Abstract
Selectins and their ligand P-selectin glycoprotein ligand-1 (PSGL-1) mediate leukocyte rolling along inflamed vessels. Cell rolling is modulated by selectin interactions with their ligands and by topographic requirements including L-selectin and PSGL-1 clustering on tips of leukocyte microvilli. Lipid rafts are cell membrane microdomains reported to function as signaling platforms. Here, we show that disruption of leukocyte lipid rafts with cholesterol chelating agents depleted raft-associated PSGL-1 and L-selectin and strongly reduced L-, P-, and E-selectin–dependent rolling. Cholesterol repletion reversed inhibition of cell rolling. Importantly, leukocyte rolling on P-selectin induced the recruitment of spleen tyrosine kinase (Syk), a tyrosine kinase associated to lipid raft PSGL-1. Furthermore, inhibition of Syk activity or expression, with pharmacologic inhibitors or by RNA interference, strongly reduced leukocyte rolling on P-selectin, but not on E-selectin or PSGL-1. These observations identify novel regulatory mechanisms of leukocyte rolling on selectins with a strong dependency on lipid raft integrity and Syk activity.
Introduction
Selectins initiate leukocyte tethering and rolling along the inflamed vascular wall.1-3 L-selectin is expressed by most leukocytes, whereas P- and E-selectin expression is induced on activated platelets and/or endothelial cells. In early inflammation, leukocyte rolling depends mainly on P-selectin interactions with P-selectin glycoprotein ligand-1 (PSGL-1).4,5 PGSL-1 is also a major ligand for L-selectin and mediates secondary interactions between circulating and adherent leukocytes or leukocyte fragments.6-9 In addition, PSGL-1 cooperates with other adhesion receptors to support leukocyte rolling on E-selectin.10-13
Several observations indicate that PSGL-1 is a signaling molecule. Engagement of leukocyte PSGL-1 induces Ras and MAPK activation as well as interleukin-8, tissue factor, or colony-stimulating factor-1 secretion.14-17 PSGL-1 interactions with P-selectin promote the binding of PSGL-1 cytoplasmic domain to actin-binding proteins ezrin and moesin.18-20 Moesin serves as an adaptor between PSGL-1 and Syk.21 PSGL-1 engagement induces Syk tyrosine phosphorylation and generates intracellular signals leading to expression of early-immediate activation genes such as c-fos.21 Whether Syk is involved in regulating leukocyte rolling has not yet been examined.
PSGL-1 and L-selectin are detectable in membrane lipid rafts of human cell lines.22,23 The lipid raft model proposes that these cholesterol- and glycosphingolipid-rich microdomains function as key cellular membrane signaling platforms.24,25 We therefore examined whether lipid rafts were required for PSGL-1 and L-selectin function. Moreover, as Syk was shown to colocalize in lipid rafts with tyrosine phosphatase CD45 upon B-lymphocyte activation via the B-cell antigen receptor,26 we examined here whether Syk was recruited in lipid rafts upon PSGL-1 engagement.
Our results show for the first time that (1) lipid raft integrity is essential to support leukocyte rolling on P- and E-selectin and PSGL-1 and (2) optimal rolling on P-selectin requires Syk-dependent signaling by raft-associated PSGL-1. These observations identify key novel regulatory mechanisms of leukocyte trafficking.
Materials and methods
Cells
Neutrophils were prepared from healthy blood donors by Ficoll centrifugation, dextran sedimentation, and erythrocyte hypotonic lysis. KG1 (CRL-246 and CRL-246.1; ATCC, Manassas, VA) and Jurkat cells were cultured in RPMI 1640 containing 10% fetal calf serum (Invitrogen, Basel, Switzerland) and 1% penicillin/streptomycin. CHODHFR– cells stably coexpressing core-2 β1,6-N-acetylglucosaminyltransferase, fucosyltransferase-VII, and PSGL-1 cDNAs were cultured as described.27
Proteins and antibodies
Anti–L-selectin monoclonal antibody (mAb) LAM1-14,28 anti–PSGL-1 mAb PS5,27 anti-CLA mAb HECA-452 (HB-11485; ATCC), and CSLEX-1 (HB-10135; ATCC) were purified from hybridoma culture media. Anti-PSGL1 mAb KPL1, PE-labeled anti–L-selectin mAb DREG-56, FITC-labeled HECA-452 mAb, and FITC- or PE-labeled rat and mouse IgM were from BD Biosciences (Basel, Switzerland). Isotypic mouse IgG1 mAbs, goat anti–mouse Ig-PE F(ab′)2, goat anti–mouse Ig-HRP, and FITC-labeled rabbit anti–human IgM heavy chain antibody were obtained from Dako Cytomation (Zug, Switzerland). Anti-Syk mAb 4D10.1 was from Upstate (Lake Placid, NY). Recombinant soluble human P-selectin, E-selectin, and ICAM-1 were purchased from R&D Systems (Abingdon, United Kingdom). PSGL-1/human IgM heavy chain chimera (PSGL-1/μ) and L-selectin/μ chimera were isolated from culture media of CHO cells stably transfected with PSGL-1, core-2 β1-6, N-acetylglucosaminyltransferase, and fucosyltransferase VII cDNAs.27
Detergent-resistant cell lysate fractions
Detergent-insoluble cell lysate fractions were isolated on discontinuous sucrose gradients.29,30 Briefly, cells (0.5-2 × 108) were washed and lysed for 20 minutes on ice in 0.2 mL to 1 mL lysis buffer. Lysis buffer composition was as follows: 25 mM Tris-HCl pH 7.6 containing 150 mM NaCl, 5 mM EDTA, 0.5% Brij 58 (Sigma-Aldrich, St Louis, MO) supplemented with protease inhibitors (2 μg/ml aprotinin [Bayer, Zurich, Switzerland], 35 μg/mL PMSF, 10 μg/mL leupeptin, and 100 μg/mL benzamidine σ) and phosphatase inhibitors (1 mM sodium orthovanadate, 30 mM disodium pyrophosphate, and 10 mM glycerophosphate). Cell lysates were homogenized with 10 strokes of a loose-fitting Dounce homogenizer (Fisher Scientific, Wohlen, Switzerland) and gently mixed for 10 minutes on ice with an equal volume of sucrose 80% (wt/vol) in MNE buffer (25 mM MES pH 6.5, 150 mM NaCl, 5 mM EDTA). Two layers of 30% and 5% sucrose in MNE buffer were added successively. The discontinuous gradient was spun for 18 hours at 200 000g at 4°C in Beckman TLS55 or SW55TI rotors. Fractions collected from the gradient top were used immediately or kept frozen at –20°C until use.
Cholera toxin assay
Detergent-insoluble cell lysate fractions collected from sucrose gradients were evaluated for the presence of lipid rafts using a cholera toxin binding assay.31 Briefly, 2 μL of each fraction was dot blotted onto Trans-Blot Transfer Medium Pure Nitrocellulose Membrane (0.45 μm; Bio-Rad Laboratories, Reinach, Switzerland). Membranes were then dried for 10 minutes, blocked for 1 hour at room temperature with 6% bovine serum albumin in PBS-0.1% Tween 20 (vol/vol) (Bio-Rad Laboratories), and incubated for 1 hour with HRP-conjugated cholera toxin (0.084 ng/mL; Sigma-Aldrich). Cholera toxin binding was revealed by chemiluminescence (ECL Plus; Amersham Pharmacia Biotech, Dübendorf, Switzerland).
Lipid raft disruption
Cells were cultured in RPMI/10% FCS supplemented with methyl-β-cyclodextrin (mβCD; Sigma-Aldrich) or filipin III (Sigma-Aldrich) for 10 minutes at 37°C. mβCD or filipin III treatment did not affect cell viability, as assessed by trypan blue exclusion and staining with propidium iodide. Raft disruption following cell exposure to mβCD or filipin III was verified using the cholera toxin assay described in “Cholera toxin assay.”
Western blotting
Fractions or whole-cell lysates were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Nitrocellulose membranes were blocked for 1 hour in TBS–Tween 20 containing 5% milk (wt/vol). PSGL-1 was detected with anti–PSGL-1 mAb PS5 (3 μg/mL), L-selectin with LAM1-14 mAb (3 μg/mL), and Syk with anti-Syk mAb 4D10.1 (0.2 μg/mL), overnight at 4°C. mAb binding was revealed with HRP-conjugated goat anti–mouse IgG (1/10 000) for 30 minutes at room temperature and enhanced chemiluminescence. Extensive washes in TBS–Tween 20 (0.1% vol/vol) were performed between all steps. Densitometric scanning was performed using an ImageScanner (Amersham Pharmacia Biotech). Results were quantified with the ImageMaster TotalLab software (Amersham Pharmacia Biotech).
Immunophenotypic analysis
Electron microscopy
Cells were fixed with 2% paraformaldehyde (Fluka, Buchs, Switzerland) and 0.2% glutaraldehyde (Electron Microscopy Systems, Fort Washington, PA) in 0.1 M Sörensen phosphate buffer, pH 7.4, for 1 hour, at 4°C. After cell centrifugation, pellets were embedded in 2% low-viscosity agarose. Specimens were then dehydrated in ethanol and embedded in LR White resin. Ultrathin sections on formwar-carbon–coated grids (Electron Microscopy Systems) were incubated for 3 minutes on a drop of 1% normal goat serum in PBS and immunoreacted for 20 hours at 4°C with PS5 mAb (100 μg/mL in PBS containing 0.5% bovine serum albumin and 0.05% Tween 20) or isotypic mAb. After rinsing with PBS/Tween, sections were incubated for 3 minutes with 1% goat serum in PBS and for 30 minutes with colloidal gold particle–conjugated goat antimouse antibody (Jackson Immunoresearch Laboratories, West Grove, PA). After staining with uranyl acetate and lead citrate, cell sections were observed in a Philips CM 12 electron microscope (Philips, Eindhoven, the Netherlands) at 80 kV, using a 50 μm objective aperture.
Flow adhesion assays
Adhesion assays were performed under constant shear stress in a parallel plate flow chamber (GlycoTech, Rockville, MD) mounted on a glass coverslip coated with recombinant P- or E-selectin (0.25 μg in 50 μL 0.1 M borate buffer pH 8.5) or PSGL1/μ chimera (0.5 μg in 50 μL 0.1 M borate buffer pH 8.5) captured by goat anti–human IgM antibody. Cells (106/mL in RPMI-1640/1% FCS) were perfused through the chamber for 10 minutes at room temperature. Cell interactions with recombinant selectins or PSGL1/μ were recorded using a phase contrast microscope (Leica Leitz DM IL, Renens, Switzerland), a high-resolution Sony CCD-IRIS videocamera (Sony, Lausanne, Switzerland) and a S-VHS-recorder (Panasonic MD830, Telecom; Panasonic, Lausanne, Switzerland) and assessed at 3 to 6 minutes of perfusion.27,32,33 For some experiments, cells were preincubated with piceatannol or genistein (15 minutes at 37°C), or with mβCD or filipin III (10 minutes at 37°C). Cells were then washed and used for rolling assays. Proteinase K treatment was performed by incubating 106 cells for 20 minutes at 37°C with proteinase K (1.7 μg/mL in PBS).34 The protease activity was inhibited by adding for 5 minutes 35 μg/mL PMSF in PBS supplemented with 6% FCS. Loss of PSGL-1 expression was verified before performing flow adhesion assays.
Adhesion assay on ICAM-1
Static adhesion assays were performed at room temperature on glass coverslips, coated with recombinant ICAM-1 (0.25 μg in 50 μL 0.1 M borate buffer pH 8.5), mounted at the bottom of a flow chamber. Neutrophils (106/mL) were perfused for 3 minutes under a constant shear stress of 0.5 dyn/cm2 in RPMI-1640 1% FCS containing 10 nM fMLP. Cells were then allowed to settle for 3 minutes. Loosely adherent cells in the chamber were removed by perfusing cell-free RPMI-1640 1% FCS at 0.5 dyn/cm2. Cell detachment was induced by increasing the shear flow every 45 seconds to a maximum of 12 dyn/cm2. Stably adherent cells were recorded by videomicroscopy and counted in 10 fields of 0.253 mm2.
Leukocyte activation assay
CHO cells stably transfected with P-selectin cDNA or mock-transfected were grown to confluence in 6-well plates (Costar, Corning, NY). U937 cells, KG1 cells, or neutrophils were suspended at 106 cells/mL in Hanks buffer and added to CHO-P cell monolayers kept under rotation (65 rpm) at 37°C. After 10 minutes, cells were gently detached with a pipette, collected, centrifuged for 2 minutes at room temperature, and lysed in MNE buffer. For lipid raft isolation, cell lysates were fractionated as described in “Detergent-resistant cell lysate fractions.”
Transfection of siRNA
KG1 cells (3 × 106) were suspended in Cell line Nucleofector Kit V solution (Amaxa Biosystems, Cologne, Germany) and mixed with 3 μg Syk-specific siRNA (final concentration 2.5 μM; HP validated SiRNA; Qiagen, Hombrechtikon, Switzerland). Nucleofections were performed according to the manufacturer recommendations (Amaxa Nucleofector II apparatus). Transfected cells were then cultured for 48 hours in 6-well plates at 37°C. Mock-transfected cells and KG1 cells transfected with a nontargeting siRNA (Dharmacon, Perbio Science SA, Lausanne, Switzerland) were used as controls. After 48 hours, KG1 cells were used to assess Syk mRNA expression, normalized to that of GAPDH, by real-time quantitative PCR (Quantitect primer assay; Qiagen), according to manufacturer instructions and to perform rolling adhesion assays. Similar proportions of apoptotic KG1 cells were observed 48 hours after transfection with Syk siRNA, nontargeted siRNA, or mock-transfected cells (∼25%). Cell apoptosis or death was assessed by cell staining with FITC-conjugated annexin V and propidium iodide (Annexin V–FITC Apoptosis Detection Kit I; BD Biosciences).
Statistical analysis
Analysis of variance, Tukey multiple comparison test, or Kruskal-Wallis nonparametric analysis of variance were used to assess statistical significance of differences between groups. Nonparametric Mann-Whitney test was used to compare medians of 2 unpaired groups. Wilcoxon or parametric paired t tests were used to compare paired groups. P values less than .05 were considered significant.
Results
L-selectin and PSGL-1 are localized in lipid rafts
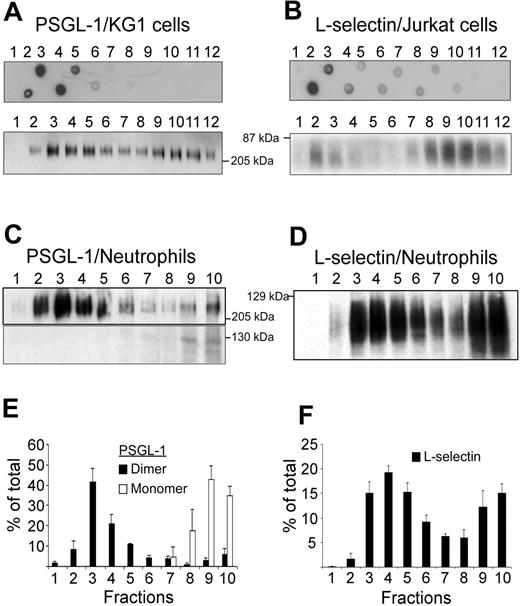
KG1 or Jurkat cell lysates were fractionated by ultracentrifugation on discontinuous sucrose gradients to isolate detergent-resistant fractions corresponding to lipid rafts. Lipid raft–containing fractions were identified by dot-blot analysis using cholera toxin B subunit as marker (Figure 1A-B, upper panels). Lipid rafts were detected in fractions 2 to 5 obtained after sedimentation of KG1 cell lysates (Figure 1A, upper panel), in fractions 2 and 3 of Jurkat cell lysates (Figure 1B, upper panel), and in fractions 2 to 5 of neutrophil lysates (not illustrated). PSGL-1 was detected in raft-containing fractions 2 to 5 from KG1 cell lysates and less abundantly in high-density fractions 10 to 12 (Figure 1A, lower panel). Only dimeric PSGL-1 was detected in membrane fractions isolated from KG1 cells. L-selectin was predominantly detectable in fractions 2 to 3 and 8 to 12 from Jurkat cells (Figure 1B, lower panel). PSGL-1 (Figure 1C,E) and L-selectin (Figure 1D,F) were also evaluated in neutrophil lysates. The distribution of neutrophil membrane PSGL-1 was bimodal and 2 molecular forms were detectable. Lipid raft fractions 2 to 5 contained dimeric PSGL-1, whereas nonraft fractions 8 to 10 contained both monomeric and dimeric forms (Figure 1C,E). Densitometric evaluation of Figure 1C showed that dimeric PSGL-1 represented 88% ± 9% and monomeric PSGL-1 12% ± 9% of total PSGL-1. Dimeric PSGL-1 was detected in fractions 2 to 5 (81% ± 16%) and in fractions 8 to 10 (9% ± 5%). L-selectin distribution in neutrophil membrane lysates was also bimodal (Figure 1D,F) with lipid raft fractions 2 to 5 containing 51% ± 7% of L-selectin and nonraft fractions 8 to 10 containing 33% ± 7%.
Leukocyte rolling on P-selectin, L-selectin, E-selectin, and PSGL-1 is dependent on membrane lipid raft integrity
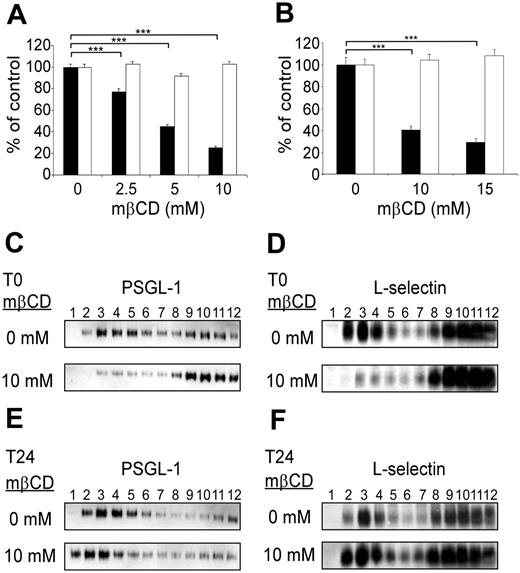
The role of PSGL-1, L-selectin, and E-selectin ligand expression in lipid rafts in regulating leukocyte rolling was evaluated by performing flow adhesion assays after lipid raft disruption with the cholesterol-depleting agent methyl-β-cyclodextrin (mβCD; Figure 2). Rolling assays were performed immediately after cell exposure to mβCD (Figure 2, black columns) or following a 24-hour recovery period in mβCD-free medium (Figure 2, open columns). mβCD strongly inhibited KG1 cell rolling on P-selectin (Figure 2A, black columns). Cell exposure to mβCD (2.5 mM, 5 mM, or 10 mM) inhibited cell rolling by 25% ± 3%, 56% ± 2%, or 76% ± 2% (mean ± SEM; n = 4; P < .001). Interestingly, mβCD treatment was associated with a loss of PSGL-1 expression in raft-containing fractions (Figure 2C, lanes 2 to 5) with more dimeric PSGL-1 being expressed in higher density fractions (Figure 2C, bottom panel, lanes 8 to 12). It has to be mentioned that mβCD did not induce monomerization of PSGL-1. After 24 hours of culture in serum-containing medium, there was a recovery of cell rolling (Figure 2A, open columns) and reexpression of dimeric PSGL-1 in lipid rafts (Figure 2E, bottom panel, lanes 1 to 5).
Detection of PSGL-1 and L-selectin in lipid rafts. (A) Detergent-insoluble fractions of KG1 or Jurkat cell lysates were isolated at the 5%/30% interface of a discontinous sucrose gradient. Lipid rafts containing fractions isolated from KG1 cells (A, top panel) or Jurkat cells (B, top panel) were revealed by dot-blot analysis, using biotinylated B-subunit of cholera toxin (A and B, top panels). After electrophoresis on a 7.5% SDS polyacrylamide gel, gradient fractions isolated from KG1 cells (A, bottom panel), Jurkat cells (B, bottom panel), or neutrophils (C and D) were examined for the presence of PSGL-1 (A and C) or L-selectin (B and D) by immunoblotting. (E) Histogram of PSGL-1 levels in fractions 1 to 10 (mean ± SEM, n = 3). (F) Histogram of L-selectin levels in fractions 1 to 10 (mean ± SEM, n = 4).
Detection of PSGL-1 and L-selectin in lipid rafts. (A) Detergent-insoluble fractions of KG1 or Jurkat cell lysates were isolated at the 5%/30% interface of a discontinous sucrose gradient. Lipid rafts containing fractions isolated from KG1 cells (A, top panel) or Jurkat cells (B, top panel) were revealed by dot-blot analysis, using biotinylated B-subunit of cholera toxin (A and B, top panels). After electrophoresis on a 7.5% SDS polyacrylamide gel, gradient fractions isolated from KG1 cells (A, bottom panel), Jurkat cells (B, bottom panel), or neutrophils (C and D) were examined for the presence of PSGL-1 (A and C) or L-selectin (B and D) by immunoblotting. (E) Histogram of PSGL-1 levels in fractions 1 to 10 (mean ± SEM, n = 3). (F) Histogram of L-selectin levels in fractions 1 to 10 (mean ± SEM, n = 4).
L-selectin–dependent rolling of Jurkat cells on PSGL-1/μ chimera was also significantly and reversibly affected by mβCD (Figure 2B, black boxes). Treatment of Jurkat cells with 10 mM or 15 mM mβCD inhibited rolling on PSGL-1 by 59% ± 2% and 68% ± 2% (mean ± SEM; n = 4; P < .001). Concomitantly, lipid raft–containing fractions were depleted in L-selectin (Figure 2D, bottom panel, lanes 2 to 5). However, after cell culture for 24 hours without mβCD, L-selectin was reexpressed in these fractions (Figure 2F, bottom panel, lanes 2 to 5).
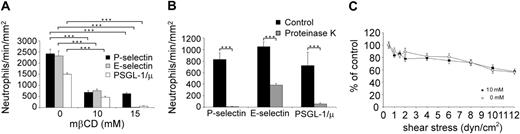
The experiments described in the preceding paragraph were repeated, substituting KG1 or Jurkat cells with neutrophils. Neutrophil treatment with 10 mM or 15 mM mβCD strongly impaired rolling on P-selectin, E-selectin, and PSGL-1 (Figure 3A). Neutrophil exposure to 10 mM versus 15 mM mβCD inhibited neutrophil rolling on P-selectin by 71% ± 8% versus 74% ± 6% (P < .001), on E-selectin by 67% ± 7% versus 99% ± 7% (P < .01), and on PSGL-1 by 69% ± 10% versus 99% ± 22% (P < .001; n = 3; Figure 3A). Similar inhibition of neutrophil rolling was obtained using filipin III (data not shown).
PSGL-1 and L-selectin localization in membrane lipid rafts is required to support KG1 cell rolling on P-selectin and Jurkat cell rolling PSGL-1. (A) KG1 cell rolling on P-selectin was evaluated under a constant shear stress (1.5 dyn/cm2) immediately (▪) or 24 hours (□) after treatment (10 minutes) with various concentrations of mβCD. (B) Jurkat cell rolling on PSGL-1/μ chimera was evaluated at constant shear stress (1.5 dyn/cm2) immediately (▪) or 24 hours (□) after treatment (10 minutes) with various concentrations of mβCD. Results in panels A and B are expressed as percentages of the number of vehicle-treated rolling cells/mm2 per minute (mean ± SEM, n = 4, ***P < .001). (C-F) Cell lysate fractions, separated by zonal sedimentation on discontinuous sucrose gradients, were evaluated by immunoblotting for PSGL-1 (C, E) or L-selectin (D, F). (C, E) KG1 cells. (D, F) Jurkat cells. Pattern observed immediately (C, D) or 24 hours (E, F) after cell treatment (10 minutes) with 10 mM mβCD.
PSGL-1 and L-selectin localization in membrane lipid rafts is required to support KG1 cell rolling on P-selectin and Jurkat cell rolling PSGL-1. (A) KG1 cell rolling on P-selectin was evaluated under a constant shear stress (1.5 dyn/cm2) immediately (▪) or 24 hours (□) after treatment (10 minutes) with various concentrations of mβCD. (B) Jurkat cell rolling on PSGL-1/μ chimera was evaluated at constant shear stress (1.5 dyn/cm2) immediately (▪) or 24 hours (□) after treatment (10 minutes) with various concentrations of mβCD. Results in panels A and B are expressed as percentages of the number of vehicle-treated rolling cells/mm2 per minute (mean ± SEM, n = 4, ***P < .001). (C-F) Cell lysate fractions, separated by zonal sedimentation on discontinuous sucrose gradients, were evaluated by immunoblotting for PSGL-1 (C, E) or L-selectin (D, F). (C, E) KG1 cells. (D, F) Jurkat cells. Pattern observed immediately (C, D) or 24 hours (E, F) after cell treatment (10 minutes) with 10 mM mβCD.
As observed with L-selectin, P-selectin, or PSGL-1, the abrogation of neutrophil rolling on E-selectin by 15 mM mβCD suggests that E-selectin ligands are mainly expressed within lipid rafts (Figure 3A). A previous study indicated that E-selectin ligands are distributed between glycoproteins and glycolipids.34 As the involvement of these lipid raft molecules in regulating neutrophils rolling has not been previously examined, we performed additional experiments to evaluate the effect of glycoprotein digestion on neutrophil rolling. Neutrophil exposure to proteinase K partially inhibited cell rolling on E-selectin (63% ± 1% of inhibition, mean ± SEM, n = 3, Figure 3B), whereas neutrophil rolling on P-selectin or PSGL-1 was almost completely inhibited (98% ± 0.3% and 89% ± 5%, respectively). These results suggest that E-selectin interacts with raft ligands that are both proteinase K–sensitive (eg, glycoproteins) and proteinase K resistant (eg, glycolipids).
Lipid raft disruption by mβCD inhibits neutrophil rolling on P-selectin, E-selectin, or PSGL-1 but does not prevent firm adhesion of fMLP-activated neutrophils on ICAM-1. (A) Freshly isolated neutrophils, treated for 10 minutes with 10 mM mβCD, were perfused on P-selectin (▪), E-selectin (▦), or PSGL-1/μ (□). Results of 3 representative experiments are shown (mean ± SEM). ***P < .001. (B) Neutrophils treated with proteinase K (1.7 μg/mL for 20 minutes at 37°C; ▦) or its vehicle (▪) were perfused on P-selectin, E-selectin, or PSGL-1/μ chimera. (C) Neutrophils were treated for 10 minutes with 10 mM mβCD (▪) or its vehicle (○). After resuspension (106 cells/mL) in medium supplemented with fMLP (10 nM), neutrophils were perfused for 3 minutes under shear stress (0.5 dyn/cm2) on a coverslip coated with ICAM-1. After a pause of 3 minutes, cell detachment was induced by perfusion of cell-free medium at various shear stress levels. Results (mean ± SEM; n = 3) are shown as percent of the number of adherent cells remaining after perfusion of cell-free medium at 0.5 dyn/cm2.
Lipid raft disruption by mβCD inhibits neutrophil rolling on P-selectin, E-selectin, or PSGL-1 but does not prevent firm adhesion of fMLP-activated neutrophils on ICAM-1. (A) Freshly isolated neutrophils, treated for 10 minutes with 10 mM mβCD, were perfused on P-selectin (▪), E-selectin (▦), or PSGL-1/μ (□). Results of 3 representative experiments are shown (mean ± SEM). ***P < .001. (B) Neutrophils treated with proteinase K (1.7 μg/mL for 20 minutes at 37°C; ▦) or its vehicle (▪) were perfused on P-selectin, E-selectin, or PSGL-1/μ chimera. (C) Neutrophils were treated for 10 minutes with 10 mM mβCD (▪) or its vehicle (○). After resuspension (106 cells/mL) in medium supplemented with fMLP (10 nM), neutrophils were perfused for 3 minutes under shear stress (0.5 dyn/cm2) on a coverslip coated with ICAM-1. After a pause of 3 minutes, cell detachment was induced by perfusion of cell-free medium at various shear stress levels. Results (mean ± SEM; n = 3) are shown as percent of the number of adherent cells remaining after perfusion of cell-free medium at 0.5 dyn/cm2.
Lipid raft integrity is not required for fMLP-activated neutrophil adhesion on ICAM-1
Leukocyte tethering and rolling are mainly mediated by selectins, whereas firm adhesion is dependent on integrins.35 Because leukocyte rolling requires lipid raft integrity (Figure 2 and Figure 3A), we examined whether the adhesion of N-formylmethionylleucyl-phenylalanine (fMLP)–activated neutrophils to ICAM-1 was affected by mβCD treatment. This experiment was considered as a control experiment. mβCD- or vehicle-treated neutrophils were activated by fMLP and then allowed to adhere on coverslips coated with ICAM-1. Shear-resistant cells were counted at progressively increasing flow shear stress. mβCD treatment did not affect neutrophil firm adhesion on ICAM-1 (number of cells/min/mm2 at 0.5 dyn/cm2, 0 mM versus 10 mM mβCD: 98% ± 8 vs 92% ± 10, n = 3, NS). Neutrophils treated with vehicle (Figure 3C, open circles) or mβCD (Figure 3C, black circles) exhibited similar resistance to detachment in response to increased shear stress, indicating that adhesion of fMLP-activated neutrophils on ICAM-1 is not affected by conditions under which lipid rafts are disrupted.
Expression of L-selectin, PSGL-1, or sLex on leukocyte microvilli is not altered by raft disruption
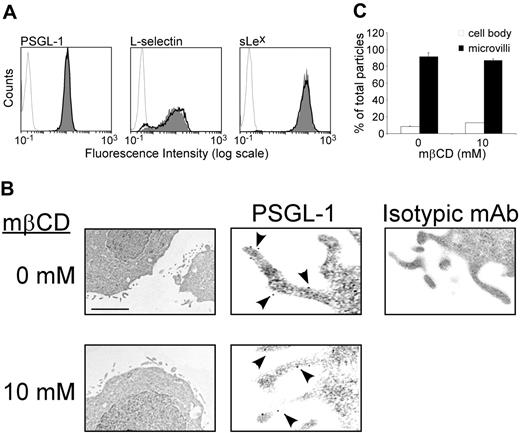
As inhibition of leukocyte rolling induced by mβCD might be due to reduced expression or altered distribution of rolling receptors, we examined the effect of neutrophil exposure to mβCD on expression of L-selectin, PSGL-1, and sialyl lewis x (sLex). Flow cytometric analyses showed (Figure 4A) that mβCD (10 mM) did not alter neutrophil cell-surface expression of PSGL-1 (10 mM vs 0 mM mβCD, mean MFI ± SEM: 15 ± 1 vs 14 ± 1, n = 15), L-selectin (8 ± 1 vs 10 ± 2, n = 9), or sLex (75 ± 6 vs 72 ± 9, n = 8, Figure 4A). These results indicate that inhibition of leukocyte rolling due to lipid raft disorganization cannot be related to reduced cell-surface expression of rolling receptors.
Expression of PSGL-1 on leukocyte microvilli is important to enhance contact initiation with selectins and stabilize rolling interactions.36,37 Immunogold labeling and electron microscopy were used to assess the effect of mβCD on PSGL-1 localization and microvilli architecture. The distribution of PSGL-1 was determined by counting gold particles in the plasma membrane of microvilli and planar surface of KG1 cells treated with 10 mM mβCD or its vehicle. mβCD did not alter KG1 cell microvilli architecture nor PSGL-1 localization (Figure 4B). Treatment with mβCD did not change cell-surface distribution of PSGL-1 (83% ± 7% vs 82% ± 6% of PSGL-1 molecules were found on microvilli treated with vehicle vs mβCD, mean ± SEM; n = 152, Figure 4C). Thus, inhibition of cell rolling by mβCD is not related to a detectable loss of rolling receptors expressed at the cell surface or to an alteration of PSGL-1 localization on microvilli.
Effect of lipid raft disruption by mβCD on cell surface expression of PSGL-1, L-selectin, or sLex. (A) Neutrophils were treated with mβCD (10 mM, 10 minutes; plain histograms) or its vehicle (bold lines), stained with antibodies against PSGL-1, L-selectin, or sLex using PE-labeled goat antimouse as secondary antibody, and analyzed by flow cytometry. Histograms are representative of 9 independent experiments. (B) KG1 cells were treated with mβCD (10 mM) or its vehicle and then immediately processed for electron microscopy. Left panels: the scale bar represents 0.7 μm (×14 000). Middle panels: the scale bar represents 0.2 μm(×50 000). Arrowheads indicate immunogold labeling of PSGL-1. Isotypic control is shown in the right panel: the scale bar represents 0.3 μm (×30 000). (C) Distribution of PSGL-1 (panel B, middle panels): percentages of gold particles in cell body (□) or microvilli (▪). Mean values plus or minus SEM of 150 counts is shown (24 microscopic fields).
Effect of lipid raft disruption by mβCD on cell surface expression of PSGL-1, L-selectin, or sLex. (A) Neutrophils were treated with mβCD (10 mM, 10 minutes; plain histograms) or its vehicle (bold lines), stained with antibodies against PSGL-1, L-selectin, or sLex using PE-labeled goat antimouse as secondary antibody, and analyzed by flow cytometry. Histograms are representative of 9 independent experiments. (B) KG1 cells were treated with mβCD (10 mM) or its vehicle and then immediately processed for electron microscopy. Left panels: the scale bar represents 0.7 μm (×14 000). Middle panels: the scale bar represents 0.2 μm(×50 000). Arrowheads indicate immunogold labeling of PSGL-1. Isotypic control is shown in the right panel: the scale bar represents 0.3 μm (×30 000). (C) Distribution of PSGL-1 (panel B, middle panels): percentages of gold particles in cell body (□) or microvilli (▪). Mean values plus or minus SEM of 150 counts is shown (24 microscopic fields).
Lipid raft disorganization partially inhibits soluble PSGL-1, E-, and P-selectin binding to neutrophils
Additional functional assays were performed using P-selectin/μ, E-selectin/μ, and PSGL-1/μ chimeras to evaluate whether cholesterol depletion had an effect on soluble ligand binding to leukocyte rolling receptors, as assessed by flow cytometry (Figure 5). Neutrophil treatment with 10 mM mβCD inhibited P-selectin/μ binding by 37% ± 5% (P < .001, n = 14). PSGL-1/μ binding was decreased by 49% ± 5% (P < .005, n = 9) and E-selectin/μ binding by 31% ± 10% (P < .05, n = 9). These results indicate that raft integrity is required for optimal binding of soluble PSGL-1, E-, and P-selectin to neutrophils. However, binding of E- and P-selectin/μ chimeras remained significantly detected despite mβCD treatment, suggesting that rolling receptors that do not reside in lipid rafts can function as ligands for E- and P-selectin (Figure 5). By comparison, leukocyte rolling under hydrodynamic flow conditions was much more severely impaired by lipid raft disruption (Figure 2 and Figure 3).
PSGL-1–mediated cell rolling on P-selectin: dependence on Syk
PSGL-1 associates with the actin-linking protein moesin,21 which acts as an adaptor molecule supporting PSGL-1 interaction with Syk. Whether PSGL-1–dependent rolling on selectin is dependent on tyrosine kinase activity (eg, Syk) has not been examined before. Flow adhesion assays were performed with KG1 cells pretreated with genistein, a nonselective tyrosine kinase inhibitor (Figure 6A). KG1 cell exposure to 5 μM, 10 μM, and 15 μM of genistein diminished cell rolling on P-selectin to 60% ± 5%, 44% ± 3%, and 25% ± 4%, respectively, of the control (mean ± SEM, n = 3, P < .001). These results indicate that PSGL-1–mediated rolling is dependent on tyrosine kinase activity. Figure 6B shows that KG1 cell exposure to piceatannol (5 μM vs 10 μM), a selective inhibitor of Syk, inhibited cell rolling on P-selectin by 72% ± 3% versus 96% ± 2% (mean ± SEM, n = 3, P < .001). By contrast, piceatannol (5 μM vs 10 μM) did not inhibit KG1 cell rolling on E-selectin (121% ± 8% vs 112% ± 9%, n = 3) or murine lymphoma 300.19-L-selectin cell rolling on PSGL-1 (111% ± 4.5% vs 117% ± 7%, n = 3; Figure 6B).
Inhibition of Syk expression by Syk-specific siRNA further established a role for Syk in regulating PSGL-1–dependent cell rolling on P-selectin (Figure 6C). At 48 hours after transfection of KG1 cells with Syk-specific siRNA, GAPDH-normalized expression of Syk was decreased to 11.6% ± 0.5% of that of mock-transfected cells (mean ± SEM, n = 2; Figure 6C), whereas nontargeting siRNA did not change Syk expression (89.4% ± 8.2% of mock-transfected cells). Treatment with Syk-specific siRNA strongly impaired KG1 cell rolling on P-selectin (compared with mock-transfected cells: 74% ± 4% of inhibition, mean ± SEM, n = 3, P < .001; Figure 6D). By contrast, KG1 cells transfected with nontargeting siRNA exhibited the same rolling efficiency as mock-transfected cells (Figure 6D).
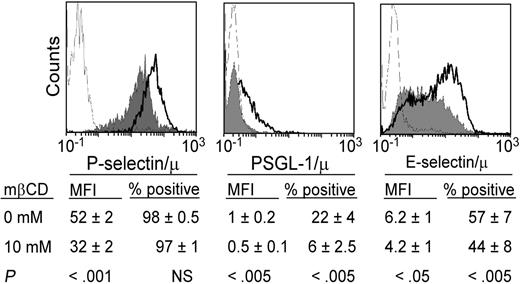
Binding of soluble PSGL-1, E-selectin/μ, or P-selectin/μ to neutrophils is inhibited by lipid raft disorganization. Neutrophils were treated with mβCD (10 mM, 10 minutes; plain histograms) or its vehicle (bold lines) and stained with PSGL1/μ, P-selectin/μ, or E-selectin/μ (5 μg/mL) preincubated with FITC-conjugated rabbit anti–human IgM. Binding was assessed by flow cytometry. A total of 5000 cells was analyzed in each experiment. Ligand binding was abolished by 5 mM EDTA (thin lines) specifically by anti–PSGL-1 mAb KPL1, anti–P-selectin mAb WAPS12.2, or anti–E-selectin mAb H18/7 (not shown). Histograms are representative of 9 to 14 experiments. Mean values of MFI and percentage of positive cells (n = 9-14) are indicated below the histograms.
Binding of soluble PSGL-1, E-selectin/μ, or P-selectin/μ to neutrophils is inhibited by lipid raft disorganization. Neutrophils were treated with mβCD (10 mM, 10 minutes; plain histograms) or its vehicle (bold lines) and stained with PSGL1/μ, P-selectin/μ, or E-selectin/μ (5 μg/mL) preincubated with FITC-conjugated rabbit anti–human IgM. Binding was assessed by flow cytometry. A total of 5000 cells was analyzed in each experiment. Ligand binding was abolished by 5 mM EDTA (thin lines) specifically by anti–PSGL-1 mAb KPL1, anti–P-selectin mAb WAPS12.2, or anti–E-selectin mAb H18/7 (not shown). Histograms are representative of 9 to 14 experiments. Mean values of MFI and percentage of positive cells (n = 9-14) are indicated below the histograms.
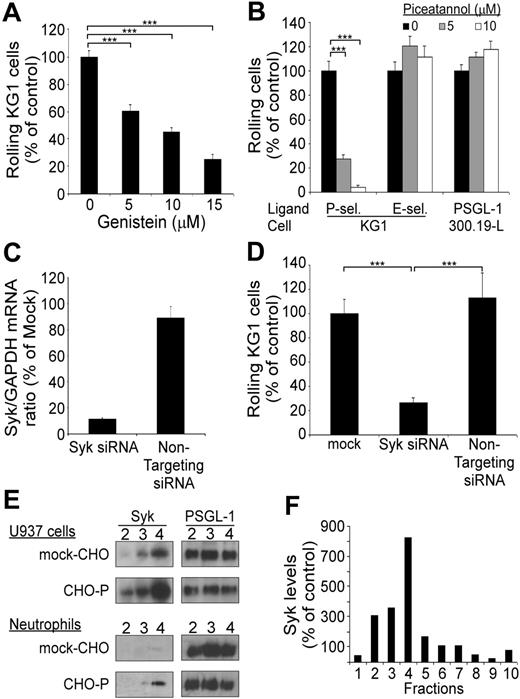
PSGL-1–mediated rolling on P-selectin is dependent on Syk. (A) Rolling of KG1 cells on P-selectin: effect of treatment with genistein. Results are expressed as mean percent (± SEM; n = 3) of control cells treated with vehicle alone. (B) Rolling of KG1 and 300.19-L-selectin cells on P-selectin, E-selectin, or PSGL-1. Cells were treated with vehicle (▪) or with piceatannol (▦ and □). Results are expressed as mean (%) plus or minus SEM (n = 3). (C) Inhibition of Syk expression by Syk-specific siRNA. KG1 cells were transfected with 3 μg Syk siRNA or of nontargeting siRNA. Syk/GAPDH mRNA ratio was assessed by RQ-PCR at 48 hours. Results are expressed as percent of mock-transfected cells, mean plus or minus SEM (n = 2). (D) Flow adhesion of KG1 cells on P-selectin. KG1 cells were transfected with 3 μg Syk siRNA or with nontargeting siRNA and compared with mock-transfected cells. Results are expressed as mean percent mock-treated cells plus or minus SEM (n = 3). ***P < .001. (E) Adhesion of U937 cells or neutrophils to confluent CHO–P-selectin monolayers or mock-transfected CHO monolayers. Lipid rafts were isolated by fractionation of cell lysates. Expression of Syk and PSGL-1 in lipid raft fractions (2 to 4) was detected by Western blot analysis using mAb 4D10.1 (0.2 μg/mL) or mAb PS5 (3 μg/mL). (F) Densitometric analysis of Syk in sucrose density fractions 1 to 10 prepared from U937 cells after rolling on CHO–P-selectin cell monolayers. Syk levels were normalized using PSGL-1 as a standard. Control values were determined by assessing Syk levels in fractions from U937 cells after rolling on mock-CHO cell monolayers. Results are representative of 3 experiments.
PSGL-1–mediated rolling on P-selectin is dependent on Syk. (A) Rolling of KG1 cells on P-selectin: effect of treatment with genistein. Results are expressed as mean percent (± SEM; n = 3) of control cells treated with vehicle alone. (B) Rolling of KG1 and 300.19-L-selectin cells on P-selectin, E-selectin, or PSGL-1. Cells were treated with vehicle (▪) or with piceatannol (▦ and □). Results are expressed as mean (%) plus or minus SEM (n = 3). (C) Inhibition of Syk expression by Syk-specific siRNA. KG1 cells were transfected with 3 μg Syk siRNA or of nontargeting siRNA. Syk/GAPDH mRNA ratio was assessed by RQ-PCR at 48 hours. Results are expressed as percent of mock-transfected cells, mean plus or minus SEM (n = 2). (D) Flow adhesion of KG1 cells on P-selectin. KG1 cells were transfected with 3 μg Syk siRNA or with nontargeting siRNA and compared with mock-transfected cells. Results are expressed as mean percent mock-treated cells plus or minus SEM (n = 3). ***P < .001. (E) Adhesion of U937 cells or neutrophils to confluent CHO–P-selectin monolayers or mock-transfected CHO monolayers. Lipid rafts were isolated by fractionation of cell lysates. Expression of Syk and PSGL-1 in lipid raft fractions (2 to 4) was detected by Western blot analysis using mAb 4D10.1 (0.2 μg/mL) or mAb PS5 (3 μg/mL). (F) Densitometric analysis of Syk in sucrose density fractions 1 to 10 prepared from U937 cells after rolling on CHO–P-selectin cell monolayers. Syk levels were normalized using PSGL-1 as a standard. Control values were determined by assessing Syk levels in fractions from U937 cells after rolling on mock-CHO cell monolayers. Results are representative of 3 experiments.
PSGL-1 triggering induces Syk enrichment within lipid rafts
Previous observations have shown that PSGL-1 interacts with moesin and Syk to transduce intracellular signals. Whether this interaction preferentially occurs within lipid raft domains remains unknown. Additional adhesion assays were performed to assess the effect of P-selectin interactions with PSGL-1 on the expression of Syk in lipid rafts. U937 cells or human neutrophils were kept under rotation on a CHO–P-selectin cell monolayer, gently detached, lysed, and centrifuged to isolate detergent-resistant fractions. Control cells were obtained from rotation assays performed on mock-transfected CHO cells. Levels of lipid raft Syk in cells (U937 and neutrophils) rolling on CHO–P-selectin (Figure 6E, CHO-P) were higher than levels in cells incubated with mock-transfected CHO cells (Figure 6E, mock-CHO). For example, with neutrophils, raft Syk was increased by 11-fold (Figure 6E, left bottom panel, fraction 4) when compared with levels measured after rotation on mock-transfected CHO cell monolayers. Moreover, densitometric analysis of detergent-insoluble fractions from U937 cells (Figure 6F) revealed that PSGL-1 triggering by P-selectin induced a 3-, 3.5-, and 8-fold increase of Syk in fractions 2, 3, and 4. Of note, the preincubation of CHO–P-selectin cells with the anti–P-selectin mAb WAPS 12.2 abrogated PSGL-1–dependent rolling of U937 cells on P-selectin and prevented Syk recruitment in lipid rafts (not illustrated).
Discussion
PSGL-1 and L-selectin are signaling molecules that regulate leukocyte rolling. As lipid rafts assemble signaling and adhesion molecules, we investigated whether they play a role in regulating leukocyte rolling. Here, we show that L-selectin, dimeric PSGL-1, and E-selectin ligands reside in lipid rafts of neutrophils and cell lines such as KG1, Jurkat, or U937. Moreover, we demonstrate that PSGL-1, L-selectin, and E-selectin ligand(s) need to be localized in leukocyte lipid rafts to support leukocyte rolling on P-selectin, PSGL-1, or E-selectin. In addition, we provide the first functional description that Syk plays a crucial role in regulating PSGL-1–dependent rolling on P-selectin, although it is not required for interactions between E-selectin and its ligand(s) or L-selectin and PSGL-1.
PSGL-1 supports primary rolling interactions on P-selectin–expressing endothelium or activated platelets, as well as secondary interactions between flowing leukocytes expressing L-selectin and adherent leukocytes or membrane fragments.9 The strong inhibition of L-selectin– and PSGL-1–dependent rolling induced by lipid raft disruption emphasizes the role of lipid rafts in regulating leukocyte rolling (Figure 2 and Figure 3). Importantly, cholesterol-chelating agents strongly reduced the localization of L-selectin and PSGL-1 in lipid rafts but did not change their global expression on leukocyte cell surface (Figure 2C-D and Figure 4A) indicating that mβCD-induced inhibition of leukocyte rolling is not due to reduced L-selectin, PSGL-1, or sLex expression. After lipid raft disruption, binding of P-selectin/μ, E-selectin/μ, and PSGL-1/μ chimeras to neutrophils was decreased but remained detectable (Figure 5). Thus, raft selectin ligand(s) are required for optimal binding as are ligands expressed in membrane domains that are not resistant to detergents. In contrast, neutrophil rolling on E- or P-selectin was much more affected by treatment with mβCD (Figure 3A). These differences may be explained by the following mechanisms: (1) assembly of P- and E-selectin ligand(s) with signal transducers in lipid rafts may be required to support rolling but not may not be essential for soluble selectin binding; (2) oligomerization of selectin ligand(s) in lipid rafts38 may be important to stabilize rolling by increasing mechanical strength of bonds formed between selectins and their ligands, whereas it may not be essential for soluble selectin binding. Lipid raft disruption did not alter PSGL-1 localization at the top of leukocyte microvilli,39-41 a prerequisite for efficient rolling under hydrodynamic flow conditions.42 Cell treatment with mβCD did not change numbers and sizes of microvilli. A majority (∼80%) of PSGL-1 was detected on KG1 cell microvilli (Figure 4C), a fraction comparable to that found for L-selectin.43 Thus, expression of lipid raft adhesion receptors had an essential role in regulating leukocyte rolling, and rolling inhibition by mβCD was not due to major changes of leukocyte architecture or receptor localization on microvilli.
Western blot analysis revealed that raft PSGL-1 migrates as an approximately 220 kDa glycoprotein, suggesting that it is expressed as a dimer (Figure 1). Neutrophil detergent-soluble plasma membrane fractions contained a majority of monomeric and a minority of dimeric PSGL-1. PSGL-1 dimerization was previously found to stabilize leukocyte tethering and rolling on P-selectin by increasing the mechanical strength of selectin-ligand bonds and by favoring rebinding within a bond cluster.44 Strong inhibition of PSGL-1–dependent leukocyte rolling following lipid raft disruption (Figure 2A and Figure 3A) suggests that raft expression of dimeric PSGL-1 may be another mechanism that contributes to the regulation of leukocyte rolling. When PSGL-1 was excluded from lipid rafts, dimeric and monomeric PSGL-1 did not efficiently support leukocyte rolling.
A major part of L-selectin is expressed in lipid rafts (Figure 1B). Strong inhibition of leukocyte rolling on PSGL-1 by lipid raft disruption suggests that L-selectin localization in microdomains is required to support L-selectin–dependent rolling (Figure 2B, Figure 3A). Previous observations indicated that localization within lipid rafts modulates L-selectin shedding and MAPK activation following L-selectin cross-linking by monoclonal antibodies.22 Like L-selectin, E-selectin is recruited in lipid rafts upon engagement, where it associates with Src family kinases, a mechanism that links E-selectin–dependent adhesion to signal transduction.45 Raft localization of rolling receptors and association with signaling molecules might be a general mechanism that regulates leukocyte rolling.
Neutrophil rolling on E-selectin was also strongly inhibited by cell treatment with mβCD, suggesting that E-selectin counter receptors—or at least part of them—are expressed in lipid rafts (Figure 3A). Several putative E-selectin ligands have been identified but their exact contribution in regulating human leukocyte migration is unknown.12,13,27,34,46-50 Although PSGL-1 is not essential to support leukocyte rolling on E-selectin, several in vivo experiments indicate that E-selectin interactions with PSGL-1 play a role in regulating leukocyte migration in inflamed tissues.11,51,52 PSGL-1 cooperates with CD44, another E-selectin ligand, to recruit neutrophils in peritonitis and inflamed skin.12 E-selectin ligands such as PSGL-1, L-selectin, and CD44 are expressed in lipid rafts.53 CD43 may also serve as an E-selectin ligand on cutaneous lymphocyte-associated (CLA)–expressing T lymphocytes13 and it can be segregated in lipid rafts.54 Plasma membrane glycolipids expressing sLex were reported to bind to E-selectin34 ; although their physiological role has not been established, they could mediate residual rolling on E-selectin after cell exposure to proteinase K (Figure 3B).
Engagement of PSGL-1 induces MAPK phosphorylation, Ras GTPase activation, and secretion of cell stimulating factors.14-16,55,56 Leukocyte exposure to genistein strongly reduced PSGL-1-dependent rolling on P-selectin, indicating that tyrosine kinase activity is required to support PSGL-1 interactions with P-selectin (Figure 6A). Previous studies demonstrated that Syk is phosphorylated upon PSGL-1 engagement and that PSGL-1 cytoplasmic tail associates with Syk indirectly though the immunoreceptor tyrosine-based activation motif (ITAM)–like motives of the actin-linking proteins moesin and ezrin.18,21 Now, we show that PSGL-1 engagement by P-selectin during leukocyte adhesion induces Syk recruitment in lipid rafts (Figure 6E) and that piceatannol strongly inhibits PSGL-1–dependent rolling on P-selectin (Figure 6B). Similar results were obtained by inhibiting Syk expression by specific siRNA (Figure 6C-D). Syk involvement in regulating leukocyte rolling was restricted to PSGL-1 interactions with P-selectin (Figure 6B). Like PSGL-1, the cytoplasmic tail of L-selectin and CD44 can bind to ezrin and moesin21,57,58 and might potentially associate to Syk. However, as Syk inhibition did not affect L-selectin interactions with PSGL-1, or E-selectin–ligand interactions with E-selectin (Figure 6B), other signal transducers could be involved in these reactions. These observations suggest that Syk specifically regulates PSGL-1–dependent rolling on P-selectin. Cell exposure to mβCD has a broader inhibitory effect on rolling than piceatannol (Figure 2, Figure 3, and Figure 6). Raft disruption impairs L-, P-, and E-selectin–dependent rolling, whereas piceatannol only inhibits P-selectin–dependent rolling. By disrupting lipid rafts, cholesterol-chelating agents appear to uncouple PSGL-1, L-selectin, and E-selectin ligand(s) from downstream kinases or other signal transducers regulating selectin-mediated rolling.
In summary, data reported here show for the first time that lipid rafts play a crucial role in regulating leukocyte rolling on selectins and PSGL-1. In addition, we demonstrate that Syk regulates leukocyte rolling on P-selectin whereas it is not required for leukocyte rolling on E-selectin or on PSGL-1. Furthermore, we show that lipid rafts serve as signaling platforms, where Syk is recruited upon PSGL-1 engagement by P-selectin. Similar mechanisms might regulate L-selectin– and E-selectin–dependent rolling, lipid rafts assembling rolling receptors, and signaling complexes that regulate leukocyte rolling. Taken together, these observations reveal novel key regulatory mechanisms of leukocyte rolling.
Prepublished online as Blood First Edition Paper, July 18, 2006; DOI 10.1182/blood-2006-04-013912.
Supported by grant no. 3200BO-105 593 from the Swiss National Foundation for Scientific Research.
The authors declare no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We would like to thank Dr S. Fakan, J. Fakan, and F. Ardizzoni from the Centre of Electron Microscopy, University of Lausanne, Switzerland, for their outstanding technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal