Interleukin-21 (IL-21) is a member of the IL-2 cytokine family, which mediates proliferation or growth arrest and apoptosis of normal B cells, depending on their activation state. Here we demonstrate that surface IL-21 receptor (R) is expressed at variable levels by chronic lymphocytic leukemia (CLL) B cells freshly isolated from 33 different patients. IL-21R expression was up-regulated following cell stimulation via surface CD40. Therefore, IL-21 effects were more evident in CD40-activated CLL B cells. IL-21 induced an early signaling cascade in CLL B cells, which included JAK-1 and JAK-3 autophosphorylation and tyrosine phosphorylation of STAT-1, STAT-3, and STAT-5. IL-21 signaling failed to stimulate CLL B-cell proliferation, but induced their apoptosis. In addition, IL-21 counteracted the proliferative and antiapoptotic signals delivered by IL-15 to CLL B cells. IL-21-mediated apoptosis involved activation of caspase-8 and caspase-3, cleavage of Bid to its active form t-Bid, and cleavage of PARP and of p27Kip-1. Recent data indicate that CLL B cells require interaction with the microenvironment for their survival and expansion. The present findings thus provide a set of new mechanisms involved in the balance between cell-survival and apoptotic signals in CLL B cells.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common type of adult leukemia, characterized by the clonal expansion of CD5+ B lymphocytes.1-3 In the past, it was assumed that CLL was caused by the accumulation of slowly proliferating cells with defective apoptosis.4 However, more recent data indicate that CLL is a dynamic disorder, where the cell accumulation depends on the rate of cell birth and death. This dynamic turnover is variable among different patients, and patients with a high turnover rate have a more rapidly progressive disease.5 In addition to the high turnover rate, several other biologic markers have been related to CLL clinical course and found to have a predictive value. High CD386-8 or ZAP-709-10 expression levels or the absence of somatic mutations of immunoglobulin V heavy (IgVH) chain genes 6,9,10 are associated to a worse prognosis in CLL.
Several factors, including soluble cytokines or membrane-bound receptor ligands, modulate the proliferation or the apoptotic rate of CLL B cells. In particular, both interleukin-2 (IL-2)11-13 and IL-1514 have been reported to promote the growth of CLL B cells and to mediate antiapoptotic effects. The receptor complex of these 2 cytokines is composed by the promiscuous IL-2 receptor (R) β (CD122) and γ (CD132) chains, involved in signal transduction, while the private IL-2Rα (CD25) or IL-15Rα chains are required for specific high-affinity binding of the respective cytokine.15,16 All these receptor chains are expressed by CLL B cells and allow them to proliferate in response to IL-2 or IL-15.11-14
IL-21 is a more recently discovered class I cytokine, which is produced by activated T-helper (Th) cells and displays structural similarities and functional overlaps with IL-2 and IL-15.17 IL-21 biologic activities are mediated by a specific IL-21R, structurally related to the IL-2Rβ chain,17,18 which associates with the common γ chain (γc) for signal transduction.19,20 Signaling via the IL-21R/γc complex may involve different JAK/STAT molecules in different responding cells.21 IL-21 exerts regulatory effects on virtually all cells of the immune system. In normal B cells, IL-21 effects are strikingly different, as IL-21 can mediate cell proliferation, growth arrest, and terminal differentiation, or apoptosis, depending on their activation status.22-24 IL-21 has nonredundant functions on B cells, as IL-21R-/- mice show an impaired production of IgG1 and enhanced IgE production in response to antigen stimulation.25 Accordingly, IL-21 has been shown to stimulate IgG1 production and to prevent IgE class-switch in IL-4-stimulated B cells in vitro.26
The effects of IL-21 are not restricted to the B-cell lineage, as IL-21 induces terminal differentiation of activated natural killer (NK) cells27 and stimulates proliferation, cytotoxic activity, and interferon-γ (IFN-γ) production by CD8+ effector T cells.28 Previous studies in different murine models showed that IL-21 can promote tumor rejection29-32 and induce immunity against tumor-associated antigens,30-32 suggesting that IL-21 represents a novel potential tool for neoplasia immunotherapy.33
Several lines of evidence indicate that CLL B-cell survival and expansion in vivo may depend upon interaction with the microenvironment.34 Both accessory cells and cytokines are involved in these interactions, which are not clearly defined in their cellular/molecular mechanisms. Considering the important regulatory effects of IL-21 on normal B cells, we have investigated here whether IL-21 was biologically active on the leukemic cells of CLL. Previously it was shown that IL-21 may act as a growth factor for myeloma and adult T-cell leukemia (ATL) cells in vitro.34,35 Indeed, we show that CLL B cells express the IL-21R, although its expression may vary in the different cases. CLL B cells respond to IL-21 stimulation by undergoing apoptosis, and their susceptibility to IL-21 effects is best observed following activation through CD40, which up-regulates IL-21R expression. The signaling cascade triggered by IL-21 in CLL B cells involves JAK-1 and JAK-3 and STAT-1, STAT-3, and STAT-5 followed by caspase-8 and caspase-3 activation and cleavage of the caspase substrates Bid, PARP, and p27Kip-1. These observations may have possible implications for the biology and the therapy of this disease.
Materials and methods
Cells and culture conditions
This study was approved by the review board of the University of Genoa, Italy. Upon informed consent, according to institutional procedure and the Declaration of Helsinki, peripheral-blood mononuclear cells (PBMCs) were isolated from heparinized blood obtained from untreated patients diagnosed with CLL on the basis of clinical and immunophenotypic criteria. PBMCs were isolated by density gradient centrifugation (Ficoll; Biochrom, Berlin, Germany) and subjected to a preliminar phenotypic characterization. When residual non-B cells exceeded 10%, B cells were enriched by negative selection with antibody-coated magnetic beads (CD2-beads; Dynal, Oslo, Norway) to obtain a purified population of CD19+/CD5+ B cells.
Coculture of CLL B cells with CD40L-transfected cells
Murine L cells stably transfected by the human CD40L (CD154) cDNA36 were cultured in RPMI 1640 (Cambrex Bio Science, Verviers, Belgium) supplemented with penicillin/streptomycin, l-glutamine, and 10% heat-inactivated fetal calf serum (FCS; Cambrex) in flasks until confluency. CLL B cells were cocultured with CD40L-transfected cells in 24-well plates for 72 hours.37 Then, cells were collected, checked for viability, and resuspended in fresh medium before use.
Immunofluorescence analysis of cell-surface antigen and of ZAP-70 expression
Flow cytometric analyses of total CLL PBMCs and of B-cell-enriched fractions were performed by 2-color immunofluorescence, by staining at 4°C, for 30 minutes, 1 × 105 cells/sample with the following monoclonal antibodies (mAbs): FITC-labeled anti-CD19, anti-CD4, anti-CD8, anti-CD3, anti-CD23, or PE-labeled anti-CD3, anti-CD8, anti-CD19, anti-CD20, anti-CD22, and anti-CD23 (Immunotools, Friesoythe, Germany), anti-CD40L and anti-CD27 (BD Biosciences, Milan, Italy), FITC-, PE-, or Cy-Chrome-labeled isotype-matched Ig's were used as negative controls. After 2 washings in phosphate-buffered saline (PBS) plus 2% FCS, cells were analyzed by flow cytometry (FACScan; BD Biosciences). The proportion of CD38+ leukemic cells was further determined by triple staining for CD19-FITC, CD38-PE, and CD5-Cy-Chrome (BD Biosciences). In selected experiments, the percentages of ZAP-70+ cells were assessed by immunofluorescence on CLL cells first stained by CD56-PE/CD3-PE (BD Biosciences), fixed and permeabilized by fix and perm reagents (Caltag Laboratories, Burlingame, CA) and then stained with 1.5 μg anti-ZAP-70-FITC conjugate mAb (Upstate Biotechnology, Lake Placid, NY) or with 1E7.2 anti-ZAP-70-Alexa-Fluor (Caltag Laboratories). Samples were analyzed by flow cytometry and the expression of ZAP-70 was determined on CD3-/CD56- cells.
RT-PCR analysis of IL-21R and γc mRNA expression
Total RNA was isolated by the use of NucleoSpin RNA II kit (Macherey-Nagel, Duren, Germany) according to instructions provided by the manufacturer. Total RNA (1 μg) was then reverse-transcribed using the SuperScript II Reverse Transcriptase (RT; Invitrogen, Milan, Italy) in a final volume of 20 μL. A portion (2 μL) of the cDNA was separately amplified, in a final volume of 25 μL, with 2.5 IU Taq polymerase (Genecraft, Lüdinghausen, Germany), in the presence of 1 μM of the primers specific for IL-21R (upper primer: CGTGGGAGTCAGCATGCC and lower: TGTCGTCGGCCATGAAGTG), for the γc,38 and for the housekeeping gene β-actin. The amplifications were carried out in a polymerase chain reaction (PCR) Sprint thermal cycler (Hybaid, Ashford, United Kingdom) for 30 cycles or 25 cycles for β-actin (15 seconds at 94°C, 15 seconds at 60°C, and 30 seconds at 72°C) with a final extension at 72°C for 5 minutes. PCR products (10 μL) were then analyzed on 1% to 2% agarose gel stained with ethidium bromide.
For quantitative RT-PCR analysis the following primers were used: IL-21R upper TCATCTGCATCCTGGAAATGTG, lower GCCTCGTCCTTCAGCTCTTCATA; and RNA polymerase II A subunit upper GACAATGCAGAGAAGCTGG, lower GCAGGAAGACATCATCATCC. Amplification was carried out in an iCycler instrument (Bio-Rad, Hercules, CA) using the SYBR green supermix system (Bio-Rad) according to the manufacturer protocol.
Western blotting analysis
Purified B cells (107) from CLL patients, either resting or CD40-activated, were incubated with or without recombinant human IL-21 (rhIL-21, 80 ng/mL; Biosource, Camarillo, CA) for 24 to 48 hours. Then, cells were collected, washed in PBS, and lysed in 50 μL lysis buffer for 30 minutes on ice. Equal amounts (50 μg) of protein extracts were analyzed under reducing conditions on 10% or 12% polyacrylamide gels and transferred onto Hybond-C membranes (Amersham Biosciences, Little Chalfont, United Kingdom). After overnight saturation, blots were stained with anti-IL-21R (R&D Systems, Minneapolis, MN), anti-β-actin (Sigma, Saint Louis, MO), anti-caspase-8 (MBL, Watertown, MA), anti-PARP, anti-p27Kip-1 (both from BD Biosciences), anti-p27Kip-1 N-terminus (N20; Santa Cruz Biotechnology, Santa Cruz, CA) mouse Abs or with anti-Bid (Santa Cruz Biotechnology) rabbit Ab. After washings with Tris-buffered saline (TBS) supplemented with 0.05% Tween 20, the blots were then incubated with horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit Ig antisera (Dako, Glostrup, Denmark) for 2 hours. Bands were visualized by enhanced chemiluminescence (ECL; Amersham Biosciences).
For the analysis of phosphorylated proteins, 107 CLL B cells were incubated for 10 and 20 minutes at 37°C with or without 80 ng/mL rhIL-21 in 1 mL RPMI 1640 supplemented with 10% FCS. Cells, washed twice in ice-cold PBS containing 400 mM Na orthovanadate, 5 mM EDTA, and 1 mM Na pyrophosphate, were then solubilized in 100 μL lysis buffer containing 1 mM Na orthovanadate, incubated 30 minutes on ice and centrifuged 10 minutes at 17 900g. A portion (20 μL) of the lysates were resolved under reducing conditions by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting using the following primary Abs: rabbit anti-phospho-STAT-1 (pY701; Cell Signaling Technology, Beverly, MA), mouse anti-STAT-1 (Santa Cruz Biotechnology), mouse anti-phospho-STAT-3 (pY705; Upstate Biotechnology), mouse anti-phospho-STAT-5 (pY694; BD Biosciences), rabbit anti-phospho-JAK1 (pY1022/1023), rabbit anti-phospho-JAK2 (pY1007/1008) (both from Biosource), rabbit anti-phospho-JAK3 (pY980; Santa Cruz Biotechnology) and rabbit anti-β-actin (Sigma). Appropriate secondary HRP-conjugated Abs were used.
IgVH gene analyses
The CLL B-cell IgVH gene usage and mutation was determined on cDNA according to previously reported methods.39
Statistical analysis
All statistical calculations were performed using the statistical package SPSS for Windows, release 11.5, 2002 software (SPSS UK, Working, Surrey, United Kingdom). When ZAP-70, CD38, mutational status, and IL-21R were considered as binary variables, statistical comparisons were performed using 2-way tables for the Fisher exact test and multiway tables for the Pearson chi-square test. The nonparametric Mann-Whitney U test was used to test the differences between 2 groups (CD38+ [> 30%] and CD38- [≤ 30%], and VH-mutated [≥ 2%] and VH-unmutated [< 2%] cases). Paired 2-tailed Student t test was used for analysis of IL-21R expression in resting or activated CLL B cells. A P value of less than .05 was considered significant. Paired 2-tailed Student t test was used for analysis of IL-21R expression in resting or activated CLL B cells. A P value of .05 or less was considered significant.
Determination of apoptosis, mitochondrial depolarization, and cell proliferation
Resting or CD40-activated CLL B cells were cultured with different concentrations of rhIL-21 (20-80 ng/mL) and apoptosis was determined at different time points (2-4 days) by annexin V-FITC and propidium iodide (PI) double staining (Bender MedSystem, Vienna, Austria), by fluorescence-activated cell-sorting (FACS) analysis, as described.37 Changes in the inner mitochondrial membrane potential (ΔΨm) were further evaluated by staining CLL B cells at 37° for 15 minutes with 40 nM DiOC6 (3,3′ dihexylocarbocyanine iodide; Sigma), a green fluorescent dye. After 2 washes, cells were analyzed by FACS. Viable cells have a high ΔΨm and display bright DiOC6 fluorescence, while apoptotic cells display dull DiOC6 fluorescence. In selected experiments, rhIL-21 (40 ng/mL) was used together with rhIL-15 (40 ng/mL) (PeproTech EC, London, United Kingdom) to determine potential synergism or inhibition between the 2 cytokines. Cell proliferation was determined by 3H-thymidine incorporation.38
Determination of caspase activation
Caspase activation was evaluated using the following cell-permeable fluoresceinated inhibitors of caspases: pancaspase inhibitor (FITC-VAD-FMK; Promega, Madison, WI), inhibitor of caspase-8 (FAM-LETD-FMK), caspase-9 (FAM-LEHD-FMK), and caspase-3/caspase-7 (FAM-DEVD-FMK), (all from B-Bridge, San Jose, CA). Cells were stained for 1 hour at 37°C in the dark. After this time cells were washed with washing buffer, resuspended in 0.5 mL buffer, and immediately analyzed by flow cytometry. In addition, the intracellular activated caspase-3 or Bcl-2 were detected by staining permeabilized CLL B cells with the Alexa-Fluor-labeled anti-human active caspase-3 rabbit antiserum (Ozyme, Cedex, France) or with FITC-conjugated anti-Bcl-2 mAb (BD Biosciences) and subsequent cytofluorimetric analysis.
Results
IL-21 receptor expression on CLL B cells is heterogeneous and is up-regulated by CD40 triggering
Expression of IL-21R was analyzed on CLL B cells before and after CD40 triggering, by immunofluorescence, Western blot, and RT-PCR analyses.
As shown in Table 1, surface IL-21R was expressed at different levels on CLL B cells from 33 patients and was “low,” which was operationally defined as 15% or less of IL-21R+ B cells, to undetectable in a fraction of cases (11 of 33 patients). Patients showing low IL-21R expression on CLL-B cells had “high” CD38 expression (≥ 30% positive cells) and statistical analysis also suggested an inverse correlation between surface IL-21R and CD38 expression levels (P < .001, Table 1). Although most cases having low IL-21R expression on CLL B cells displayed high (> 20%) ZAP-70 expression (9 of 11 patients) and unmutated IgVH genes (6 of 10 patients tested), the statistical correlation of IL-21R with these parameters was not significant (Table 1).
Expression of IL-21R and prognostic markers of the CLL patients studied
Patient no. . | IL-21R, % . | CD38, % . | ZAP70, % . | VH mutations, % . |
|---|---|---|---|---|
| 1 | 34 | 12 | 32 | 9.7 |
| 2 | 30 | 6 | 89 | 0.0 |
| 3 | 33 | 9 | 95 | 9.5 |
| 4 | 13 | 37 | 90 | ND |
| 5 | 26 | 14 | 35 | 5.3 |
| 6 | 10 | 53 | 24 | 1.4 |
| 7 | 10 | 96 | 88 | 0.0 |
| 8 | 6 | 34 | 46 | 9.7 |
| 9 | 10 | 30 | 55 | 2.7 |
| 10 | 15 | 47 | 99 | 0.7 |
| 11 | 14 | 69 | 19 | 9.5 |
| 12 | 16 | 67 | 64 | 0.0 |
| 13 | 29 | 2 | 1 | 7.6 |
| 14 | 19 | 5 | 25 | 3.8 |
| 15 | 34 | 6 | 21 | 5.9 |
| 16 | 18 | 3 | 5 | 5.6 |
| 17 | 48 | 6 | 55 | 3.2 |
| 18 | 35 | 6 | 52 | 0.0 |
| 19 | 49 | 3 | 15 | 0.0 |
| 20 | 47 | 4 | 67 | 4.1 |
| 21 | 2 | 74 | 65 | 0.0 |
| 22 | 28 | 52 | 68 | 0.0 |
| 23 | 40 | 7 | 16 | 7.0 |
| 24 | 20 | 1 | 28 | 10.4 |
| 25 | 25 | 1 | 25 | 8.1 |
| 26 | 24 | 87 | 70 | 0.0 |
| 27 | 16 | 10 | 15 | ND |
| 28 | 30 | 1 | 28 | 8.3 |
| 29 | 13 | 49 | 78 | 0.0 |
| 30 | 40 | 52 | 48 | 0.0 |
| 31 | 18 | 1 | 16 | 9.8 |
| 32 | 8 | 31 | 6 | 9.1 |
| 33 | 13 | 85 | 39 | 1.0 |
Patient no. . | IL-21R, % . | CD38, % . | ZAP70, % . | VH mutations, % . |
|---|---|---|---|---|
| 1 | 34 | 12 | 32 | 9.7 |
| 2 | 30 | 6 | 89 | 0.0 |
| 3 | 33 | 9 | 95 | 9.5 |
| 4 | 13 | 37 | 90 | ND |
| 5 | 26 | 14 | 35 | 5.3 |
| 6 | 10 | 53 | 24 | 1.4 |
| 7 | 10 | 96 | 88 | 0.0 |
| 8 | 6 | 34 | 46 | 9.7 |
| 9 | 10 | 30 | 55 | 2.7 |
| 10 | 15 | 47 | 99 | 0.7 |
| 11 | 14 | 69 | 19 | 9.5 |
| 12 | 16 | 67 | 64 | 0.0 |
| 13 | 29 | 2 | 1 | 7.6 |
| 14 | 19 | 5 | 25 | 3.8 |
| 15 | 34 | 6 | 21 | 5.9 |
| 16 | 18 | 3 | 5 | 5.6 |
| 17 | 48 | 6 | 55 | 3.2 |
| 18 | 35 | 6 | 52 | 0.0 |
| 19 | 49 | 3 | 15 | 0.0 |
| 20 | 47 | 4 | 67 | 4.1 |
| 21 | 2 | 74 | 65 | 0.0 |
| 22 | 28 | 52 | 68 | 0.0 |
| 23 | 40 | 7 | 16 | 7.0 |
| 24 | 20 | 1 | 28 | 10.4 |
| 25 | 25 | 1 | 25 | 8.1 |
| 26 | 24 | 87 | 70 | 0.0 |
| 27 | 16 | 10 | 15 | ND |
| 28 | 30 | 1 | 28 | 8.3 |
| 29 | 13 | 49 | 78 | 0.0 |
| 30 | 40 | 52 | 48 | 0.0 |
| 31 | 18 | 1 | 16 | 9.8 |
| 32 | 8 | 31 | 6 | 9.1 |
| 33 | 13 | 85 | 39 | 1.0 |
ND indicates not determined.
IL-21R expression was assessed by immunoflurescence with a PE-conjugated anti–IL-21R mAb. The percentage of positive cells is shown for each case. ZAP-70 and CD38 expression was evaluated as described in “Materials and methods.”
P < .001, IL-21R versus CD38; P = .6, IL-21R versus ZAP-70; and P = .9, IL-21R versus IgVH mutations.
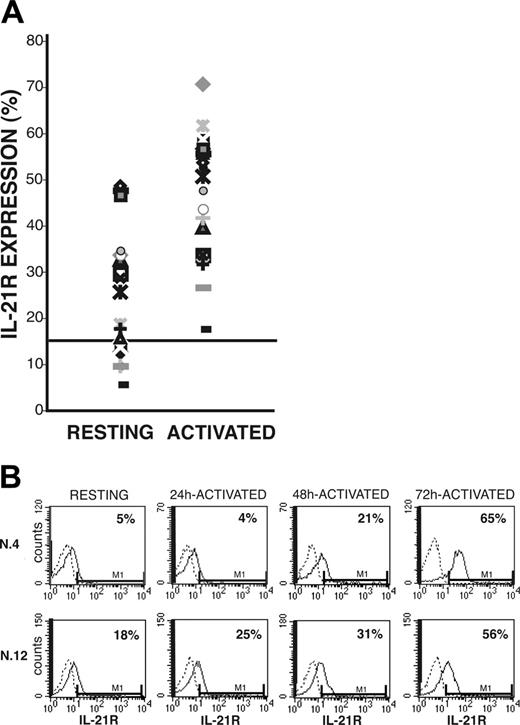
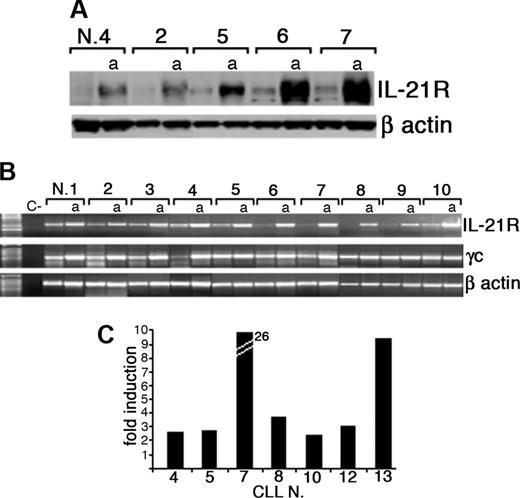
After coincubation of CLL B cells with CD40L-transfected murine fibroblasts, surface IL-21R was significantly up-regulated (activated = 46% ± 13% vs resting = 24% ± 8% IL-21R+ CLL-B cells; n = 20, P < .005) and was then expressed by leukemic cells in all the cases analyzed (Figure 1A). Induction of IL-21R expression was time dependent and progressively increased from 24 hours until 72 hours after starting coincubation with CD40L-expressing cells (Figure 1B). In control experiments the mock-transfected murine fibroblasts (not expressing CD40L) failed to trigger IL-21R expression (data not shown). Western blot analysis further confirmed that CD40 triggering induced up-regulation of IL-21R protein expression, which was associated with an increased IL-21R mRNA expression, as detected by semiquantitative and quantitative RT-PCR analyses (Figure 2). In addition, all CLL B cells expressed mRNA for the γc (Figure 2B), which is required for signaling by IL-2 cytokine family members. However, IL-21 mRNA and protein were not expressed by either resting or CD40-activated CLL B cells (data not shown), suggesting that CLL B cells could be the target of the paracrine activity of IL-21, but denying the possibility of an autocrine IL-21/IL-21R loop in these cells.
Surface IL-21R expression on resting and CD40-activated CLL B cells. Purified CLL B cells, either resting or stimulated by coculture with CD40L-transfected murine fibroblasts, were stained with PE-labeled anti-IL-21R or with an irrelevant PE-labeled Ig as control. (A) The percentage of IL-21R+ resting or CD40-activated B cells from all 20 patients with CLL. The cut-off value, arbitrarely defined as 15%, is indicated by a horizontal line. (B) Time-dependent induction of IL-21R surface expression in 2 representative patients.
Surface IL-21R expression on resting and CD40-activated CLL B cells. Purified CLL B cells, either resting or stimulated by coculture with CD40L-transfected murine fibroblasts, were stained with PE-labeled anti-IL-21R or with an irrelevant PE-labeled Ig as control. (A) The percentage of IL-21R+ resting or CD40-activated B cells from all 20 patients with CLL. The cut-off value, arbitrarely defined as 15%, is indicated by a horizontal line. (B) Time-dependent induction of IL-21R surface expression in 2 representative patients.
IL-21 induces signal transduction through JAK-1 and JAK-3, and STAT-1, STAT-3, and STAT-5 tyrosine phosphorylation in CD40-activated CLL B cells
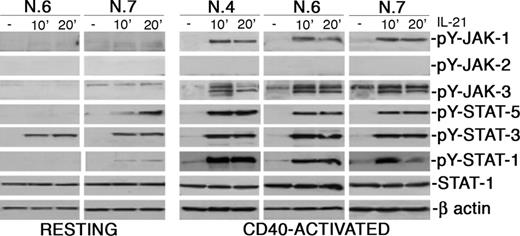
Since IL-21R may activate different signaling pathways in different cell types, we first studied early signaling events triggered by this receptor in CLL B cells. Within 10 minutes from IL-21 stimulation of CD40-preactivated CLL B cells, autophosphorylation of JAK-1 and JAK-3 tyrosine kinases was observed by the use of Ab specific for ptyr 1022/1023 of JAK-1 and ptyr 980 of JAK-3, while JAK-2 phosphorylation seemed not involved (Figure 3). In addition, tyrosine phosphorylation of STAT-1 (substrate of JAK-1), STAT-3, and STAT-5 (both substrates of JAK-3) was rapidly induced (Figure 3). It is noteworthy that signaling via JAK-1 and STAT-1 was undetectable or barely detectable in resting CLL B cells, while signaling through STAT-3 was clearly evident.
IL-21 induces apoptosis of CD40-activated CLL B cells
Since IL-21 can induce maturation and proliferation or, on the contrary, apoptosis of normal B cells, depending on their activation status,17,22-24 we analyzed the possible effects induced by IL-21 signaling on the survival of CLL B cells.
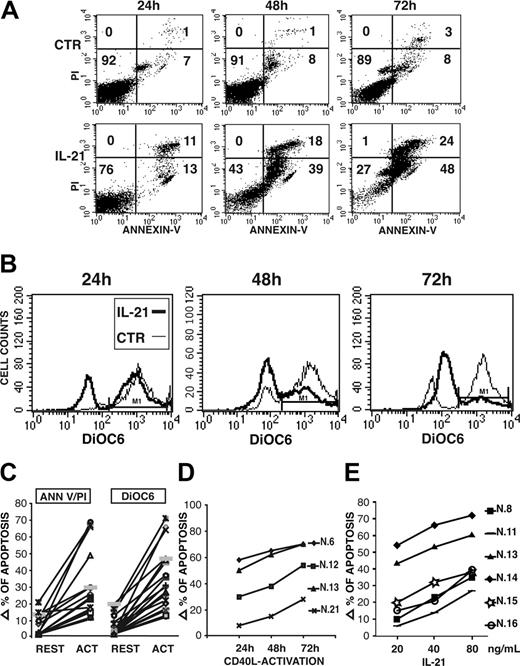
On resting CLL B cells, IL-21 induced a slight increase of the apoptotic cell rate in 6 of 20 cases, while a clearly more potent proapoptotic effect was observed in all CD40-activated CLL B cells (Figure 4A,C), as determined by annexin V/PI staining. Moreover, staining by DiOC6, a green cell-permeable fluorochrome that is concentrated in the charged mitochondria of living cells but not in the depolarized mitochondria of apoptotic cells, showed that IL-21 induces a dramatic drop of membrane mitochondrial potential in CD40-activated CLL B cells (Figure 4B-C). Time-course analysis revealed that apoptosis induced by IL-21 is a slow-rate process, since in most cases significant levels of apoptosis were detectable at 48 hours and further increased at 72 hours after IL-21 treatment (Figure 4A-B). A progressive transition from early apoptotic cells (annexin V+/PI-) to late apoptotic cells (annexin V+/PI+) was also observed. We then studied the time-dependent activation by CD40L-expressing cells on the subsequent induction of apoptosis by IL-21 treatment for 72 hours. In most cases a 24-hour preactivation period by CD40L is sufficient to sensitize CLL B cells to IL-21-induced apoptosis, although effects were maximal by a 72-hour preactivation (Figure 4D). It is noteworthy that CLL B cells of patient 21 had a low constitutive expression of IL-21R and required a longer preactivation period to be sensitized. In addition, the proapoptotic activity of IL-21 was dose dependent, as the percentage of apoptotic cells increased by enhancing IL-21 concentrations from 20 to 80 ng/mL (Figure 4E).
Expression of IL-21R protein and mRNA by CLL B cells is up-regulated by CD40 triggering. (A) Western blot analysis of total protein extracts from resting or CD40-activated (a) purified CLL B cells using an anti-IL-21R or an anti-β-actin antibody. (B) RT-PCR analysis of IL-21R and γc mRNA expression on freshly purified or CD40-activated (a) CLL B cells. A negative control (C-) without RNA was also included. Amplification of the same cDNAs with β-actin-specific primers is also shown. (C) Quantitative RT-PCR analysis indicates an increase of IL-21R mRNA in CD40L-activated CLL B cells with respect to resting cells. Data represent fold of increase and were normalized for the expression of RNA polymerase II mRNA as a housekeeping gene.
Expression of IL-21R protein and mRNA by CLL B cells is up-regulated by CD40 triggering. (A) Western blot analysis of total protein extracts from resting or CD40-activated (a) purified CLL B cells using an anti-IL-21R or an anti-β-actin antibody. (B) RT-PCR analysis of IL-21R and γc mRNA expression on freshly purified or CD40-activated (a) CLL B cells. A negative control (C-) without RNA was also included. Amplification of the same cDNAs with β-actin-specific primers is also shown. (C) Quantitative RT-PCR analysis indicates an increase of IL-21R mRNA in CD40L-activated CLL B cells with respect to resting cells. Data represent fold of increase and were normalized for the expression of RNA polymerase II mRNA as a housekeeping gene.
IL-21 induces tyrosine phosphorylation of JAK-1 and JAK-3 and of STAT-1, STAT-3, and STAT-5, but not of JAK-2 upon ligand binding. CLL B cells were stimulated with rhIL-21 (80 ng/mL) for the indicated time intervals, lysed in appropriate buffers, and analyzed by Western blotting using mAbs specific for phosphorylated or unphosphorylated forms of signal transducer proteins, or for β-actin.
IL-21 induces tyrosine phosphorylation of JAK-1 and JAK-3 and of STAT-1, STAT-3, and STAT-5, but not of JAK-2 upon ligand binding. CLL B cells were stimulated with rhIL-21 (80 ng/mL) for the indicated time intervals, lysed in appropriate buffers, and analyzed by Western blotting using mAbs specific for phosphorylated or unphosphorylated forms of signal transducer proteins, or for β-actin.
IL-21 induces apoptosis of CLL B cells, as detected by annexin V/PI 2-color fluorescence or by analysis of mitochondrial depolarization using DiOC6 staining. IL-21 (40 ng/mL) induces a time-dependent apoptosis of a CD40-activated (72 hours) CLL B-cell population from a representative patient (no. 13) evaluated as 2-color fluorescence analysis by PI and annexin V staining (A) or as mitochondrial depolarization by DiOC6 staining (B). (C) Summary of IL-21-induced apoptosis in either resting or CD40-activated CLL B cells studied is shown. Data are expressed as increments of apoptotic-cell percentages (by PI and annexin V staining) over spontaneous apoptotic rates using a 40-ng/mL concentration of rhIL-21 and a 72-hour stimulation. (D) CLL B cells from 4 representative patients were cocultured with CD40L-transfected cells for different time intervals before treatment with IL-21 (40 ng/mL for 72 hours) and evaluation of apoptosis as in panel A. (E) Induction of apoptosis by IL-21 is dose dependent at concentrations ranging from 20 to 80 ng/mL. Data are expressed as in panel C.
IL-21 induces apoptosis of CLL B cells, as detected by annexin V/PI 2-color fluorescence or by analysis of mitochondrial depolarization using DiOC6 staining. IL-21 (40 ng/mL) induces a time-dependent apoptosis of a CD40-activated (72 hours) CLL B-cell population from a representative patient (no. 13) evaluated as 2-color fluorescence analysis by PI and annexin V staining (A) or as mitochondrial depolarization by DiOC6 staining (B). (C) Summary of IL-21-induced apoptosis in either resting or CD40-activated CLL B cells studied is shown. Data are expressed as increments of apoptotic-cell percentages (by PI and annexin V staining) over spontaneous apoptotic rates using a 40-ng/mL concentration of rhIL-21 and a 72-hour stimulation. (D) CLL B cells from 4 representative patients were cocultured with CD40L-transfected cells for different time intervals before treatment with IL-21 (40 ng/mL for 72 hours) and evaluation of apoptosis as in panel A. (E) Induction of apoptosis by IL-21 is dose dependent at concentrations ranging from 20 to 80 ng/mL. Data are expressed as in panel C.
In accordance with these data, IL-21 failed to stimulate proliferation of resting (data not shown) or CD40-activated CLL B cells (Figure 5A), while IL-15, the cytokine mostly related to IL-21, induced CLL B-cell proliferation, in agreement to previous reports.14 Moreover, IL-21 (40 ng/mL) strongly inhibited the IL-15-triggered proliferation of activated CLL B cells (Figure 5A) and also counteracted the antiapoptotic effects of IL-15 (Figure 5B).
Since induction of apoptosis of CLL B has been recently associated with decreased CD23 expression,40,41 and IL-21 has been reported to down-regulate CD23 expression on normal B cells,22 we also analyzed whether IL-21 may induce such phenotypic changes in CLL B cells. Indeed, IL-21 treatment induced a significant inhibition of CD23 expression in CD40-activated CLL B cells (162 ± 85 vs 35 ± 30, means ± SD of mean fluorescence intensity [MFI] values, n = 5).
IL-21 induces activation of caspases and cleavage of Bid, p27Kip-1, and PARP in CD40-activated CLL B cells
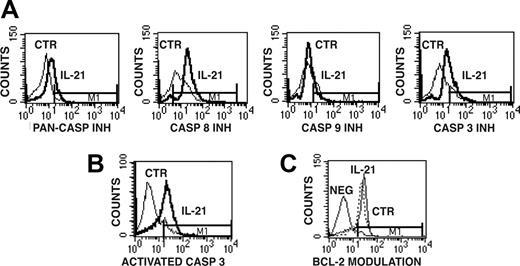
We then analyzed whether IL-21 mediates the activation of caspases in CD40-activated CLL B cells by the use of cell-permeable fluorescent caspase inhibitors, which can covalently bind to active caspases, as probes for cytofluorimetric analyses. The use of the FITC-labeled pancaspase inhibitor (FITC-VAD-FMK) preliminarily indicated that the apoptotic process induced by IL-21 is caspase dependent (Figure 6A). The use of other fluorescent caspase-specific inhibitors, such as FAM-LETD-FMK and FAM-DEVD-FMK, further suggested the involvement of caspase-8 and caspase-3/caspase-7, respectively (Figure 6A), while activation of caspase-9 detected by FAM-LEHD-FMK was less evident. Caspase-3 activation was further verified by immunofluorescence on permeabilized cells using a fluorochrome-labeled Ab specific for the active fragment of caspase-3 (Figure 6B).
IL-15 but not IL-21 induces antiapoptotic effects and CLL B-cell proliferation. The antiapoptotic and mitogenic activities of IL-15 are counteracted by IL-21. (A) CD40-activated CLL B cells were cultured in triplicate wells of 96-well plates in the presence of different concentrations of rhIL-15 and/or rhIL-21 for 48 hours and then pulsed with 3H-thymidine (0.037 MBq/well [1 μCi/well]) for an additional 18 hours of culture. Data are expressed as mean ± SD of 3 repeated experiments. (B) Apoptosis was measured by DiOC6 staining of CLL B cells after 72 hours of culture in the presence, or absence of rhIL-21 and/or rhIL-15 (40 ng/mL). Horizontal bar M1 indicates fluorescence intensity corresponding to signals from viable cells.
IL-15 but not IL-21 induces antiapoptotic effects and CLL B-cell proliferation. The antiapoptotic and mitogenic activities of IL-15 are counteracted by IL-21. (A) CD40-activated CLL B cells were cultured in triplicate wells of 96-well plates in the presence of different concentrations of rhIL-15 and/or rhIL-21 for 48 hours and then pulsed with 3H-thymidine (0.037 MBq/well [1 μCi/well]) for an additional 18 hours of culture. Data are expressed as mean ± SD of 3 repeated experiments. (B) Apoptosis was measured by DiOC6 staining of CLL B cells after 72 hours of culture in the presence, or absence of rhIL-21 and/or rhIL-15 (40 ng/mL). Horizontal bar M1 indicates fluorescence intensity corresponding to signals from viable cells.
Detection of caspase-8 and caspase-3 activation and of Bcl-2 levels in IL-21-treated or untreated CD40-activated CLL B cells. (A) Caspase activation was detected by staining CLL B cells with cell-permeable fluorescent inhibitors of all caspases (PAN-CASP INH) or specific for caspase-8, caspase-3, and caspase-9. The bold line represents IL-21-treated cells, while the thin line represents untreated cells. (B) Permeabilized CLL B cells were analyzed for activated caspase-3 expression by the use of a fluorescent mAb specific for the active form of caspase-3. (C) Intracellular expression of Bcl-2, evaluated by the use of a Bcl-2-specific FITC-conjugated mAb, did not show significant modulation in IL-21-treated cells. Horizontal bar M1 is the marker of positive signal.
Detection of caspase-8 and caspase-3 activation and of Bcl-2 levels in IL-21-treated or untreated CD40-activated CLL B cells. (A) Caspase activation was detected by staining CLL B cells with cell-permeable fluorescent inhibitors of all caspases (PAN-CASP INH) or specific for caspase-8, caspase-3, and caspase-9. The bold line represents IL-21-treated cells, while the thin line represents untreated cells. (B) Permeabilized CLL B cells were analyzed for activated caspase-3 expression by the use of a fluorescent mAb specific for the active form of caspase-3. (C) Intracellular expression of Bcl-2, evaluated by the use of a Bcl-2-specific FITC-conjugated mAb, did not show significant modulation in IL-21-treated cells. Horizontal bar M1 is the marker of positive signal.
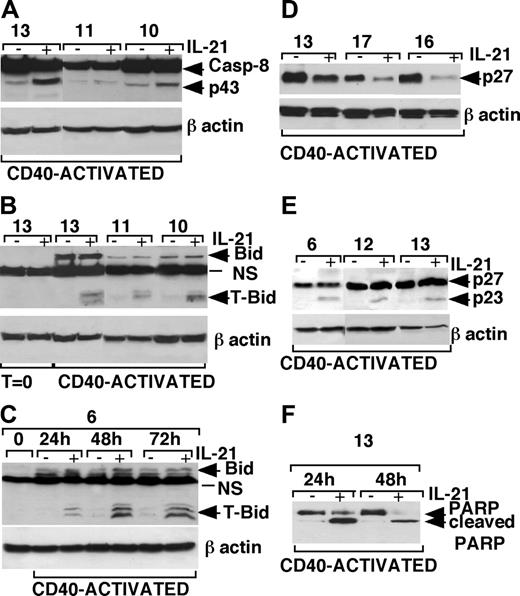
The involvement of caspase-8 was also confirmed by the detection of 43- to 41-kDa intermediate cleavage product of caspase-8 by Western blot analysis of IL-21-treated CLL B cells (Figure 7A). Consistent with this finding, we could also detect cleavage of Bid (Figure 7B), a caspase-8 substrate, to the proapoptotic form t-Bid, which can translocate to the mitochondrial membrane.42,43 It is of note that Bid was undetectable in resting CLL B cells, while it was expressed in CD40-activated CLL B cells, in agreement with a recent report showing induction of Bid after CD40 activation of CLL B cells.44 A 24-hour stimulation with CD40L-expressing cells was sufficient to induce Bid expression in CLL B cells of a representative patient (no. 6) and to sensitize these cells to IL-21-mediated apoptosis and progressive induction of t-Bid (Figure 7C). Moreover, IL-21 treatment resulted in a clear-cut reduction of the caspase-3 substrates p27Kip-145,46 and full-length PARP (Figure 7D,F). The appearance of the low-molecular-weight (23-kDa) breakdown products of p27Kip-1 (detected by an antibody reacting to a N-teminal epitope of p27Kip-1) and of PARP in IL-21-stimulated CLL B cells was strongly suggestive of cleavage of these proteins by activated caspase-3 (Figure 7E-F).
Western blot analysis of caspase-8, Bid, p27Kip-1, and PARP in IL-21-treated or untreated CLL B cells. (A) An increase of caspase-8 intermediate cleavage fragments (p43/p41) was detected in cell lysates of CD40-activated CLL B cells cultured with rhIL-21 (80 ng/mL) from 3 representative patients. (B) The truncated form of Bid (t-Bid) was observed in cell lysates of CD40-activated CLL B cells cultured in the presence of rhIL-21 (80 ng/mL) from 3 representative patients. (C) Bid expression is induced by 24-hour stimulation with CD40L-expressing cells and persisted for 72 hours, and treatment with IL-21 induces the appearance of t-Bid. (D) p27Kip-1 down-regulation was observed after rhIL-21 treatment (80 ng/mL for 72 hours) in lysates from CD40-activated CLL B cells of 3 different patients. (E) The use of a different Ab, recognizing a p27/Kip1 breakdown product, showed a cleavage product of 23 kDa of p27Kip-1 48 hours after IL-21 stimulation. (F) The cleaved form of PARP observed in CD40-activated CLL B cells after 24 hours of culture with IL-21, was further increased after 48 hours of culture in relationship with the increase of apoptotic cells.
Western blot analysis of caspase-8, Bid, p27Kip-1, and PARP in IL-21-treated or untreated CLL B cells. (A) An increase of caspase-8 intermediate cleavage fragments (p43/p41) was detected in cell lysates of CD40-activated CLL B cells cultured with rhIL-21 (80 ng/mL) from 3 representative patients. (B) The truncated form of Bid (t-Bid) was observed in cell lysates of CD40-activated CLL B cells cultured in the presence of rhIL-21 (80 ng/mL) from 3 representative patients. (C) Bid expression is induced by 24-hour stimulation with CD40L-expressing cells and persisted for 72 hours, and treatment with IL-21 induces the appearance of t-Bid. (D) p27Kip-1 down-regulation was observed after rhIL-21 treatment (80 ng/mL for 72 hours) in lysates from CD40-activated CLL B cells of 3 different patients. (E) The use of a different Ab, recognizing a p27/Kip1 breakdown product, showed a cleavage product of 23 kDa of p27Kip-1 48 hours after IL-21 stimulation. (F) The cleaved form of PARP observed in CD40-activated CLL B cells after 24 hours of culture with IL-21, was further increased after 48 hours of culture in relationship with the increase of apoptotic cells.
Conversely, we were unable to detect significant changes in Bcl-2 (Figure 6C), Bcl-XL and Bcl-XS, and BAX (data not shown), which are involved in distinct pathways of apoptosis.
Discussion
In this study we show that IL-21R is expressed by leukemic cells freshly collected from most of the B-CLL patients studied and that its expression is up-regulated by stimulation through the CD40 molecule. Although the analysis of the correlation of IL-21R with prognostic markers was beyond the purpose of this study, surface IL-21R expression appeared inversely related to CD38 levels, but not to ZAP-70 or VH gene mutational status, albeit in a relatively small cohort of CLL patients.
Because the IL-21R is a functional signaling molecule in CLL B cells, the existence of a paracrine loop involving IL-21, which is produced by activated T helper cells,17 cannot be excluded in the immune control of IL-21R+ CLL. Indeed, IL-21 induced the rapid activation of the JAK-1/STAT-1 and of the JAK-3/STAT-3 and STAT-5 pathways, followed by the induction of an apoptotic process. The proapoptotic activity of IL-21 was capable of counteracting the antiapoptotic and growth factor activity of the structurally related cytokine IL-1514 in vitro, indicating an opposite function of these 2 factors in the regulation of CLL B-cell survival and proliferation.
It must be emphasized that IL-21R has no death domain and therefore it cannot directly trigger apoptosis via Fas-associated death domain (FADD)/caspase-8. However, IL-21 has been reported to induce apoptotic cell death in normal resting or CD40-activated B lymphocytes.22,24 Our present in vitro data indicate that IL-21 induced apoptosis in resting B cells of some CLL patients, although this effect was more evident in CD40-activated CLL B cells. This finding may reflect the increased expression of IL-21R in CD40-activated CLL B cells, although CD40 stimulation may also potentiate IL-21-activated apoptotic pathways, such as those involving Bid.42,43 This possibility was suggested by the finding that Bid is undetectable in resting CLL B cells but its expression is induced as soon as 24 hours after CD40 triggering, in agreement with a previous report.44 Indeed, time-course experiments indicated that 24-hour prestimulation by CD40L-expressing cells is also sufficient to sensitize CLL B cells to the proapoptotic activity of IL-21 in most patients studied, with the exception of those cases lacking constitutive IL-21R expression.
Our data suggest a possible involvement of STAT-1 in IL-21-induced apoptosis, although the lack of available STAT-1-specific inhibitors (to the best of our knowledge) did not allow us to directly prove this hypothesis. Thus, STAT-1 tyrosine phosphorylation levels were very low or undetectable in IL-21-treated resting CLL B cells, while IL-21 significantly increased pSTAT-1 levels in CD40-activated CLL B cells, which undergo apoptosis. In addition, a previous report showed that IL-21 induced proliferation of human myeloma cells through tyrosine phosphorylation of STAT-3, while STAT-1 phosphorylation was not involved.35 It is well known that the JAK-1/STAT-1 pathway may deliver proapoptotic signals in different cell types,47 and STAT-1 has been proposed to act as a tumor suppressor gene.48 The role of STAT-1 as inducer of lymphoid-cell apoptosis is also supported by the observation that lymphocytes from STAT-1-deficient mice display increased proliferation and diminished apoptosis.49 Interestingly, previous reports showed that gene therapy by reinfusion of CD40L-transduced autologous CLL B cells induced clinical and immunologic responses,50 which were associated with tyrosine phosphorylation of STAT-1 in CLL B cells.51 Since in vitro contact with CD40L-transduced cells failed to induce substantial STAT-1 tyrosine phosphorylation in CLL B cells, it has been proposed that cytokines induced in vivo by CD40L gene therapy could be responsible for STAT-1 pathway activation. Our present in vitro data support this previous hypothesis and suggest IL-21 as a potential candidate cytokine involved in the CD40L gene therapy effects. On the other hand, the possibility of using recombinant IL-21 in combination with CD40L gene therapy, or with other stimuli up-regulating IL-21R expression, may be considered as a potential approach for CLL B treatment.
The results obtained by staining with a fluorescent caspase-8 inhibitor and the detection of cleavage products of caspase-8 by Western blot suggested that IL-21-triggered apoptosis in CD40-activated CLL B cells is associated with caspase-8 activation. In addition, we also detected cleavage of Bid, a caspase-8 substrate, to its proapoptotic form t-Bid, which has the ability to translocate to the mitochondrial membrane and act as mediator of caspase-8 apoptosis.42,43 In keeping with this observation, IL-21 induced an evident drop in mitochondrial membrane polarization in activated CLL B cells. The finding that resting CLL B cells respond poorly to IL-21 proapoptotic signals could also depend on the lack of Bid expression in these cells, while Bid was induced upon stimulation via CD40, in agreement with a recent report.44 Our data further indicate the involvement of caspase-3 in IL-21-mediated apoptosis. Thus, activation of caspase-3 was associated with cleavage of its substrates p27Kip-1 and PARP. P27Kip-1, an inhibitor of cell-cycle progression, is highly expressed in CLL B cells, and high expression levels have been related to a worse prognosis of B-CLL.52 Interestingly, p27Kip-1 degradation by caspase-3 was also observed in fludarabine- or flavopiridol-treated CLL B cells,45,46 thus suggesting overlaps of apoptotic pathways in drug- or IL-21-triggered apoptosis of CLL B cells.
In conclusion, our data indicate that IL-21 may play a relevant role in the control of the balance between proapoptotic and survival/proliferation signals in CLL B cells by promoting apoptosis and counteracting the activity of CLL growth factors, such as IL-15.
Prepublished online as Blood First Edition Paper, January 3, 2006; DOI 10.1182/blood-2005-09-3535.
Supported by L'Associazione Italiana per la Ricerca sul Cancro (AIRC), the Italian Ministry of Health, and Comitato Interministeriale per la Programmazione Economica (CIPE).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 5. IL-15 but not IL-21 induces antiapoptotic effects and CLL B-cell proliferation. The antiapoptotic and mitogenic activities of IL-15 are counteracted by IL-21. (A) CD40-activated CLL B cells were cultured in triplicate wells of 96-well plates in the presence of different concentrations of rhIL-15 and/or rhIL-21 for 48 hours and then pulsed with 3H-thymidine (0.037 MBq/well [1 μCi/well]) for an additional 18 hours of culture. Data are expressed as mean ± SD of 3 repeated experiments. (B) Apoptosis was measured by DiOC6 staining of CLL B cells after 72 hours of culture in the presence, or absence of rhIL-21 and/or rhIL-15 (40 ng/mL). Horizontal bar M1 indicates fluorescence intensity corresponding to signals from viable cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/9/10.1182_blood-2005-09-3535/2/m_zh80090694950005.jpeg?Expires=1769164739&Signature=G6Md1XyZKWcBvevf3fN0e1cXWHqrXO6tpJryqkPA-dIiz2vFmTHygTLqc5mmidrjb4FsIcT8NyBm2RrEA9iXpzaU0TaZRutTg-EBeeTNwVBJVSJeNretkARAmbBW9psVf~bLkbIgPjdqxGeAYTHEJVyFvpUGw5CF-KvXKAGMQKIGhGDrP0NcN6LCnJSmWlPoukQZN2696oj2qcM5fPE8ilcLBDhA5aZkVj6QhtPPfXexxycVrvFUcQ8abrktSeFCAa6lcwLD3zuTS9ElixxZVwDpC138d3LPk8bASA2PIkq1RVCNKQXIhFZX4sr8mCF4I74EVE-theCVvNlvHE1Oig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal