Comment on Martin et al, page 270
The role self-MHC recognition by naive CD4+ T cells plays in their maintenance has been the subject of a see-saw debate for the past decade. The study by Martin and colleagues provides new insight into this question that should satisfy both sides.
The limited ability of a thymocyte's T-cell antigen receptor to recognize self-peptide MHC complexes has long been recognized as the key event that triggers positive selection and maturation of T cells in the thymus. More recently, it has become an attractive concept that the same low-avidity self-recognition should continue to play a role in the physiology of naive T cells in peripheral tissues. One suggested function for self-recognition has been to regulate T-cell survival.1,2 Such a concept has important implications for how the peripheral T-cell repertoire might be shaped and maintained and how it might react to environmental traumas that affect its size and composition. More recent studies using different or refined model systems, however, challenged these earlier findings, and failed to detect any such role for self-recognition in naive T-cell survival.3,4 Providing a definitive answer to such an important question has been hindered by the complex interactions that exist between the various components of a normal T-cell compartment (CD4 vs CD8, naive vs memory), each of which has both unique and shared survival requirements. A further complication comes from the fact that cytokines such as IL-7 also play a key role in regulating naive T-cell survival and that IL-7 levels can vary greatly depending on the host environment.
In this issue of Blood, Martin and colleagues provide data that can account for the apparent conflicts in the literature and, in doing so, provide a definitive answer. Self-recognition does play a role in survival of CD4+ T cells but only when other survival factors are limiting. The authors' insight comes from 2 key observations. First, that the Aβ-/- mice commonly used as class II MHC-deficient hosts are not in fact completely devoid of such molecules. Class II Aα Eβ heterodimers can still form in these mice. Therefore, using mice that lack both the α and β chains of I-A and I-E class II MHC molecules, the authors were able to examine the survival of naive CD4+ T cells in the complete absence of any class II MHC molecules. Second, Martin et al compare the survival of CD4+ T cells in either lymphopenic or replete class II-deficient hosts, using either CD3ϵ-/- mice that lack any T cells or mice with an intact CD8 T-cell compartment. In the latter case, the host CD8+ T cells provide competition for other survival factors, such as IL-7.
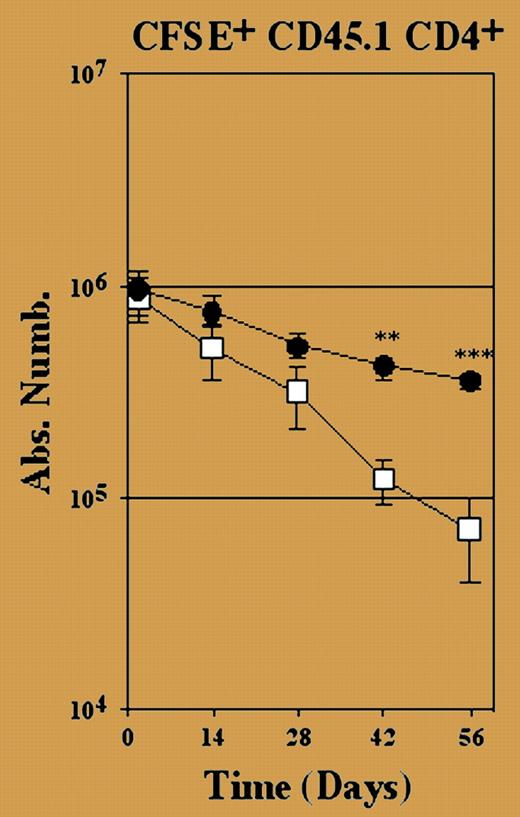
Consistent with previous reports,3,4 naive CD4+ T cells survived and indeed underwent lymphopenia-induced proliferation in the complete absence of class II MHC molecules, but only when hosts lacked any endogenous T cells. In hosts with an intact CD8+ T-cell compartment, naive CD4+ T cells were rapidly lost. Crucially, Martin et al show that this loss was even greater than observed when the same cells were transferred to normal “full” hosts (see figure). Under these circumstances, transferred cells gradually decline due to competition with existing and newly generated host T cells for survival factors, thus suggesting that competition for self-MHC is constantly shaping our T-cell repertoire. ▪
Survival of CD4+ T cells depends on interactions with MHC class II molecules in a nonlymphopenic environment. See the complete figure in the article beginning onpage 270.
Survival of CD4+ T cells depends on interactions with MHC class II molecules in a nonlymphopenic environment. See the complete figure in the article beginning onpage 270.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal