We describe the cloning and characterization of Siglec-H, a novel murine CD33-related siglec-like molecule with 2 immunoglobulin domains. Unlike other CD33-related siglecs, Siglec-H lacks tyrosine-based signaling motifs in its cytoplasmic tail. Although Siglec-H has the typical structural features required for sialic acid binding, no evidence for carbohydrate recognition was obtained. Specific monoclonal and polyclonal antibodies (Abs) were raised to Siglec-H and used to define its cellular expression pattern and functional properties. By flow cytometry, Siglec-H was expressed specifically on plasmacytoid dendritic cell (pDC) precursors in bone marrow, spleen, blood, and lymph nodes. Staining of tissue sections showed that Siglec-H was also expressed in a subset of marginal zone macrophages in the spleen and in medullary macrophages in lymph nodes. Using bone marrow-derived pDC precursors that express Siglec-H, addition of Abs did not influence cytokine production, either in the presence or absence of synthetic oligodeoxynucleotides containing unmethylated cytosine-guanine motifs (CpG). In comparison, Siglec-H functioned as an endocytic receptor and mediated efficient internalization of anti–Siglec-H Abs. By immunizing mice with ovalbumin-conjugated anti–Siglec-H Ab in the presence of CpG, we demonstrate generation of antigen-specific CD8 T cells in vivo. Targeting Siglec-H may therefore be a useful way of delivering antigens to pDC precursors for cross-presentation.
Introduction
Siglecs are sialic acid-binding immunoglobulin-like lectins expressed mostly in the immune system and are involved in both adhesive and signaling functions.1 A characteristic feature of siglecs is their lineage-restricted expression patterns. For example, sialoadhesin/Siglec-1 is expressed by tissue macrophage subsets,2 myelin-associated glycoprotein is found exclusively on myelinating glial cells,3 CD22 is a B cell–specific marker,4 and Siglec-8 is specifically expressed by eosinophils.5,6 The CD33-related siglecs are a distinct subset of molecules that are primarily expressed in the innate immune system and characteristically possess a cytoplasmic immune receptor tyrosine-based inhibitory motif (ITIM) and an ITIM-like motif that collectively mediate recruitment and activation of protein tyrosine phosphatases SHP-1 and SHP-2.1 Where studied, the CD33-related siglecs can mediate inhibitory signaling following co–cross-linking with activatory receptors7-10 and trigger apoptosis in the cases of Siglecs-8 and -9 expressed on eosinophils and neutrophils, respectively.11,12
There are significant differences in the repertoires of CD33-related siglecs among different mammalian species, including the great apes,13 and detailed phylogeny analyses indicate that this is a rapidly evolving subset of genes. Whereas there are 8 bona fide CD33-related siglecs in humans, only 5 were predicted following the completion of the mouse genome.14 Three of the murine proteins have the conserved ITIM and ITIM-like motifs present in the human CD33-related siglecs and have been designated Siglec-E, Siglec-F, and Siglec-G.14 Collectively, these siglecs are expressed on diverse cells of the innate immune system.15,16 The other 2 murine CD33-related siglecs are the mouse CD33/Siglec-3 ortholog expressed on neutrophils17 and an uncharacterized gene, Siglec-H. In this paper, we describe the molecular features, expression pattern, and properties of Siglec-H. We show that Siglec-H is expressed predominantly on a specialized subset of leukocytes, the plasmacytoid dendritic cell (pDC) precursors. Although Siglec-H has all the typical features required for sialic acid binding, we were unable to demonstrate recognition of sialylated glycoconjugates, but this receptor was shown to mediate efficient uptake of anti–Siglec-H antibodies (Abs) and is therefore likely to function in endocytosis. To investigate whether the endocytic properties and selective expression of Siglec-H on pDC precursors could be used to target antigens and prime the generation of specific CD8 T cells, we gave mice injections of ovalbumin (OVA)–anti–Siglec-H immunoconjugates. When combined with synthetic oligodeoxynucleotides containing unmethylated cytosineguanine motifs (CpG), a significant expansion of antigen-specific CD8 T cells was apparent compared with a preimmune IgG-OVA conjugate that was poorly immunogenic.
Materials and methods
Cloning of Siglec-H cDNA
Full-length Siglec-H cDNA was amplified by polymerase chain reaction (PCR) from mouse brain first-strand cDNA using Pyrococcus furiosus (P furiosus) polymerase and the following forward and reverse primers (5′ to 3′): ACTAAGCTTAGACAGGAGCCCAGGCCATC and GTGGATCCCTGGTGTAATGCC and cloned into pcDNA3. To identify proteins related to Siglec-H, we performed BLAST searches of the GenBank nucleotide and protein sequence databases (http://www.ncbi.nlm.nih.gov/BLAST/). Manipulations of sequences and alignments were performed using Baylor College of Medicine molecular biology software. The cDNA sequence encoding Siglec-H has GenBank accession number AAZ81614.
Production of Fc fusion proteins
To prepare Siglec-H-Fc recombinant protein, we amplified the region encoding the 2 extracellular immunoglobulin-like domains of Siglec-H by PCR from the full-length Siglec-H cDNA using the P furiosus polymerase and the following forward and reverse primers (5′ to 3′): ACTAAGCTTAGACAGGAGCCCAGGCCATC and ACTGGATCCACTTACCTGTGGTGACATTGAGCTGGATAG. The PCR product was cloned into the HindIII and BamHI sites of pEE14-hSiglec-9-3C-Fc18 to give pEE14-Siglec-H-3C-Fc, which was used to generate Siglec-H-Fc in transiently transfected COS cells. To derive a stable CHO cell line, we cloned a HindIII-BamHI fragment into the corresponding sites of pDEF-hSiglec-9-3C-Fc18 to give pDEF-Siglec-H-3C-Fc. CHO cells stably secreting Siglec-H-Fc at about 2 mg/L were selected with hygromycin. mCD33-Fc, mSiglec-E-Fc, mSiglec-F-Fc, and mSiglec-G-Fc fusion proteins were prepared as described.16 Fc chimeras were used either unpurified as tissue culture supernatants or following purification with protein A–Sepharose.
Preparation of monoclonal rat and polyclonal sheep anti–Siglec-H Abs
Lou rats (Harlan, Indianapolis, IN) were immunized intraperitoneally with Siglec-H-Fc. Briefly, rats were immunized 3 times with 50 μg immunogen in Titermax Gold (Cytrx, Norcross, GA) followed by a final boost with 50 μg immunogen in phosphate-buffered saline (PBS). After fusion of the spleen cells with Yb/20 myeloma cells, the hybridomas were screened by enzyme-linked immunosorbent assay (ELISA) on antigen-coated plates. MB15 (H009-2330) supernatant bound selectively to Siglec-H-Fc and the hybridoma was cloned twice and isotyped (BD Biosciences PharMingen, San Diego, CA). A sheep polyclonal antibody (pAb) was raised against purified Siglec-H-Fc protein following standard approved immunization protocols (Diagnostics Scotland, Edinburgh, United Kingdom). Sheep IgG was purified using protein G-Sepharose, anti–human Fc reactivity removed over a human IgG-Sepharose CL-4B column, and the sheep anti–Siglec-H IgG affinity purified using a Siglec-H-Fc-Sepharose CL-4B column as described previously.16 IgG was labeled with FITC, Alexa 488 (Molecular Probes, Invitrogen, Paisley, United Kingdom), or biotin (Pierce, Rockford, IL) following protocols recommended by the suppliers.
Characterization of Siglec-H+ cells by FACS analysis
Unless otherwise stated, all commercial Abs and conjugates were from BD Biosciences PharMingen. For characterization of Siglec-H+ cells using the MB15 monoclonal antibody (mAb), bone marrow, spleen, and peripheral lymph node cells from Balb/c, C57BL/6, C3H, DBA, and μH-deficient mice (Jackson ImmunoResearch Laboratories, Bar Harbor, ME) were incubated with 2.4G2 mAb to block Fc receptors and then labeled with either MB15 mAb or control rat IgG2a (R35-95) followed by biotin-labeled anti–rat IgG2a (RG7/1.30). Cells were then stained with CD45R/B220-APC (RA3-6B2) in combination with CD11b-FITC (M1/70) or CD11c-FITC (HL3) and streptavidin-PE. For characterization of Siglec-H+ cells with the sheep anti–Siglec-H pAb, Fc receptor-blocked cells were stained either with sheep anti–Siglec-H-FITC (5 μg/mL) or preimmune sheep Ig-FITC, and counterstained with CD4-allophycocyanin (APC; RM4-5, Caltag, Towcester, United Kingdom), CD8α-APC (5H10, Caltag), CD11c-biotin (N418, eBioscience, San Diego, CA), CD40-PE (3/23, Caltag), CD45R(B220)–biotin (RA3-6B2, eBiosience), CD80-biotin (B7-1, eBioscience), CD86-PE (PO3.1, eBioscience), or I-Ab-biotin (AF6-120.1, BD Biosciences). All biotinylated Ab stainings were followed by labeling with streptavidin-APC. Cells were finally resuspended in 7-amino actinomycin D (7-AAD) prior to fluorescence-activated cell sorting (FACS) analysis to gate out dead cells. Cells were acquired on a FACSCalibur (BD Biosciences, San Jose, CA), and the profiles analyzed using the CellQuest software (BD Biosciences).
Immunofluorescence labeling of cryostat sections
Acetone-fixed 7-μm cryostat sections of spleen and lymph nodes were stained with MB15 mAb (6 μg/mL) or rat IgG2a (6 μg/mL; eBR2a; eBioscience), followed by goat anti–rat FITC (Caltag). The sections were counterstained with CD4-biotin (5 μg/mL, GK1.5; eBioscience) or CD11c-biotin (5 μg/mL) followed by streptavidin-tetramethylrhodamine isothiocyanate (TRITC) (Serotec, Kidlington, United Kingdom), or rabbit antisialoadhesin followed by goat anti–rabbit IgG-TRITC (Sigma, Dorset, United Kingdom). For staining with sheep anti–Siglec-H immunoglobulin-biotin or preimmune sheep immunoglobulin-biotin, the sections were first blocked with 10% mouse serum and counterstained with ER-TR9 (BMA, Augst, Switzerland), followed by streptavidin–Texas red and rabbit anti–rat immunoglobulin-FITC (Dako, Glostrup, Denmark). For staining using sheep anti–Siglec-H immunoglobulin-FITC (5 μg/mL) or preimmune sheep immunoglobulin-FITC, the sections were first blocked with 5% sheep serum and counterstained with B220-biotin followed by streptavidin-TRITC or rabbit anti-Sn followed by goat anti–rabbit IgG-TRITC. Images of cryostat sections were taken using an Axiocam camera linked to an Axioskop microscope equipped with an Achroplan 40 ×/0.65 numeric aperture (NA) or Planar Neofluar 10 ×/0.30 NA objective and a 10 ×/20 eyepiece (all from Carl Zeiss, Thornwood, NY). Images were then analyzed using Zeiss AxioVision 3.1.
Cytokine measurements
Bone marrow–derived pDC precursors were obtained following culture with FMS-like tyrosine kinase 3 ligand (Flt-3-L) as described.19 On day 10 to14 of culture, Siglec-H+ cells were selectively labeled with Ly6c-biotin (AL-21; BD Biosciences) followed by streptavidin-APC and B220-PE (RA3-6B2; BD Biosciences) and purified by FACS using a FACSVantage (Becton Dickinson, San Jose, CA). The purified cells were then incubated in tissue culture medium at 37°C with no Ab, MB15 mAb (10 μg/mL), or sheep anti–Siglec-H IgG (10 μg/mL) either in the absence or the presence of 10 μM CpG 166819 for 18 hours and the tissue culture supernatants were harvested for cytokine analysis using the BD Cytometric Bead Array (Mouse Inflammation Kit; BD Biosciences).
Internalization assays
pDC precursor–enriched primary bone marrow cells were used for studying Siglec-H internalization by FACS or by DeltaVision deconvolution microscopy. Following erythrocyte lysis, mouse bone marrow cells were immunomagnetically depleted of CD11b+, CD19+, or TER119+ cells using an autoMACS (Miltenyi Biotec, Bisley, United Kingdom) followed by removal of adherent monocytes by a 20-minute incubation on tissue culture flasks. For FACS analysis of internalization, pDC precursor–enriched cell suspensions were incubated on ice for 1 hour with sheep preimmune IgG, sheep preimmune F(ab′)2, sheep anti–Siglec-H IgG, or sheep anti–Siglec-H F(ab′)2 at 10 μg/mL, either labeled or not with Alexa 488. The cells were washed and then either kept on ice or incubated for 3 hours at 37°C. The levels of anti–Siglec-H IgG remaining at the cell surface were detected using donkey anti–sheep IgG-FITC (Jackson ImmunoResearch Laboratories). Cells were counterstained with B220-PE and 7-AAD and subjected to FACS analysis. To measure the time course of anti–Siglec-H internalization, we incubated total bone marrow cells on ice for 1 hour with 20 μg/mL biotinylated sheep anti–Siglec-H or biotinylated mPDCA-1 (Miltenyi Biotec), washed, and either kept on ice or incubated for varying periods of time at 37°C and then placed back on ice. After 3 hours, all cell samples were stained with streptavidin-APC, B220-PE, and CD11c-FITC and cells analyzed by FACS. The median fluorescent values of Siglec-H+ B220+ cells were determined and used to calculate the percent remaining anti–Siglec-H IgG at the surface, with the 3-hour ice-incubated cells taken as 100%. For deconvolution restoration microscopy, 4 × 105 pDC precursor–enriched bone marrow cells were stained on ice for 1 hour with preimmune sheep IgG-Alexa 488 (10 μg/mL), sheep anti–Siglec-H IgG-Alexa 488, preimmune sheep F(ab′)2-Alexa 488, or sheep anti–Siglec-H F(ab′)2-Alexa 488, in PBS containing 0.5% BSA (Sigma). The cells were then either kept on ice or incubated at 37°C for 3 hours and then attached to poly-l-lysine–coated slides for 30 minutes on ice. This was followed by membrane staining with 5 μg/mL cholera toxin subunit B-Alexa 594 (Molecular Probes), washing with PBS, and fixation with 4% paraformaldehyde for 10 minutes at room temperature. Finally, the cells were permeabilized with 0.05% saponin for 15 minutes and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Z stack images of single cells were acquired using a Coolsnap HQ camera (Photometrics, Tucson, AZ) linked to an Olympus IX70 inverted microscope (Olympus, Melville, NY), a DeltaVision deconvolution system (Applied Precision, Issaquah, WA) using an Olympus 100 ×/1.4 NA oil immersion objective, and oil with refractive index of n = 1.514 (Cargille Laboratories, Cedar Grove, NJ). Images were deconvoluted and analyzed using softWoRx 3.4.0 (Applied Precision).
Targeting of Siglec-H+ cells in vivo
Mice received intravenous injections of 50 μg sheep anti–Siglec-H-FITC or 50 μg sheep anti–Siglec-8-FITC (negative control). After 3 hours, the mice were humanely killed and the bone marrow cells and splenocytes were Fc receptor blocked and stained with B220-PE in combination with either CD11c-biotin or Ly6c-biotin. This was followed by incubation with streptavidin-APC and 7-AAD and the cells analyzed by 4-color flow cytometry.
Preparation of anti–Siglec-H-OVA immunoconjugate and in vivo priming of OVA-cytotoxic T lymphocytes
OVA-coupled immunoconjugates were prepared as described.20 Briefly, purified sheep anti–Siglec-H IgG or preimmune IgG was reduced using 2-mercaptoethanolamine/HCl and separated from the reducing agent over a desalting column. The reduced IgGs were conjugated to maleimide-activated OVA (Pierce) according to the manufacturer's instructions and the IgG-OVA conjugates were purified over a protein G-Sepharose column and concentrated. Conjugation was judged to have gone to completion as evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with anti-OVA Abs (data not shown). The OVA-conjugated anti–Siglec-H IgG reacted with pDCs in an indistinguishable manner from the unconjugated Ab when analyzed by flow cytometry (data not shown). To prime OVA257-264-specific CD8 T cells, we immunized C57BL/6 mice intravenously with 15 μg OVA-conjugated anti–Siglec-H IgG or preimmune IgG in the presence or absence of 200 μg CpG 2216 (Coley Pharmaceutical, Wellesley, MA). After 7 days, mice were boosted by intravenous injection of 106 P furiosus UV-inactivated recombinant vaccinia virus encoding the full length OVA protein. Seven days after vaccinia boosting, antigen-specific T cells were enumerated in the blood by tetramer staining, as described.21
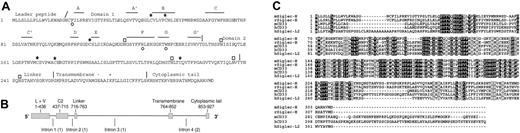
Molecular features of Siglec-H. (A) Predicted protein sequence of Siglec-H. Asterisks indicate positions of the 3 cysteine residues in domain 1 that are characteristic of siglecs. Open circles underlie residues in Siglec-H that are predicted to be important for sialic acid binding, including the critical arginine on the F β-strand.22 Potential N-linked glycosylation sites are shown by open boxes. The β-strands (A, A′, B, and C etc) shown in domain 1 are predicted by alignment with hSiglec-7.23 The slanted line indicates the predicted leader peptide cleavage site and the vertical lines show positions of intron-exon boundaries deduced from comparisons of the cDNA and gDNA sequences. Domains 1 and 2, the linker, the transmembrane region, and cytoplasmic tail are indicated. (B) Genomic organization of Siglec-h. Exons are shown as filled bars and introns as lines. L indicates leader peptide; V, domain 1 (V-set immunoglobulin-like); and C2, domain 2 (C2-set immunoglobulin-like). Numbers underlie nucleotide residues in cDNA, beginning at the start codon. Numbers in brackets indicate intron phases. (C) Alignment of the mouse Siglec-H with the closest matching rat, mouse, and human siglecs and a human siglec-like sequence. Identical residues are boxed in black and similar residues in gray. Accession numbers are as follows: mSiglec-H, AAZ81614; rSiglec-H, XP_341867; mCD33, AAB30843; hCD33, AAA51948; and hSiglec-L2, XP_290822.
Molecular features of Siglec-H. (A) Predicted protein sequence of Siglec-H. Asterisks indicate positions of the 3 cysteine residues in domain 1 that are characteristic of siglecs. Open circles underlie residues in Siglec-H that are predicted to be important for sialic acid binding, including the critical arginine on the F β-strand.22 Potential N-linked glycosylation sites are shown by open boxes. The β-strands (A, A′, B, and C etc) shown in domain 1 are predicted by alignment with hSiglec-7.23 The slanted line indicates the predicted leader peptide cleavage site and the vertical lines show positions of intron-exon boundaries deduced from comparisons of the cDNA and gDNA sequences. Domains 1 and 2, the linker, the transmembrane region, and cytoplasmic tail are indicated. (B) Genomic organization of Siglec-h. Exons are shown as filled bars and introns as lines. L indicates leader peptide; V, domain 1 (V-set immunoglobulin-like); and C2, domain 2 (C2-set immunoglobulin-like). Numbers underlie nucleotide residues in cDNA, beginning at the start codon. Numbers in brackets indicate intron phases. (C) Alignment of the mouse Siglec-H with the closest matching rat, mouse, and human siglecs and a human siglec-like sequence. Identical residues are boxed in black and similar residues in gray. Accession numbers are as follows: mSiglec-H, AAZ81614; rSiglec-H, XP_341867; mCD33, AAB30843; hCD33, AAA51948; and hSiglec-L2, XP_290822.
Results
Cloning and molecular features of Siglec-H
A partial cDNA-encoding Siglec-H was amplified from mouse brain cDNA during a search for novel murine siglec-like genes as described previously16 and a full-length clone was isolated from a murine spleen cDNA library. Siglec-H cDNA is predicted to encode a type 1 membrane protein of 309 amino acid residues composed of an N-terminal leader peptide, a V-set immunoglobulin-like domain, a C2-set immunoglobulin-like domain, a short linker, a transmembrane region and a short cytoplasmic tail (Figure 1A). Siglec-h maps to mouse chromosome 7B4 in a region that is syntenic with human chromosome 19q13 where human CD33-related siglecs are located. Examination of the completed mouse genomic sequence showed that Siglec-H cDNA is encoded by 5 exons (Figure 1B).
Siglec-H has all the characteristic features of siglecs, including the essential arginine at position 125 (β strand F) that is expected to interact with the carboxyl group of sialic acid, the aromatic amino acids phenylalanine immediately before strand A and tryptophan within strand G that are predicted to make hydrophobic contacts with sialic acid, and the unusual pattern of cysteines in domains 1 and 2 that form intra-β sheet and interdomain disulfide bonds22,23 (Figure 1). Siglec-H is predicted to have a basic amino acid residue(K) and an acidic residue (E) within its transmembrane region. This unusual feature is also shared by mCD33, but not by other siglec or siglec-like molecules (Figure 1C).
Blast searches of the human, rat, chimp, cow, pig, dog, sheep, and cat genome sequence databases identified a clear-cut rat Siglec-H ortholog sharing 69% sequence identity, but the closest matches in the other databases were CD33 orthologs (∼42% identity) and a previously uncharacterized 2-immunglobulin domain human siglec-like sequence, designated hSiglec-L2 (42% identity; Figure 1C). The gene encoding hSiglec-L2 was originally reported as a pseudogene (Siglec-P3),14 but the identification of an alternative upstream and in-frame start codon suggests this gene may code an authentic protein. However, hSiglec-L2 has a tryptophan residue instead of the essential arginine required for sialic acid binding. In addition, this predicted protein also has a lysine residue within its transmembrane region, suggesting it may function as an activatory receptor. Interestingly, the rat Siglec-H ortholog also lacks the critical arginine, being replaced by a serine residue.
Sialic acid-binding analysis of Siglec-H
Siglec-H-Fc recombinant protein was used to investigate the potential sialic acid-binding capacity of Siglec-H. The protein was used either purified or not purified, produced in COS cells or CHO cells, and either treated or not treated with sialidase to remove potentially inhibitory sialic acids. All other siglecs characterized previously in our laboratory were found to mediate sialic acid-dependent binding to human red blood cells (RBCs) in solid-phase assays, but no RBC binding to Siglec-H-Fc was observed under any conditions. Similarly, no significant sialic acid binding was detected in binding assays with sialylated-polyacrylamide conjugates that bind avidly to other siglecs (data not shown). Finally, Siglec-H-Fc protein was submitted to the Glycan array Core of the Consortium for Functional Glycomics but no carbohydrate binding was observed, in contrast to several other CD33-related siglecs. In summary, it seems unlikely that Siglec-H is a sialic acid-binding protein.
Characterization of Siglec-H expression by FACS analysis
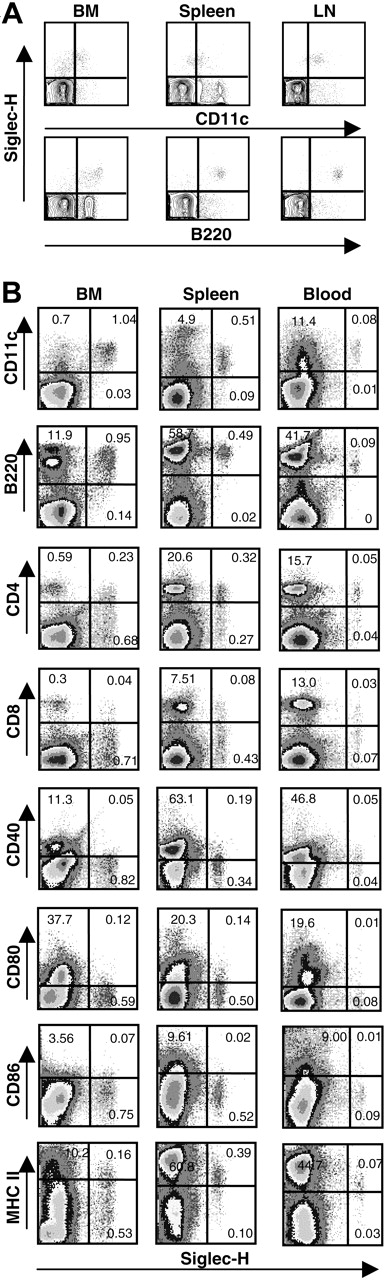
A rat anti–Siglec-H mAb, MB15 (IgG2a), was raised against the recombinant protein and shown by ELISA to be specific for Siglec-H using a panel of mouse Fc-fusion proteins (Siglec-H, mCD33, mSiglec-E, mSiglec-F, and mSiglec-G; data not shown). To characterize Siglec-H expression in mice, we used MB15 in FACS analysis to stain single-cell suspensions from bone marrow, spleen, and peripheral lymph nodes of different mouse strains (BALB/c, C57BL/6, C3H, and DBA). Small subsets (< 1%) of Siglec-H+ cells were identified in all samples and were found to be exclusively pDC precursors24 as defined by B220+CD11clow. However, interpretation of the FACS results was complicated by high background staining of B cells due to reactivity with antirat conjugates used to detect MB15 (data not shown). Using μH-deficient C57Bl/6 mice that lack B cells, specific labeling of B220+CD11clow pDC precursors was confirmed for cell suspensions of bone marrow, spleen, and lymph node (Figure 2A).
To further characterize the Siglec-H+ cells, we stained single-cell suspensions of wild-type C57Bl/6 mouse bone marrow, spleen, and blood with a sheep pAb anti–Siglec-H-FITC in combination with a panel of Abs. As shown in Figure 2B, FACS confirmed that Siglec-H+ cells were present at about 1% in bone marrow, about0.5% in spleen, and about 0.1% in blood. Regardless of tissue origin, Siglec-H+ cells were CD11clow and B220+, mostly CD8–, expressed low or undetectable levels of costimulatory molecules CD40, CD80, and CD86. Despite these similarities, there were notable differences among Siglec-H+ cells from different tissues. First, whereas Siglec-H+ cells from bone marrow were mostly CD4–, over half of the Siglec-H+ cells from spleen and blood were CD4+. Second, compared with Siglec-H+ cells in bone marrow, Siglec-H+ cells in blood and spleen expressed significantly higher levels of major histocompatibility complex (MHC) class II (Figure 2B).
Siglec-H is expressed specifically on pDC precursors in FACS analyses. (A) Single-cell suspensions of bone marrow (BM), spleen, and lymph node (LN) from μH gene-deficient mice were stained sequentially with MB15, biotinylated mouse anti-rat IgG2a, and streptavidin-PE. Cells were counterstained with CD11c-FITC and B220-APC. About 50 000 events were acquired for each sample. (B) Single-cell suspensions from bone marrow (BM), spleen, and blood were stained with FITC-labeled sheep anti–Siglec-H pAb in combination with the indicated mAbs. The percentages of cells relative to total live cells are shown in the quadrants. About 200 000 events were acquired for each sample.
Siglec-H is expressed specifically on pDC precursors in FACS analyses. (A) Single-cell suspensions of bone marrow (BM), spleen, and lymph node (LN) from μH gene-deficient mice were stained sequentially with MB15, biotinylated mouse anti-rat IgG2a, and streptavidin-PE. Cells were counterstained with CD11c-FITC and B220-APC. About 50 000 events were acquired for each sample. (B) Single-cell suspensions from bone marrow (BM), spleen, and blood were stained with FITC-labeled sheep anti–Siglec-H pAb in combination with the indicated mAbs. The percentages of cells relative to total live cells are shown in the quadrants. About 200 000 events were acquired for each sample.
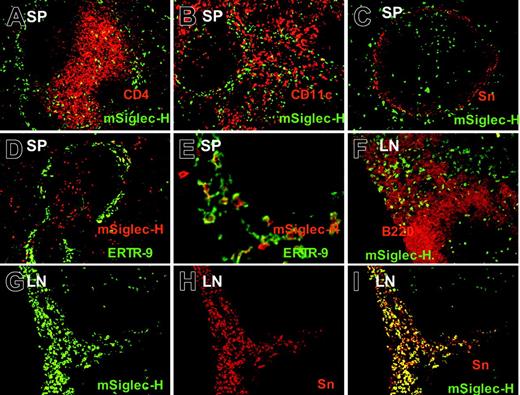
Siglec-H is expressed on pDC precursor and macrophage subsets in spleen and lymph nodes. (A-C) Spleen cryostat sections were stained with MB15 (rat anti–Siglec-H) mAb followed by goat anti–rat-FITC. Sections were counter-stained with CD4 (A), CD11c (B), or sialoadhesin (Sn), (C). Siglec-H+ cells are codistributed with T cells (A) and CD11c+ DCs (B), and some larger Siglec-H+ cells are present within the marginal zone, adjacent to Sn+ metallophilic macrophages (C). (D-E). Spleen sections were stained with biotinylated sheep anti–Siglec-H pAb and ER-TR9 mAb (rat anti–SIGN-R1) followed by streptavidin–Texas red and rabbit anti–rat immunoglobulin-FITC; panel D is low-power view, while panel E is higher-power view. In the marginal zone, there are large macrophages double labeled for both Siglec-H and SIGN-R1, as well as smaller pDC precursors that are only labeled with Siglec-H (E arrows). (F-I) Mesenteric lymph node (LN) sections were stained with sheep anti–Siglec-H–FITC and either counterstained with B220 (F) or anti-Sn (G-I). In panel F, small Siglec-H+ pDC precursors are labeled (arrowheads), whereas in the medullary cord (MC), larger Siglec-H+ cells can be seen adjacent to B220+ B cells. Siglec-H+ pDC precursors are also present in a T-cell zone (arrowheads). In panels G-I, many Siglec-H+ macrophages are present in the medullary cords (MCs) that colocalize with Sn (I). Siglec-H+ pDC precursor can also be seen in a T-cell zone(T). Magnification: 100× (A-D, F-I); 400× (E).
Siglec-H is expressed on pDC precursor and macrophage subsets in spleen and lymph nodes. (A-C) Spleen cryostat sections were stained with MB15 (rat anti–Siglec-H) mAb followed by goat anti–rat-FITC. Sections were counter-stained with CD4 (A), CD11c (B), or sialoadhesin (Sn), (C). Siglec-H+ cells are codistributed with T cells (A) and CD11c+ DCs (B), and some larger Siglec-H+ cells are present within the marginal zone, adjacent to Sn+ metallophilic macrophages (C). (D-E). Spleen sections were stained with biotinylated sheep anti–Siglec-H pAb and ER-TR9 mAb (rat anti–SIGN-R1) followed by streptavidin–Texas red and rabbit anti–rat immunoglobulin-FITC; panel D is low-power view, while panel E is higher-power view. In the marginal zone, there are large macrophages double labeled for both Siglec-H and SIGN-R1, as well as smaller pDC precursors that are only labeled with Siglec-H (E arrows). (F-I) Mesenteric lymph node (LN) sections were stained with sheep anti–Siglec-H–FITC and either counterstained with B220 (F) or anti-Sn (G-I). In panel F, small Siglec-H+ pDC precursors are labeled (arrowheads), whereas in the medullary cord (MC), larger Siglec-H+ cells can be seen adjacent to B220+ B cells. Siglec-H+ pDC precursors are also present in a T-cell zone (arrowheads). In panels G-I, many Siglec-H+ macrophages are present in the medullary cords (MCs) that colocalize with Sn (I). Siglec-H+ pDC precursor can also be seen in a T-cell zone(T). Magnification: 100× (A-D, F-I); 400× (E).
Expression of Siglec-H on pDC precursors and in macrophage subsets in lymphoid tissues by immunofluorescence microscopy
The preceding FACS analysis demonstrated that anti–Siglec-H Ab exclusively stained pDC precursors in single-cell suspensions from hemopoietic and lymphoid tissues. To investigate whether additional cell types that are not present in the single-cell suspensions are also Siglec-H+, we stained cryostat tissue sections of spleen and lymph node with either MB15 mAb or sheep anti–Siglec-H pAb. As shown in Figure 3, many small, round Siglec-H+ cells were located in the T-cell zones of both spleen and lymph nodes (Figure 3) and were codistributed with CD4+ (Figure 3A) or CD11c+ DCs (Figure 3B). In the spleen, there was additional staining of Siglec-H+ cells within the marginal zone (Figure 3A-E). A subset of the marginal zone Siglec-H+ cells was bigger and had a more irregular shape than mSiglec-H+ cells in the T-cell zones (Figure 3E). These cells were distinct from sialoadhesin (Sn)–positive metallophilic macrophages in the inner marginal zone (Figure 3C) and costained with ER-TR9, an anti–SIGN-R1–specific mAb expressed on marginal zone macrophages (Figure 3D-E). Siglec-H labeling of marginal zone macrophages appeared to be predominantly intracellular, being surrounded by a rim of surface ER-TR9 staining (Figure 3E). This was confirmed in FACS analyses of collagenase-digested spleens in which the released ER-TR9+ macrophages were unlabeled with anti–Siglec-H in contrast to the pDCs (data not shown). In lymph nodes, Siglec-H was expressed in large macrophages in the medullary cords, lying in close proximity to B220+ B cells (Figure 3F). These medullary cord macrophages were also labeled with anti-Sn Ab (Figure 3G-I), although the Sn+ subcapsular sinus macrophages were negative for Siglec-H (data not shown). In conclusion, staining of cryostat sections revealed that Siglec-H was expressed not only on pDC precursor, but also by specialized subsets of macrophages in lymphoid organs.
Siglec-H ligation with Abs does not modulate pDC precursor activation or survival
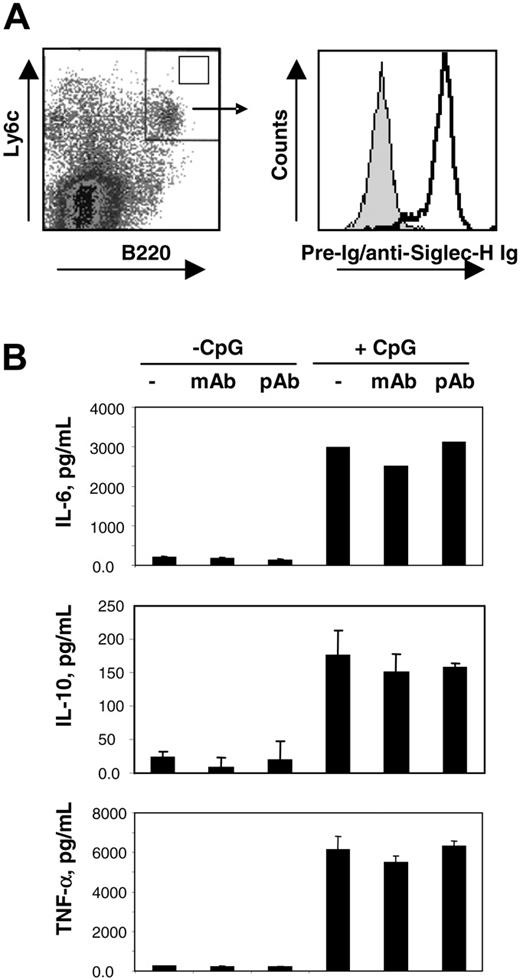
Having established that mSiglec-H is a novel marker for mouse pDC precursors, we next asked whether ligation of this molecule could modulate pDC precursor activation. For this, we generated pDC precursors from bone marrow precursors grown in the presence of Flt-3 ligand.19 After 10 to 14 days of culture, Siglec-H was induced on B220 and Ly6c double-positive pDC precursors (Figure 4A). These cells were purified by FACS and cultured with 10 μg/mL MB15 mAb, 10 μg/mL sheep anti–mSiglec-H pAb or without Abs, in both the presence and absence of CpG oligonucleotides as a pDC precursor activator. Following CpG activation, the purified pDC precursor underwent a dramatic change in morphology and up-regulation of MHC class II antigens as expected, but neither property was affected in the presence of anti–Siglec-H mAb and pAb (data not shown). The rapid apoptosis of pDC precursors in culture was also not affected (data not shown). We next measured cytokine secretion by activated pDCs in the presence and absence of anti–Siglec-H Abs. As shown in Figure 4B, CpG treatment of pDC precursors resulted in the secretion of significant levels of TNF-α, IL-10, and IL-6, but addition of the mAb or pAb anti–Siglec-H had no effect. We also coated the Abs onto tissue culture plastic but again no effect on pDC activation was seen (data not shown). Finally, production of interferon-α by bone marrow pDCs stimulated with inactivated Sendai or influenza viruses was not consistently affected by addition of anti–Siglec-H Abs when compared with control Abs (data not shown). Thus, ligation of mSiglec-H did not modulate pDC activation under the conditions used.
Ligation of Siglec-H does not modulate pDC activation. (A) Bone marrow cells were expanded for 14 days with Flt-3 ligand and labeled with anti-B220 and anti-Ly6c mAbs to identify pDC precursor (left panel). Double-positive cells (top right quadrant, left panel) were sorted and an aliquot stained with either sheep anti–Siglec-H+ (open histogram, right panel) or preimmune immunoglobulin (gray histogram, right panel). (B) FACS-sorted B220+ Ly6c+ cells were cultured with no Ab, MB15 mAb (10 μg/mL), or sheep anti–Siglec-H pAb (10 μg/mL) in the presence or absence of 10 μM CpG 1668 for 18 hours. The concentrations of the indicated cytokines in the cell culture supernatants were determined by flow cytometry.
Ligation of Siglec-H does not modulate pDC activation. (A) Bone marrow cells were expanded for 14 days with Flt-3 ligand and labeled with anti-B220 and anti-Ly6c mAbs to identify pDC precursor (left panel). Double-positive cells (top right quadrant, left panel) were sorted and an aliquot stained with either sheep anti–Siglec-H+ (open histogram, right panel) or preimmune immunoglobulin (gray histogram, right panel). (B) FACS-sorted B220+ Ly6c+ cells were cultured with no Ab, MB15 mAb (10 μg/mL), or sheep anti–Siglec-H pAb (10 μg/mL) in the presence or absence of 10 μM CpG 1668 for 18 hours. The concentrations of the indicated cytokines in the cell culture supernatants were determined by flow cytometry.
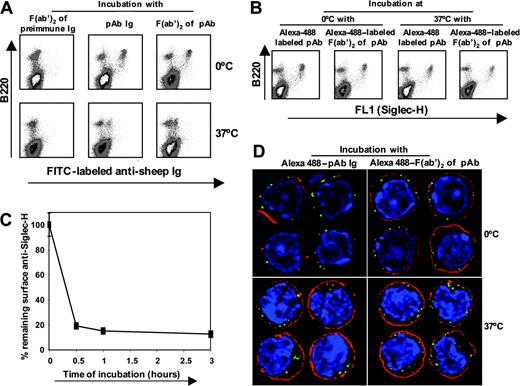
pDC precursors can mediate efficient internalization of anti–Siglec-H Abs. (A) Bone marrow cells enriched in pDC precursors were incubated with sheep anti–Siglec-H IgG or F(ab′)2 fragments or preimmune F(ab′)2 fragments for 1 hour on ice and then either incubated on ice or at 37°C for 3 hours. At the end of the incubation period, the remaining surface anti–Siglec-H IgG or F(ab′)2 was detected using donkey anti–sheep IgG-FITC. (B) To determine whether anti–Siglec-H IgG or F(ab′)2 was internalized or shed at 37°C, we treated bone marrow cells enriched in pDC precursors as in panel A, using Alexa 488–labeled sheep anti–Siglec-H IgG or F(ab′)2 fragments and total cell-associated Ab (surface + internalized) was measured after 3 hours at either 0°C or 37°C. (C) Time course of internalization. Bone marrow cells were incubated with biotinylated sheep anti–Siglec-H IgG for 1 hour on ice and then incubated either on ice for 3 hours or at 37°C for the indicated time points. After 3 hours, percent remaining surface anti–Siglec-H was measured using streptavidin-APC. Data show means ± 1 SD of triplicate measurements and are representative of 3 experiments performed. (D) Bone marrow cells enriched in pDC precursors were labeled with Alexa 488-labeled sheep anti–Siglec-H IgG or F(ab′)2 fragments treated as in panel B, counterstained with the plasma membrane marker cholera toxin subunit B-Alexa 594 (red) and the DNA marker DAPI (blue). Cells were examined on a DeltaVision deconvolution fluorescence microscope and images from the central z-section of representative cells are shown. Magnification: 1000×.
pDC precursors can mediate efficient internalization of anti–Siglec-H Abs. (A) Bone marrow cells enriched in pDC precursors were incubated with sheep anti–Siglec-H IgG or F(ab′)2 fragments or preimmune F(ab′)2 fragments for 1 hour on ice and then either incubated on ice or at 37°C for 3 hours. At the end of the incubation period, the remaining surface anti–Siglec-H IgG or F(ab′)2 was detected using donkey anti–sheep IgG-FITC. (B) To determine whether anti–Siglec-H IgG or F(ab′)2 was internalized or shed at 37°C, we treated bone marrow cells enriched in pDC precursors as in panel A, using Alexa 488–labeled sheep anti–Siglec-H IgG or F(ab′)2 fragments and total cell-associated Ab (surface + internalized) was measured after 3 hours at either 0°C or 37°C. (C) Time course of internalization. Bone marrow cells were incubated with biotinylated sheep anti–Siglec-H IgG for 1 hour on ice and then incubated either on ice for 3 hours or at 37°C for the indicated time points. After 3 hours, percent remaining surface anti–Siglec-H was measured using streptavidin-APC. Data show means ± 1 SD of triplicate measurements and are representative of 3 experiments performed. (D) Bone marrow cells enriched in pDC precursors were labeled with Alexa 488-labeled sheep anti–Siglec-H IgG or F(ab′)2 fragments treated as in panel B, counterstained with the plasma membrane marker cholera toxin subunit B-Alexa 594 (red) and the DNA marker DAPI (blue). Cells were examined on a DeltaVision deconvolution fluorescence microscope and images from the central z-section of representative cells are shown. Magnification: 1000×.
Siglec-H is an endocytic receptor
Many surface proteins expressed by macrophages and DCs are important endocytic receptors for delivery of ligands to intracellular compartments for targeted degradation or antigen processing. To investigate the endocytic capacity of Siglec-H, we developed a flow cytometry-based assay using sheep anti–Siglec-H IgG or F(ab′)2 fragments and pDC precursor–enriched primary bone marrow cells (Figure 5). Following incubation of pDC precursor with IgG or F(ab′)2 for 1 hour at 4°C, the levels of surface Ab after 3 hours at 37°C were greatly reduced compared with incubation on ice (Figure 5A). The reduction in surface anti–Siglec-H Ab was due to internalization rather than shedding because the levels of Alexa 488-labeled Abs remained constant over 3 hours either on ice or at 37°C (Figure 5B). In time-course experiments, anti–Siglec-H IgG was rapidly internalized, with about 80% removed from the cell surface within 30 minutes of warming to 37°C (Figure 5C). In comparison, internalization of the pDC-specific mAb, PDCA-1 was less efficient, with 39% ± 9% (mean ± 1 SD) being internalized after a 3-hour incubation. By deconvolution fluorescence microscopy, staining of pDC precursor with Alexa 488-labeled sheep anti–Siglec-H IgG or F(ab′)2 fragments led to large punctuate surface structures at the plasma membrane. These were efficiently internalized at 37°C but remained associated with the plasma membrane during an equivalent incubation on ice (Figure 5D). Colocalization with the early endosomal marker, anti–EEA-1, showed that most of the anti–Siglec-H was present in endosomes (data not shown).
Targeting Siglec-H in vivo with immunoconjugates results in generation of antigen-specific CD8 T cells
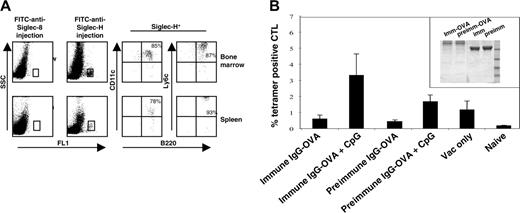
Having established that Siglec-H is an endocytic receptor expressed on mouse pDCs, we next asked whether delivery of an antigen via Siglec-H could result in generation of CD8 T cells in vivo. As a first step, we injected mice intravenously with FITC-labeled sheep anti–Siglec-H Ab or, as a control, FITC-labeled sheep anti–Siglec-8 and analyzed the cell types labeled in vivo. More than 90% of the anti–Siglec-H–labeled cells in both the spleen and bone marrow were defined as pDC precursors, as demonstrated by multiparameter labeling with mAbs to B220, CD11c, and Ly6c (Figure 6A). To see whether delivery of OVA via Siglec-H could lead to antigen presentation and generation of CD8 antigen-specific T cells, we coupled OVA covalently to either sheep anti–Siglec-H IgG or preimmune sheep IgG as a control (Figure 6B inset). When CpG was used as an adjuvant, mice we had injected with anti–Siglec-H–OVA and boosted with vaccinia virus had a significantly higher percentage of OVA257-264-specific cytotoxic T lymphocytes (CTLs) compared with the preimmune control (Figure 6B). These results suggest that pDCs are capable of cross-presenting antigens targeted via surface receptors, as opposed to antigens delivered via fluid phase uptake.21
Discussion
In this paper, we describe the cloning and characterization of a novel mouse siglec-like molecule, designated Siglec-H. Siglec-H is predicted to contain 2 extracellular immunoglobulin-like domains, a transmembrane region and a short cytoplasmic tail that lacks ITIM-related sequences. Siglec-H is therefore unlikely to function as an inhibitory receptor, in contrast to most other CD33-related siglecs.1 Interestingly, however, Siglec-H in both mouse and rat contains 2 oppositely charged amino acids in the cytoplasmic tail that are also present in mouse CD33 but none of the other siglecs so far characterized. Many activatory receptors of the immune system have a single basic residue in the transmembrane region that is required for association with adaptors like DAP12.25 In the present study, we did not obtain any evidence that ligation of Siglec-H led to activation of pDC precursors, using either the mAb or pAb reagents. Therefore, the significance of the charged pair of amino acids in Siglec-H and mCD33 is unclear, but they could be involved in charge-dependent cis-interactions with other membrane receptors to create complexes at the cell surface. In this regard, our attempts to express Siglec-H in heterologous cells such as COS cells, CHO cells, or 293 T cells have so far been unsuccessful, suggesting that additional binding partners such as DAP12 are required for surface expression (J.Z., unpublished observations, June 2004). Interestingly, the staining of macrophage subsets in splenic and lymphoid tissues appeared to be predominantly intracellular, perhaps due to either inefficient targeting to the plasma membrane or continuous endocytosis from the plasma membrane.
Targeting of anti–Siglec-H Ab to pDC precursors in vivo and priming of OVA-specific CTLs by sheep anti–Siglec-H–OVA immunoconjugate. (A) Mice were given intravenous injections of either sheep anti–Siglec-H IgG-FITC or, as a control, sheep anti–hSiglec-8 IgG-FITC. After 3 hours, bone marrow cells (top dot plots) and spleen cells (bottom dot plots) were isolated and labeled with anti-B220, anti-CD11c, and anti-Ly6C. The FITC-labeled cells were gated (boxes, left dot plots) and analyzed for expression of pDC markers B220, CD11c, and Ly6c. The percentage values are shown for each marker. (B) Anti–Siglec-H-OVA complex primes OVA-specific CD8+ T cells in vivo. C57BL/6 mice (n = 5) were primed by intravenous injection of anti–Siglec-H–OVA complexes or irrelevant complexes, in the presence or absence of CpG, and boosted after 7 days with vaccinia virus encoding full-length ovalbumin. CTL responses were assessed in the blood by FACS analysis using SIINFEKL-H2Kb tetramers 7 days after boosting. Mean proportions of tetramer-positive cells as a percentage of CD8 cells (± SEM) for each group are shown. The inset shows SDS-PAGE of sheep anti–Siglec-H IgG-OVA (imm-OVA) and preimmune IgG-OVA (preimm-OVA), together with the corresponding unconjugated IgGs (imm and preimm) run under nonreducing conditions. Molecular weight markers in kilodaltons are shown.
Targeting of anti–Siglec-H Ab to pDC precursors in vivo and priming of OVA-specific CTLs by sheep anti–Siglec-H–OVA immunoconjugate. (A) Mice were given intravenous injections of either sheep anti–Siglec-H IgG-FITC or, as a control, sheep anti–hSiglec-8 IgG-FITC. After 3 hours, bone marrow cells (top dot plots) and spleen cells (bottom dot plots) were isolated and labeled with anti-B220, anti-CD11c, and anti-Ly6C. The FITC-labeled cells were gated (boxes, left dot plots) and analyzed for expression of pDC markers B220, CD11c, and Ly6c. The percentage values are shown for each marker. (B) Anti–Siglec-H-OVA complex primes OVA-specific CD8+ T cells in vivo. C57BL/6 mice (n = 5) were primed by intravenous injection of anti–Siglec-H–OVA complexes or irrelevant complexes, in the presence or absence of CpG, and boosted after 7 days with vaccinia virus encoding full-length ovalbumin. CTL responses were assessed in the blood by FACS analysis using SIINFEKL-H2Kb tetramers 7 days after boosting. Mean proportions of tetramer-positive cells as a percentage of CD8 cells (± SEM) for each group are shown. The inset shows SDS-PAGE of sheep anti–Siglec-H IgG-OVA (imm-OVA) and preimmune IgG-OVA (preimm-OVA), together with the corresponding unconjugated IgGs (imm and preimm) run under nonreducing conditions. Molecular weight markers in kilodaltons are shown.
Sialic acid binding by siglecs is dependent on several conserved features within the N-terminal V-set domain,22,23 all of which were present in Siglec-H. However, despite numerous attempts to demonstrate sialic acid recognition with a variety of different assays, no binding signals were seen. A possible molecular explanation for this is the presence of 2 unpaired cysteines in domain 1, one immediately upstream of strand A, the other immediately downstream of strand G (Figure 1A). These could potentially form an intersheet disulfide bond that might reduce the distance between the 2 β sheets and prevent interactions with sialic acids.22,23 These unusual cysteines are also conserved in rat Siglec-H which, in addition, has the key arginine required for sialic acid binding replaced by a serine residue (Figure 1C). Although we cannot exclude the possibility that mouse Siglec-H interacts with a very specific type of carbohydrate ligand, it seems more likely that it has evolved to interact primarily with ligands other than sialic acids.
A striking feature of Siglec-H is its highly restricted expression pattern on pDC presursors and subsets of macrophages in lymphoid tissues. Using a rat anti–Siglec-H mAb and a sheep anti–Siglec-H pAb, we demonstrate here by FACS analysis that Siglec-H is specifically expressed on pDC precursors from single-cell suspensions. It therefore provides an excellent marker for these cells. Indeed, the Ab reagents developed here allowed us to directly analyze pDC phenotypes, including those of blood pDC precursors, which make up only about 0.1% of blood leukocytes. Our findings were consistent with previous phenotypic analyses of mouse pDC precursors, showing that Siglec-H+ cells from bone marrow, spleen, and blood are B220+, CD11clow, with variable levels of CD4 and MHC class II and low levels of the costimulatory molecules CD40, CD80, and CD86 (for a review, see Colonna et al26 ). The increase in levels of markers like CD4 on pDCs in tissues like spleen and lymph node compared with bone marrow may reflect a natural maturation process.27 However, recent studies have demonstrated the existence of 2 distinct populations of pDC precursors in the bone marrow of mice that differ in their expression of the inhibitory receptor, Ly49Q, and activation potential.28,29 Furthermore, pDC precursors in bone marrow have been shown to have a long half-life of about 2 weeks,30 similar to that of pDCs in other tissues.31
One of the major functions of pDCs is in first-line defense to viruses. These cells produce extremely high levels of type I interferon following activation of Toll-like receptor-dependent and -independent signaling pathways.24 Besides inhibiting viral replication, type I interferon has a number of important effects on the immune system and can modulate the functions of T cells, B cells, natural killer (NK) cells, and conventional DC populations.24 Thus, pDCs are considered as a key link between the innate and adaptive immune systems. Resting pDC presursors have an immature phenotype and are inefficient at presenting antigen and inducing primary T-cell responses, but may play a role in T-cell tolerance and generation of regulatory T cells (for a review, see Colonna et al26 ). However, following activation, levels of MHC class II and accessory molecules are dramatically increased and pDC precursors acquire the ability to induce primary T-cell activation in vitro32,33 and in vivo.21,34
Despite the rapid advances in understanding pDC functions, the specific roles of pDC surface receptors are less well understood. Several pDC-restricted molecules have been described on human and mouse pDCs, including BDCA-2 and BDCA-4 in humans,35 Ly49Q in mice,28,29 and 3 additional pDC-restricted antigens in mice defined by the mAbs 120G8, 440c, and mPDCA-1 whose molecular features are unknown.36-38 Ab-mediated ligation of several of these receptors can modulate pDC activation35-37 and for BDCA-2, a role in endocytosis has also been shown.39 From the experiments reported here, the biologic functions of Siglec-H on pDC precursors are more likely to be related to binding or uptake of exogenous ligands rather than regulating cellular activation. Based on antibody staining, Siglec-H appears to be the only murine siglec expressed on pDC precursors (J.Z., unpublished observations, June 2004). Further studies are required to determine if any of the other murine CD33-related siglecs expressed by myeloid cells16 are able to mediate endocytosis and cross-presentation in a similar manner to Siglec-H.
The presence of a Siglec-H ortholog in rats, and its apparent absence from several mammals, suggests that this molecule evolved relatively late during mammalian evolution. It is likely to have arisen from gene duplication and loss of the exon encoding the canonical ITIM and ITIM-like motifs of CD33-related siglecs. This would be analogous to the way that NK cell inhibitory receptors are thought to have given rise to activating counterparts during evolution.40 It has been suggested that certain activating receptors of NK cells and myeloid cells have evolved from inhibitory receptors to bind pathogen ligands.41-43 A similar scenario could be envisioned for Siglec-H and would be consistent with its remarkable restriction to pDCs and specialized subsets of macrophages positioned on the “front line” of the innate immune system. Thus, it is possible that Siglec-H has evolved in rodents to function as a pattern recognition molecule that binds viral or other pathogen ligands. By delivering these to the endosomal compartment, it could favor interactions with Toll-like receptors 7 and 9 as well as the antigen-processing machinery. The possibility that Siglec-H could deliver antigens for T-cell priming was supported in the present study by our demonstration that OVA-anti–Siglec-H conjugates in the presence of CpG could prime CD8 T-cell responses in vivo. This cross-priming is likely to have been due to pDC rather than marginal zone macrophages because pDC precursors were strongly labeled by Ab after intravenous injection, whereas surface expression of Siglec-H was undetectable on marginal zone macrophages. Clearly, an important area for future studies on the biology of Siglec-H will be the identification of ligands, either pathogen-derived or otherwise, and to relate this to host defense functions mediated by pDCs.
Supported by a Wellcome Trust Senior Fellowship GR047677MA (P.R.C.), by Cancer Research United Kingdom (Programme Grant C399), and by research funding from BD Biosciences (R.H.).
R.H. is employed by BD Biosciences, whose (potential) product was studied in the present work.
J.Z., A.R., N.S., R.H., M.J.P., and M.S. performed and analyzed the research; P.R.C. and V.C. designed the research; and J.Z. and P.R.C. wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-09-3842.
Note added in proof. While this paper was under review, Colonna's group44 showed independently that Siglec-H is recognized by the 440c monoclonal antibody and requires DAP12 for cell-surface expression.
We thank Joanna Warren for assistance with Ab injections, Rosemary Clarke for help with cytokine measurements, and Neil Barclay for discussions on the structural features of Siglec-H.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal