Comment on Haberichter et al, page 3344
Haberichter and colleagues describe a specific subset of patients with type 1 von Willebrand disease in 4 different families and suggest that an increased ratio between the von Willebrand propeptide and von Willebrand factor antigen may indicate a true genetic defect and decreased VWF survival.
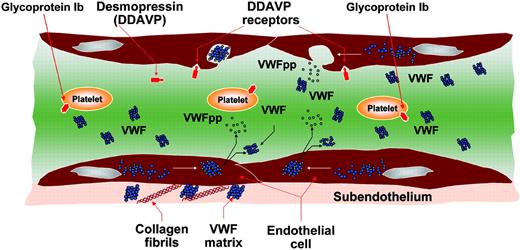
Von Willebrand factor (VWF) is a large adhesive multimeric glycoprotein that plays major roles in hemostasis by mediating platelet adhesion and aggregation at the sites of vessel wall injury and serving as the carrier of factor VIII. The primary product of the VWF gene, located at chromosome 12p13.2, is a 2813–amino acid protein made of a signal peptide of 22 amino acids, a large propeptide of 741 amino acids, and a mature VWF molecule of 2050 amino acids. The pre-pro VWF, synthesized in endothelial cells (ECs) and megakaryocytes (MKs), undergoes intracellular modifications including signal peptide cleavage, C-terminal dimerization, glycosylation, sulfation, and aminoterminal multimerization. Then proteolysis occurs in the trans-Golgi where the VWF propeptide (VWFpp) is cleaved but remains stored together with mature VWF in alpha-granules (MKs) and Weibel-Palade bodies (ECs). After secretion into plasma, VWFpp dissociates from VWF and can be measured with specific antibodies. VWFpp circulates at a concentration of about 1 μg/mL with a half-life of 2 to 3 hours, whereas mature VWF circulates at about 10 μg/mL with a half-life of 8 to 12 hours.1,2 Several physiologic or pathologic stimuli can release VWF and VWFpp from ECs: desmopressin (DDAVP) is the first-choice treatment of mild forms of von Willebrand disease (VWD) because it can increase plasma VWF levels by releasing it from ECs.3 These events are summarized in figure (see below). Type 1 VWD is characterized by partial quantitative deficiency of VWF with autosomal dominant inheritance and a high variable phenotype.3 Most patients with type 1 VWD show good biologic responses to DDAVP; however, heterogeneous half-lives of VWF activities can be found (see figure on the next page). Recently, a novel mechanism for type 1 VWD has been linked to increased clearance of VWF from plasma.4 VWFpp has also been recommended to identify patients with acquired von Willebrand syndrome who might have increased clearance of VWF because of autoantibodies to VWF.5 FIG1
Pictorial representation of the events related to VWF biosynthesis and release induced by desmopressin (DDAVP).
Pictorial representation of the events related to VWF biosynthesis and release induced by desmopressin (DDAVP).
Haberichter and colleagues report a peculiar decreased survival of VWF in 4 families with moderately severe inherited type 1 VWD. In fact, VWF half-lives were significantly shorter (1-3 hours) than in healthy individuals, whereas the half-life of VWFpp was normal. Affected individuals of these 4 families were characterized by low plasma VWF antigen (VWF:Ag) and factor VIII levels, proportionately low ristocetin cofactor activity, and dominant inheritance. Single novel heterozygous mutations were found in affected members: S2179F in 2 families and W1144G in 2 families.
In conclusion, data by Haberichter et al suggest that plasma VWFpp can be a useful additional marker for identifying inherited type 1 VWD patients with a shortened VWF half-life and open new insights in the field of VWD management. However, since this VWFpp assay has not been standardized yet, more patients with inherited and acquired defects of VWF should be evaluated in controlled multicenter laboratory studies.
Dr. Federici is associate professor of hematology at the University of Milan School of Medicine.▪FIG2
Biologic responses to DDAVP in 26 patients with type 1 VWD. Changes of factor VIII (FVIII:C), ristocetin cofactor (VWF:RCo), and bleeding time (BT) before and following DDAVP.3
Biologic responses to DDAVP in 26 patients with type 1 VWD. Changes of factor VIII (FVIII:C), ristocetin cofactor (VWF:RCo), and bleeding time (BT) before and following DDAVP.3


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal