antibodies gave similar staining on OT-II and wild-type (WT) CD4+ T cells and thus results obtained with these antibodies were combined.
Cell isolation and flow cytometry analysis
Cells were isolated from MLNs, PLNs (axillary, brachial, inguinal), spleen, liver, and small intestinal LP as previously described.6 Flow cytometry was performed essentially as previously described.6 Briefly, cells were incubated for 20 minutes in fluorescence-activated cell sorting (FACS) buffer containing anti–murine FcRII/III (2.4G2) antibody to block Fc receptors or 10% normal goat serum (NGS; Sigma-Aldrich) or 10% normal mouse serum (NMS; Sigma-Aldrich) where appropriate to prevent unspecific binding of the secondary antibody. Cells were incubated with saturating concentrations of primary antichemokine receptor antibody for 45 minutes, washed, and incubated with secondary biotin-conjugated antibodies for 20 minutes. After washing, cells were incubated with 10% normal rat serum (NRS; Sigma-Aldrich) for 15 minutes, and FITC- and PE-conjugated monoclonal antibodies (mAbs) were added together with SA-APC for 20 minutes. Dead cells were excluded from the analysis after staining with 7-AAD. Cells were analyzed on a flow cytometer (FACSCalibur; BD Biosciences Europe, Erembodegem, Belgium) equipped with CellQuest software (BD Biosciences). For calculating the percentage of chemokine receptor-positive cells, the percentage of cells staining with the isotype control antibody, or for CCR9 with the polyclonal CCR9 antibody that had been preincubated with the immunizing CCR9 peptide, were subtracted. All antichemokine receptor antibodies stained positive control cell populations using the staining protocol described.
Cell isolation, labeling, and adoptive transfers
Splenic or MLN dendritic cells (DCs) and CD4+ T cells were purified, after removal of red blood cells with Ficoll-Paque density centrifugation (Amersham Pharmacia Biotech, Uppsala, Sweden), by magnetic cell sorting using biotinylated anti-CD11c and anti-CD4 antibody, respectively, and streptavidin-coated beads according to the manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). For CFSE labeling, cells were incubated with CFSE (1 μM, Molecular Probes, Eugene, OR) in PBS for 8 minutes at room temperature.
For adoptive transfers, CD4+ T cells (2-6 × 106) were injected intravenously into recipient mice and 1 day later mice received intraperitoneal injections of 200 μL PBS containing OVA (500 μg, grade VI; Sigma-Aldrich) and LPS (100 μg, Escherichia coli, serotype 055:B5, Sigma-Aldrich). Transfer of CCR9–/– and WT OT-II cells positively selected with an anti-CD4 antibody showed a similar tissue distribution pattern and similar ratios in the LNs and intestinal LP as unselected OT-II cells (data not shown).
Immunohistochemistry
Cryostat-sectioned acetone-fixed small intestinal tissue slices (8 μm) were quenched with 0.5% H2O2, preincubated with 10% NMS (Sigma-Aldrich) in PBS, and blocked with an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) according to manufacturer's instructions. Sections were incubated for 1 hour with biotinylated anti-CD4 (GK1.5) antibody (2.5 μg/mL) in PBS containing 10% NMS, washed, and incubated for a further 1 hour with biotinylated mouse anti–rat IgG2b (2.5 μg/mL) in PBS containing 10% NMS. Sections were incubated with horseradish peroxidase-labeled streptavidin followed by biotinylated-conjugated tyramide using the tyramide signal amplification biotin system kit (PerkinElmer Life Science, Boston, MA). Finally, sections were incubated with SA-Alexa568 (Molecular Probes) for 1 hour, counterstained with DAPI (Molecular Probes), and mounted; images were acquired using an Olympus BX60 microscope (Olympus, Hamburg, Germany), equipped with a 40 ×/0.75 numeric aperture objective lens and an Olympus V-CMAD-2 camera. AnalySIS-Pro software version 3.2 (Soft Imaging System, Münster, Germany) was used for image processing. No staining was observed with an isotype-matched control antibody.
In vitro T-cell stimulation
Purified DCs were pulsed with OVA (1 mg/mL) for 2 hours at 37°C, washed extensively, and incubated together with CFSE-labeled OT-II cells in flat-bottom 96-well plates (1 × 105 DCs and 2 × 105 OT-II cells/well) in complete culture medium (RPMI supplemented with 10% FCS, 10 mM HEPES, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin, and 20 μg/mL gentamicin; Gibco BRL, Paisley, United Kingdom) at 37°C. After 3.5 days of coculture, cells were removed and chemokine receptor expression on responding OT-II cells determined by flow cytometry. For analysis of OT-II cell proliferation, OT-II cells (50 000 cells/well) were cultured in round-bottom 96-well plates together with a graded number of OVA-pulsed MLN DCs for 48 hours with 3H-thymidine (1 μCi [0.037 MBq]/well, Amersham Pharmacia Biotech) added during the last 16 hours.
Results
Small intestinal CD4+ LPLs express a restricted and heterogeneous array of chemokine receptors
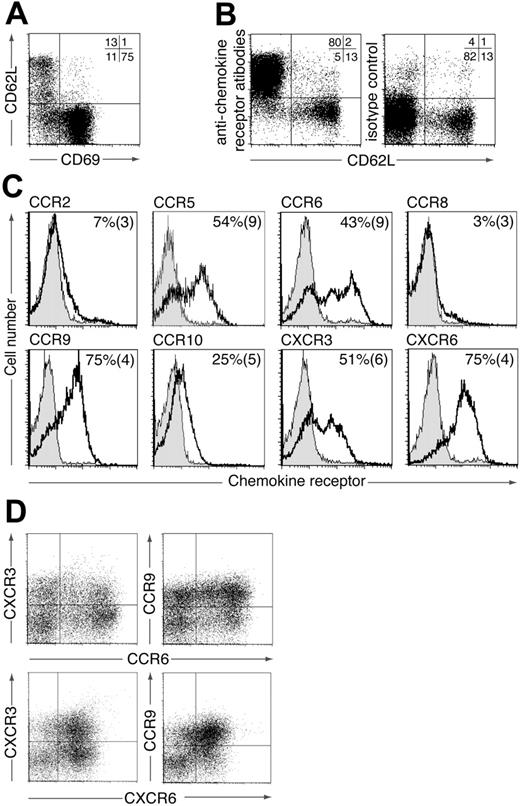
To identify potential chemokine receptors involved in the localization and function of CD4+ LP lymphocytes (LPLs), murine small intestinal LPLs were stained with a panel of antibodies to chemokine receptors previously found to be expressed on subsets of activated/memory T cells (CCR2, CCR5, CCR6, CCR8, CCR9, CCR10, CXCR3, CXCR6). The majority of CD4+ LPLs expressed CD69 (Figure 1A) and thus bore the phenotype of effector/memory T cells. In addition, a small but variable population of CD4+ LPLs were CD69–CD62Lhi (Figure 1A). These cells probably represent naive CD4+ T cells derived from contaminating blood or isolated lymphoid follicles (ILFs) within the LP,22 because they failed to stain with any of the chemokine receptor antibodies (Figure 1B). We therefore focused on identifying chemokine receptors expressed on CD69+CD4+ LPLs (Figure 1C). The large majority of CD69+CD4+ LPLs expressed CCR9 and CXCR6 and approximately 50% expressed CCR5, CCR6, and CXCR3 (Figure 1C). In contrast, CCR2 and CCR8 were expressed on less than 10% of cells. CD69+CD4+ LPLs also showed weak staining for CCR10; however, levels were extremely low compared with those found on IgA+ LP cells23 (data not shown). Similar weak staining for CCR10 was observed on naïve and activated CD4+ and CD8+ T cells (data not shown) indicating that this was nonspecific staining of the anti-CCR10 antibody compared with the isotype control. Dual staining for CCR6, CCR9, CXCR3, and CXCR6 demonstrated a wide heterogeneity in chemokine receptor expression on endogenous CD4+ LPLs (Figure 1D). Together these results implicate a potential role for CCR5, CCR6, CCR9, CXCR3, and CXCR6 in modulating the localization or function (or both) of small intestinal CD4+ LPLs.
Expression of chemokine receptors by murine CD4+ LPLs. Murine CD4+ small intestinal LPLs were stained with antibodies to (A) CD69 and CD62L or (B) CD62L and a pool of chemokine receptors (CCR2, CCR5, CCR6, CCR8, CCR9, CCR10, CXCR3, CXCR6). Numbers represent percentage of CD4+ LPLs in each quadrant and are from one representative experiment of 2 to 4 performed. (C) CD4+ CD69+ LPLs were stained with antichemokine receptor (blank) or isotype control antibody (shaded). Representative plots from one representative experiment of 3 to 6 performed. Numbers are the mean (SEM) from 3 to 6 experiments using pooled cells from 3 to 10 mice/experiment except CCR5 (1-4 mice/experiment). (D) CD4+ LPLs were stained with antibodies to 2 chemokine receptors. Results are from 1 representative experiment of 3 to 4 using pooled cells from 4 to 10 mice/experiment.
Expression of chemokine receptors by murine CD4+ LPLs. Murine CD4+ small intestinal LPLs were stained with antibodies to (A) CD69 and CD62L or (B) CD62L and a pool of chemokine receptors (CCR2, CCR5, CCR6, CCR8, CCR9, CCR10, CXCR3, CXCR6). Numbers represent percentage of CD4+ LPLs in each quadrant and are from one representative experiment of 2 to 4 performed. (C) CD4+ CD69+ LPLs were stained with antichemokine receptor (blank) or isotype control antibody (shaded). Representative plots from one representative experiment of 3 to 6 performed. Numbers are the mean (SEM) from 3 to 6 experiments using pooled cells from 3 to 10 mice/experiment except CCR5 (1-4 mice/experiment). (D) CD4+ LPLs were stained with antibodies to 2 chemokine receptors. Results are from 1 representative experiment of 3 to 4 using pooled cells from 4 to 10 mice/experiment.
Differential regulation of chemokine receptors during CD4+ T-cell activation in secondary lymphoid organs
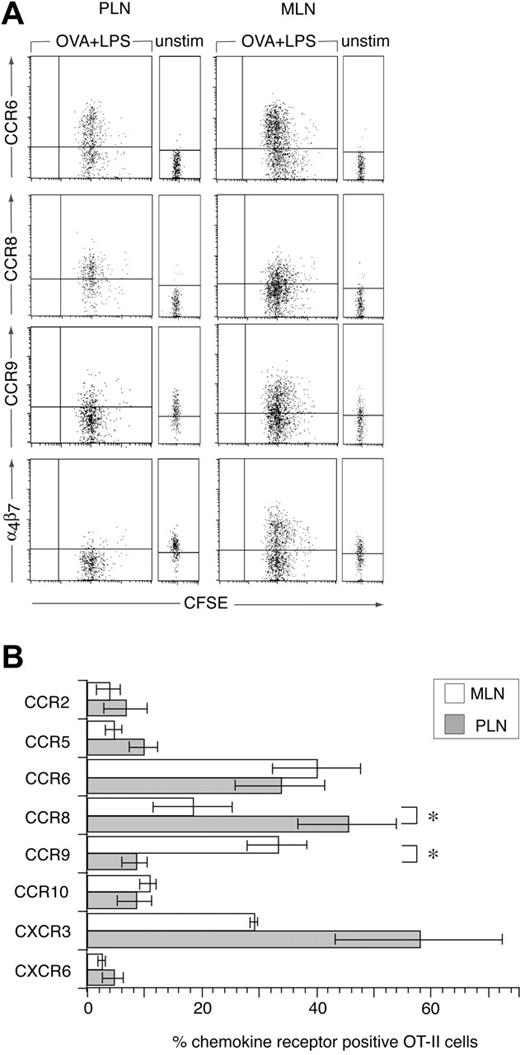
Because T cells enter intestinal effector sites after activation in secondary lymphoid organs, we used an OVA-specific T-cell receptor (TCR) transgenic CD4+ T cell (OT-II) adoptive transfer model to examine chemokine receptor induction during CD4+ T-cell priming in vivo. OT-II cells were labeled with CFSE and adoptively transferred into C57BL/6J.Ly.5.1 mice and the chemokine receptor profile on donor cells in MLNs and PLNs determined 2 days after immunization with OVA and LPS intraperitoneally, a time point where antigen-specific lymphocytes remain sequestered in LNs.15,24 Antigen was administered intraperitoneally to compare chemokine receptor induction on OT-II cells in the MLNs and PLNs within the same mice and at the same time. LPS was included as an adjuvant because we have previously demonstrated efficient generation of gut tropic effector CD8+ T cells in the MLNs after administration of antigen and adjuvant intraperitoneally.7 In the absence of stimulation, OT-II cells failed to express any of the chemokine receptors, with the exception of low levels of CCR9 (Figure 2A). CCR9 expression on adoptively transferred OT-II cells in the PLNs was consistently slightly higher than in the MLNs; however, we do not currently understand the reason for this (Figure 2A). The expression of CCR9 by naïve OT-II cells was surprising given that naïve nontransgenic CD4+ T cells fail to express this receptor.6 CD4+ DO11.10.RAG–/– cells also expressed low levels of CCR9 (data not shown), demonstrating that expression of this receptor is not specific to the OT-II TCR transgenic cells and is independent of endogenous TCR expression. Naïve splenic OT-II and DO11.10.RAG–/– cells failed to respond to CCL25 in chemotaxis assays (data not shown), indicating that levels of CCR9 expressed by these cells were insufficient to mediate chemotaxis.
Chemokine receptor induction on CD4+ T cells following their activation in MLNs and PLNs. (A-B) CFSE-labeled OT-II cells were injected into C57BL/6J.Ly5.1 mice and their expression of chemokine receptors in MLNs and PLNs was determined by flow cytometry, in the absence of or 2 days after intraperitoneal administration of OVA plus LPS. (A) Representative chemokine receptor staining (lines represent level at which < 2% cells stained with isotype-matched control antibody) and (B) mean (±SEM) chemokine receptor-positive cells among 7-AAD– Ly5.2+ OT-II cells. Results are from 3 to 5 experiments, using pooled cells from 3 mice/experiment. *P < .03, paired 2-tailed Student t test, for differences between priming in MLNs and PLNs.
Chemokine receptor induction on CD4+ T cells following their activation in MLNs and PLNs. (A-B) CFSE-labeled OT-II cells were injected into C57BL/6J.Ly5.1 mice and their expression of chemokine receptors in MLNs and PLNs was determined by flow cytometry, in the absence of or 2 days after intraperitoneal administration of OVA plus LPS. (A) Representative chemokine receptor staining (lines represent level at which < 2% cells stained with isotype-matched control antibody) and (B) mean (±SEM) chemokine receptor-positive cells among 7-AAD– Ly5.2+ OT-II cells. Results are from 3 to 5 experiments, using pooled cells from 3 mice/experiment. *P < .03, paired 2-tailed Student t test, for differences between priming in MLNs and PLNs.
Two days after immunization, OT-II cells had undergone a similar number of divisions in MLNs and PLNs (Figure 2A). CXCR3 and CCR6 were induced on OT-II cells activated in MLNs and PLNs, although the percentage of cells expressing CXCR3 in PLNs varied considerably (Figure 2A-B). CCR9 and α4β7 were induced on OT-II cells activated in MLNs but down-regulated on OT-II cells in the PLNs (Figure 2A-B). Down-regulation of CCR9 and α4β7 in the PLNs may require specific factors within the PLNs although OT-I cells down-regulate these receptors after activation with anti-CD3 and anti-CD28 antibody in vitro.7 In contrast to CCR9 and α4β7, CCR8, a chemokine receptor expressed on human skin resident T cells,25 was preferentially induced on OT-II cells in PLNs (Figure 2A-B). CCR2, CCR5, and CXCR6 were induced on less than 10% of OT-II cells in both MLNs and PLNs (Figure 2B). Finally OT-II cells stained weakly with the anti-CCR10 antibody in both MLNs and PLNs, in the absence or presence of immunization presumably due to some nonspecific staining of this antibody as described (Figure 2B). Together these results demonstrate that priming of CD4+ T cells in the MLNs induces the expression of a restricted and distinct panel of chemokine receptors on responding cells.
Induction of chemokine receptors on MLN DC–primed CD4+ T cells in vitro
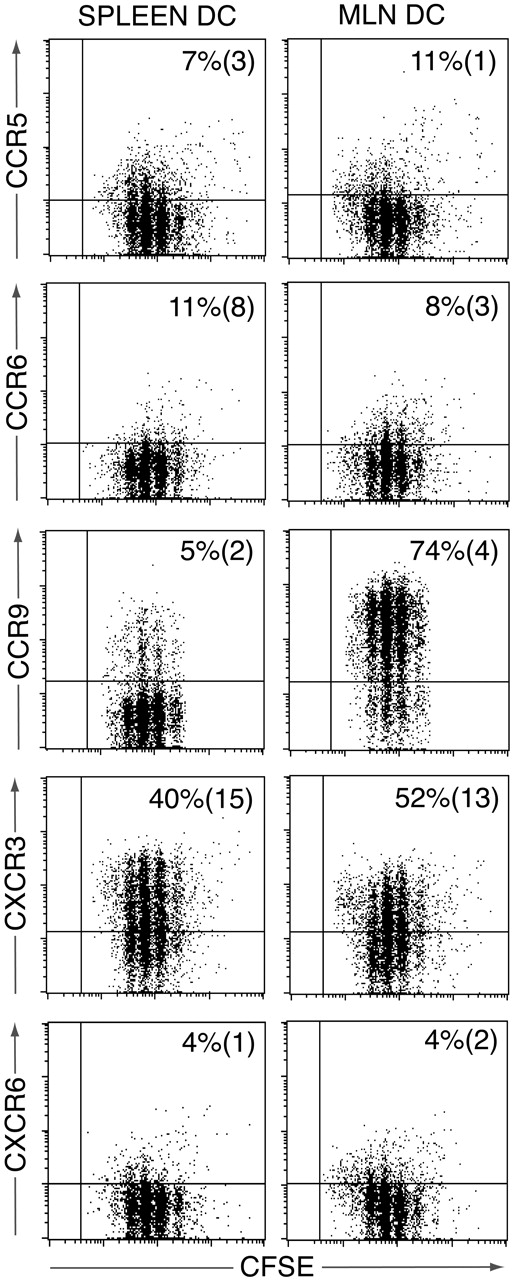
We have recently demonstrated a critical role for MLN DCs in the generation of CCR9+CD8+ effector T cells in MLNs.7 To determine whether the chemokine receptor profile adopted by activated OT-II cells in the MLNs could be reproduced using MLN DCs, CFSE-labeled OT-II cells were activated with OVA-pulsed MLNs or splenic DCs and receptor expression determined by flow cytometry 3.5 days later (Figure 3). A similar percentage of OT-II cells was induced to express CXCR3 after activation with MLN or splenic DCs, whereas CCR9 was induced on OT-II cells activated by MLN but not splenic DCs (Figure 3). In contrast, there was little induction of CCR2, CCR5, CCR6, CCR8, or CXCR6 (Figure 3 and data not shown). Similar results were obtained when OT-II cells were analyzed 6 days after activation (data not shown). Thus, whereas the chemokine receptor profile adopted by OT-II cells in MLNs was largely reproduced using MLN DCs as antigen-presenting cells, induction of CCR6 appears to require additional non–DC-derived signals within the LNs.
CCR9–/– CD4+ T cells exhibit normal homing capacity to MLNs and antigen-induced proliferation
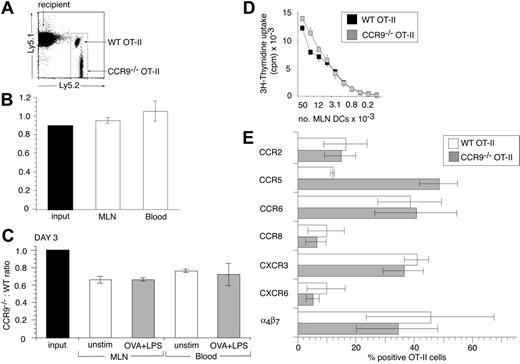
The selective induction of CCR9 on OT-II cells activated in MLNs, together with the expression of CCR9 on the majority of endogenous CD4+ LPLs, indicated that CCR9 may be involved in regulating CD4+ T-cell entry to the intestinal LP. To explore this possibility, CCR9–/– OT-II (Ly5.2+) cells and WT OT-II (Ly5.1+ Ly5.2+) cells were coinjected into C57BL/6J.Ly5.1 recipients and the ratio of CCR9–/– to WT cells determined in different organs. Both input populations contained a similar proportion of CD62L+ cells (CCR9–/– OT-II, 81% [SEM 2.3] and WT OT-II, 85% [SEM 2.7]) and small lymphocytes (CCR9–/– OT-II, 88% [SEM 1.5] and WT OT-II, 84% [SEM 2.6]). At 24 hours after adoptive transfer, the ratio of small CD62L+ CCR9–/– to WT OT-II cells in the blood and MLNs was similar to that of the input population (Figure 4A-B), demonstrating that CCR9 is not required for entry of these cells into the MLNs. To determine whether CCR9–/– and WT OT-II cells proliferated equally in response to antigen in vivo, mice received intraperitoneal injections of OVA plus LPS, and the ratio of CCR9–/– to WT OT-II cells was examined 3 days later. There was a slight but consistent reduction in the CCR9–/–/WT OT-II cell ratio in the MLNs and blood 3 days after cell transfer in the absence of antigen administration compared with the input population, when gating on the total OT-II cell population; however, we do not currently understand the reason for this (Figure 4C). Nevertheless, the CCR9–/–/WT OT-II cell ratio in the MLNs of mice that received OVA plus LPS was the same as in the MLNs of mice that had not received antigen (Figure 4C), indicating that CCR9–/– OT-II cells proliferated as efficiently as their WT counterparts in response to antigen stimulation in vivo. Consistent with this interpretation, CCR9–/– OT-II cells proliferated at least as efficiently as their WT counterparts in vitro after activation with OVA-pulsed MLN DCs, splenic DCs, or plate-bound anti-CD3/anti-CD28 antibody (Figure 4D and data not shown). Similar results were obtained using purified CD4+ T cells from nontransgenic CCR9–/– or WT mice stimulated with allogeneic DCs (data not shown).
Chemokine receptor induction on OT-II cells following activation with splenic or MLN DCs in vitro. CFSE-labeled OT-II cells were cultured with OVA-pulsed splenic or MLN DCs, and chemokine receptor expression assessed on responding OT-II cells 3.5 days later. Plots are from one representative experiment of 3 to 5 performed using pooled DCs from 5 to 10 mice/experiment. Numbers represent mean (SEM) of 3 to 5 experiments.
Chemokine receptor induction on OT-II cells following activation with splenic or MLN DCs in vitro. CFSE-labeled OT-II cells were cultured with OVA-pulsed splenic or MLN DCs, and chemokine receptor expression assessed on responding OT-II cells 3.5 days later. Plots are from one representative experiment of 3 to 5 performed using pooled DCs from 5 to 10 mice/experiment. Numbers represent mean (SEM) of 3 to 5 experiments.
To determine whether activation of CCR9–/– OT-II cells induced a similar chemokine receptor profile as observed with WT OT-II cells, chemokine receptor expression was examined on both cell populations in the MLNs, 2 days after administration of OVA plus LPS. CD62L+ CCR9–/– and WT OT-II cells in the input population failed to express CCR2, CCR5, CCR6, CCR8, CCR10, CXCR3, or CXCR6 (data not shown), whereas WT OT-II cells expressed low levels of CCR9 (as in Figure 2A). Following their activation in MLNs, CCR9–/– OT-II cells were induced to express similar levels of α4β7, CCR2, CCR6, CCR8, CXCR3, and CXCR6 as WT OT-II cells (Figure 4E). In contrast, CCR5 was induced on a greater proportion of CCR9–/– compared with WT-OT-II cells (Figure 4E). Thus, CCR9–/– OT-II cells adopt a similar chemokine receptor profile as WT OT-II cells after activation in MLNs but express higher levels of CCR5.
CCR9–/– OT-II cells are as efficient as WT OT-II cells in their homing to and proliferation within MLNs. (A-B) Short-term homing of CCR9–/– and WT OT-II cells into MLNs. CCR9–/– and WT OT-II cells were coinjected into C57BL/6J.Ly5.1 recipients and the CCR9–/–/WT OT-II cell ratio in the MLNs and blood assessed 24 hours later. (A) Representative flow cytometry analysis of WT and CCR9–/– OT-II cells in MLNs. (B) Ratio of small CD62Lhi CCR9–/– to WT OT-II cells in the input population, MLNs, and blood. Mean (SEM) of 3 mice from one representative experiment of 4 performed. (C) In vivo proliferation of CCR9–/– and WT OT-II cells. The CCR9–/–/WT OT-II cell ratio in the MLNs and blood in the absence of antigen (□) or 3 days after intraperitoneal administration of OVA plus LPS (▦). Mean (SEM) of 3 to 4 mice from one representative experiment of 2 performed. (D) In vitro proliferation of WT and CCR9–/– OT-II cells. WT (▪) and CCR9–/– (▦) OT-II cells were cultured together with the indicated number of OVA-pulsed MLN DCs. Mean (SD) of triplicate wells from one representative experiment of 3 performed. (E) Chemokine receptor expression on CCR9–/– and WT OT-II cells in MLNs, 2 days after intraperitoneal administration of OVA plus LPS. Mean (SEM) of 3 experiments using cells pooled from 2 to 3 mice/experiment except for CCR8, which is the mean of 2 experiments.
CCR9–/– OT-II cells are as efficient as WT OT-II cells in their homing to and proliferation within MLNs. (A-B) Short-term homing of CCR9–/– and WT OT-II cells into MLNs. CCR9–/– and WT OT-II cells were coinjected into C57BL/6J.Ly5.1 recipients and the CCR9–/–/WT OT-II cell ratio in the MLNs and blood assessed 24 hours later. (A) Representative flow cytometry analysis of WT and CCR9–/– OT-II cells in MLNs. (B) Ratio of small CD62Lhi CCR9–/– to WT OT-II cells in the input population, MLNs, and blood. Mean (SEM) of 3 mice from one representative experiment of 4 performed. (C) In vivo proliferation of CCR9–/– and WT OT-II cells. The CCR9–/–/WT OT-II cell ratio in the MLNs and blood in the absence of antigen (□) or 3 days after intraperitoneal administration of OVA plus LPS (▦). Mean (SEM) of 3 to 4 mice from one representative experiment of 2 performed. (D) In vitro proliferation of WT and CCR9–/– OT-II cells. WT (▪) and CCR9–/– (▦) OT-II cells were cultured together with the indicated number of OVA-pulsed MLN DCs. Mean (SD) of triplicate wells from one representative experiment of 3 performed. (E) Chemokine receptor expression on CCR9–/– and WT OT-II cells in MLNs, 2 days after intraperitoneal administration of OVA plus LPS. Mean (SEM) of 3 experiments using cells pooled from 2 to 3 mice/experiment except for CCR8, which is the mean of 2 experiments.
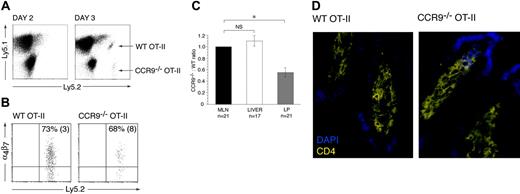
CCR9–/– OT-II cells are compromised in their ability to localize to the small intestinal LP
To determine whether CCR9–/– OT-II cells were deficient in their ability to localize to the small intestinal LP, the CCR9–/– and WT OT-II cells were cotransferred into recipient animals and their relative numbers determined in the MLNs, small intestinal LP, and liver. The liver was examined, since effector CD8+ T-cell accumulation within this organ is CCR9/CCL25 independent.6 In the absence of immunization, OT-II cells were not detected in the intestinal LP (data not shown), consistent with a requirement for prior activation in secondary lymphoid organs. OT-II cells (both WT and CCR9–/–) first appeared in the small intestinal LP 3 days after immunization with OVA plus LPS (Figure 5A). The mean number of OT-II cells isolated from the intestinal LP at this time was 3714 (324) cells/mouse (mean [SEM] of 8 mice from 2 experiments). A similar percentage of WT and CCR9–/– OT-II cells expressed α4β7 (Figure 5B), indicating that they both derived primarily from gut-associated lymphoid tissue (GALT). In comparing the CCR9–/–/WT OT-II cell ratio in the LP, MLNs, and liver, CCR9–/– cells showed a selective and significant disadvantage in their ability to localize to the intestinal LP compared with their WT counterparts (Figure 5C). Thus, CCR9 plays an important role in effector CD4+ T-cell localization to the intestinal LP. We were unable to detect sufficient numbers of transferred cells by immunohistochemistry to expose potential differences in the sublocalization of CCR9–/– and WT OT-II in the LP. Nevertheless, immunohistochemical analysis of the small intestine of CCR9–/– and WT OT-II mice clearly demonstrated that CCR9–/– OT-II cells are capable of gaining entry to villous LP (Figure 5D).
Role of CCR9 in OT-II cell recruitment to the small intestinal LP. (A) CCR9–/– and WT OT-II cells are first detected in the small intestinal LP 3 days after intraperitoneal administration of OVA plus LPS. (B) The majority of CCR9–/– and WT OT-II cells entering the small intestinal LP 3 days after intraperitoneal administration of OVA plus LPS express α4β7. Numbers represent mean (SEM) positive cells from 3 separate experiments. (C) The CCR9–/–/WT OT-II ratio was determined in the MLNs, liver, and small intestinal LP 3 days after intraperitoneal administration of OVA plus LPS. CCR9–/–/WT OT-II cell ratio was calculated by dividing the percentage of CCR9–/– OT-II cells with the percentage of WT OT-II cells in each organ. The ratios for liver and LP were normalized to the MLN ratio for each animal. Mean (SEM) CCR9–/–/WT OT-II ratio from 21 (MLNs), 17 (liver), and 21 (small intestinal LP) mice from 5 (liver) and 6 (LP) experiments, respectively. *P < .001, paired 2-tailed Student t test. NS indicates not significant. (D) CD4+ T cells within the small intestinal villous LP of CCR9–/– and WT OT-II mice as assessed by immunohistochemistry analysis.
Role of CCR9 in OT-II cell recruitment to the small intestinal LP. (A) CCR9–/– and WT OT-II cells are first detected in the small intestinal LP 3 days after intraperitoneal administration of OVA plus LPS. (B) The majority of CCR9–/– and WT OT-II cells entering the small intestinal LP 3 days after intraperitoneal administration of OVA plus LPS express α4β7. Numbers represent mean (SEM) positive cells from 3 separate experiments. (C) The CCR9–/–/WT OT-II ratio was determined in the MLNs, liver, and small intestinal LP 3 days after intraperitoneal administration of OVA plus LPS. CCR9–/–/WT OT-II cell ratio was calculated by dividing the percentage of CCR9–/– OT-II cells with the percentage of WT OT-II cells in each organ. The ratios for liver and LP were normalized to the MLN ratio for each animal. Mean (SEM) CCR9–/–/WT OT-II ratio from 21 (MLNs), 17 (liver), and 21 (small intestinal LP) mice from 5 (liver) and 6 (LP) experiments, respectively. *P < .001, paired 2-tailed Student t test. NS indicates not significant. (D) CD4+ T cells within the small intestinal villous LP of CCR9–/– and WT OT-II mice as assessed by immunohistochemistry analysis.
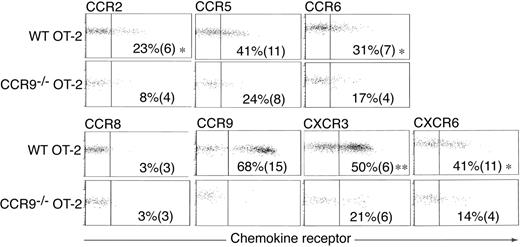
Chemokine receptor expression on OT-II cells entering the intestinal LP. Representative chemokine receptor staining on WT and CCR9–/– OT-II cells in the intestinal LP 3 days after intraperitoneal administration of OVA plus LPS. Numbers represent the mean (SEM) chemokine receptor-positive OT-II cells from 4 to 6 experiments except for CCR8 (3 experiments). *P < .03, **P < .001, paired 2-tailed Student t test for differences between WT and CCR9–/– OT-II cells.
Chemokine receptor expression on OT-II cells entering the intestinal LP. Representative chemokine receptor staining on WT and CCR9–/– OT-II cells in the intestinal LP 3 days after intraperitoneal administration of OVA plus LPS. Numbers represent the mean (SEM) chemokine receptor-positive OT-II cells from 4 to 6 experiments except for CCR8 (3 experiments). *P < .03, **P < .001, paired 2-tailed Student t test for differences between WT and CCR9–/– OT-II cells.
Chemokine receptor expression on CCR9–/– OT-II cells within the small intestinal LP
Because CCR5 was induced on a greater percentage of CCR9–/– OT-II cells than WT OT-II cells following their activation in MLNs, we determined whether CCR5 or other chemokine receptors may be compensating for the lack of CCR9 to mediate CCR9–/– OT-II cell migration to the LP, we examined chemokine receptor expression on adoptively transferred CCR9–/– and WT OT-II cells after their entry into the LP (Figure 6). The majority of WT OT-II cells that had entered the LP expressed CCR9 and CXCR3, and about 40% expressed low levels of CCR5 and CXCR6, whereas 23% and 31% expressed low levels of CCR2 and CCR6, respectively (Figure 6A). WT OT-II cells failed to express CCR8 (Figure 6A), and cells showed similar background staining with the anti-CCR10 antibody similar to that described in Figure 1C. CCR9–/– OT-II cells that had entered the intestinal LP expressed lower levels of CCR2, CCR5, CCR6, CXCR3, and CXCR6 than their WT counterparts (Figure 6A). Importantly CCR9–/– OT-II and WT OT-II cells in bulk LP preparations from these adoptive transfers failed to up-regulate these receptors after 3 hours of culture in vitro (data not shown) indicating that their lack of expression was not a result of ligand-induced receptor internalization.
Discussion
The entry of effector CD4+ T cells into the intestinal LP is critical to the generation of an effective mucosal immune response. Here we demonstrate that CD4+ T cells activated in MLNs express a highly restricted array of chemokine receptors and a subset of these cells, expressing the α4β7 integrin, enter the intestinal LP. CCR9 was required for maximal CD4+ T-cell localization to the small intestinal LP; however, α4β7+ CCR9–/– CD4+ T cells were clearly capable of entering this site. Remarkably the majority of CCR9–/– CD4+ T cells in the LP failed to express CCR2, CCR5, CCR6, CCR8, CCR10, CXCR3, or CXCR6. Together these results demonstrate for the first time the dynamics of chemokine receptor induction following CD4+ T-cell priming in MLNs and that effector CD4+ T cells generated in GALT use both CCR9-dependent and CCR9-independent mechanisms of entry to the small intestinal LP.
Although alterations in chemokine receptor expression following T-cell activation is thought to underlie the ability of an effector T cell to enter nonlymphoid tissues, little information is available regarding their induction following CD4+ T-cell priming in vivo. The current study compared the induction of a wide range of chemokine receptors on CD4+ T cells following their priming in MLNs and PLNs. Chemokine receptors could be broadly divided into 3 groups based on their pattern of induction in the LNs. The first group, consisting of CXCR3 and CCR6, were induced on CD4+ T cells primed in both MLNs and PLNs. Induction of CXCR3 on CD4+ T cells primed in PLNs has been observed previously,6,11 and this receptor was also induced on CD4+ T cells primed in vitro with splenic or MLN DCs. CCR6 was also recently shown to be induced on a subset of TCR transgenic CD4+ T cells following their adoptive transfer and activation in vivo, although the site of CCR6 induction was not clear because analysis was performed 9 days after immunization.26 Interestingly, CCR6 was not induced on CD4+ T cells after activation in vitro with MLN or splenic DCs, consistent with the reported inability of anti-CD3 antibody or monocyte-derived DCs to induce CCR6 on human CD4+ T cells.27,28 Thus, it appears that additional factors present in secondary lymphoid organs are required for CCR6 induction. The second group consisted of CCR9 and CCR8 that were selectively induced on CD4+ T cells activated in distinct LNs. The induction of CCR9 on CD4+ T cells in the MLNs and down-regulation of this receptor in the PLNs is consistent with previous results demonstrating enhanced responsiveness of MLN- but not PLN-primed CD4+ T cells to CCL25.15 In addition, MLN DCs but not splenic DCs were capable of inducing CCR9 on responding CD4+ T cells, as previously demonstrated for CD8+ T cells.7 In contrast to CCR9, CCR8 was preferentially induced on CD4+ T cells primed in PLNs. This finding is particularly intriguing in light of recent evidence that CCR8 may selectively target immune surveillance T cells to the human skin.25 The final group of receptors consisted of CCR2, CCR5, CCR10, and CXCR6 that were poorly induced on CD4+ T cells during their activation in LNs or after activation with MLN or splenic DCs. This latter finding is consistent with previous in vitro studies demonstrating induction of CCR2, CCR5, and CXCR6 on human CD4+ T cells only after prolonged culture and after multiple cell divisions.14,28 In one of these studies, plasmacytoid but not myeloid DCs were capable of inducing CXCR6, whereas CCR5 expression was enhanced when CD4+ T cells were primed under Th1 polarizing conditions.14 In this regard, it will be of considerable interest to determine chemokine receptor induction on CD4+ cells in vivo under Th1- and Th2-polarizing conditions, with different antigen doses, and using different antigen administration routes. Together our results demonstrate a considerable heterogeneity in chemokine receptor regulation during CD4+ T-cell priming in vivo and show that CD4+ T cells activated in MLNs are induced to express a highly restricted chemokine receptor profile that is distinct but partially overlapping with that induced on PLN-primed CD4+ T cells.
The majority of effector OT-II cells entering the intestinal LP expressed CCR9 and CCR9–/– OT-II cells were selectively disadvantaged in their ability to enter this site compared with WT OT-II cells. These results suggest that CCL25 is present within the intestinal LP at functionally relevant concentrations to mediate transendothelial CD4+ T-cell migration or at the intestinal microvascular endothelial surface to mediate effector CD4+ T-cell arrest. Consistent with this possibility, CCR9/CCL25 has recently been implicated in the recruitment of IgA-committed B cells into the small intestinal LP,9,29 and immunohistochemical analysis has shown CCL25 protein to be present on intestinal endothelial cells.29,30 Because CCL25 mRNA expression is restricted to the murine small intestinal epithelium,31,32 CCL25 protein detected at these sites likely derives from the epithelium.
While CCR9 was required for optimal CD4+ T-cell recruitment to the intestinal LP, the difference in CCR9–/–/WT OT-II ratio in the LP was not as dramatic as previously observed for effector CD8+ (OT-1) cell localization to the small intestinal epithelium.7 Thus, CCR9 appears to play a less dominant role in T-cell recruitment to the intestinal LP compared with the small intestinal epithelium. We were unable to determine whether CCR9–/– OT-II cell entry into the intestinal LP was dependent on signaling through Gαi, and thus potentially a chemokine receptor-dependent event, as the use of Gαi inhibitors, such as pertussis toxin, in this model would be expected to influence both CCR9–/– OT-II cell entry and exit from lymph nodes.33 CCR9–/– OT-II cells in the LP expressed little CCR2, CCR5, CCR6, CCR8, CCR10, CXCR3, and CXCR6, suggesting that entry into the LP occurs independently of these chemokine receptors. Indeed, CCR9–/– OT-II cells entering the intestinal LP expressed reduced levels of additional chemokine receptors compared with WT OT-II cells, indicating that T cells entering the LP in a CCR9-dependent fashion express greater levels of these receptors. However, while the chemokine receptor profile of CCR9–/– OT-II cells remained unaltered after culture of bulk LP preparations in vitro, since there were insufficient CCR9–/– OT-II cells in the LP to perform such analysis on purified populations, we cannot exclude the possibility that some of these receptors undergo ligand-induced down-regulation on entry into the LP in vivo. The exact role of these, or additional chemokine receptors, in mediating CCR9–/– OT-II entry into the intestinal LP will be important to determine.
Finally, a higher percentage of endogenous CD4+ LPLs expressed CCR5, CCR6, and CXCR6, and at higher levels, compared with recently activated OT-II cells in MLNs or effector OT-II cells that had entered the LP. These results suggest either that effector/memory CD4+ T cells entering the LP under alternative priming conditions express a different chemokine receptor profile or that the intestinal environment itself has a profound influence on the chemokine receptor profile adopted by resident T cells. Consistent with the latter possibility, CCR5, CCR6, and CXCR6 can be induced on human previously activated/memory CD4+ T cells by IL-15,34 a cytokine constitutively expressed in the intestinal mucosa.35 Furthermore, endogenous CD4+ LPLs from CCR9–/– mice express similar levels of these receptors as their WT counterparts (H. S., unpublished observations, March 2005). Because the CXCR6 ligand, CXCL16, and the CCR6 ligand, CCL20, are expressed in the intestine,36,37 these receptors may nevertheless be functionally relevant in the LP. In this regard, it will be of considerable interest to determine whether the heterogeneity in chemokine receptor expression on endogenous murine CD4+ LPLs reflects functional distinct subsets of cells and/or differences in the localization of these subsets.
In conclusion, in the current study we demonstrate that CD4+ T cells activated in MLNs are induced to express a distinct and restricted array of chemokine receptors and that they display CCR9-dependent and CCR9-independent entry into the intestinal LP. Our results suggest that it will be important to target both CCR9-dependent and CCR9-independent entry mechanisms to therapeutically inhibit CD4+ T-cell accumulation to the small intestinal mucosa. The challenge now is to identify the mechanisms of CCR9-independent CD4+ T-cell entry to the intestinal LP and to determine the relative contribution of each pathway in the setting of small intestinal inflammation.
Prepublished online as Blood First Edition Paper, January 3, 2006; DOI 10.1182/blood-2005-07-2860.
Supported by grants from the Swedish Medical Research Council, Crafoordska, Österlund, Åke Wiberg, Richard and Ruth Julins, Nanna Svartz and Kocks foundations, Royal Physiographic and Swedish Medical Society, Swedish Foundation for Strategic Research INGVAR II, and “Microbes and Man” programs.
H.S., A.E., and B.J.-L. performed the research; H.S., A.E., B.J.-L., M.S., and J.M. developed the in vivo and in vitro model systems; M.M., D.P., D.S., G.M., and M.B. provided important reagents; and H.S. and W.W.A. designed the study, analyzed the data, and wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr M.-J. Wick (Gothenburg University, Gothenburg, Sweden) for providing the OT-II mice and Drs A. Wurbel and B. Malissen (INSERM, Marseille, France) for providing the CCR9–/– mice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal