Abstract
The promiscuous CD11b/CD18 (Mac-1) integrin has important roles in regulating many immunologic functions such as leukocyte adhesion and emigration from the bloodstream via interactions with the endothelial ligands ICAM-1 and ICAM-2, iC3b-mediated phagocytosis, and apoptosis. However, the mechanisms for Mac-1 inside-out activation have remained poorly understood. Phosphorylation of integrin cytoplasmic domains is emerging as an important mechanism of regulating integrin functions. Here, we have studied phosphorylation of human CD11b, which takes place on the cytoplasmic Ser1126 in neutrophils. We show that mutation of the serine phosphorylation site leads to inability of Mac-1 to become activated to bind the cellular ligands ICAM-1 and ICAM-2. However, CD11b-mutant cells are fully capable of binding other studied CD11b ligands (ie, iC3b and denatured BSA). Activation epitopes expressed in the extracellular domain of the integrin and affinity for soluble ICAM ligands were decreased for the mutated integrin. Additionally, the mutation resulted in inhibition of chemokine-induced migration in a transendothelial assay in vitro and significantly reduced the accumulation of intravenously administered cells in the spleen and lungs of Balb/c mice. These results characterize a novel selective mechanism of Mac-1–integrin activation, which mediates leukocyte emigration from the bloodstream to the tissues.
Introduction
The large family of heterodimeric cell surface receptors called integrins mediates adhesion to diverse ligands in the extracellular matrix, on other cells, as well as in solution. The 4 members of the CD11/CD18 family of integrins are exclusively expressed on leukocytes, and they mediate many important immunobiologic functions.1,2 Such functions include the recruitment of leukocytes to inflammatory sites through interactions with intercellular adhesion molecules (ICAMs) expressed on endothelium. Human patients with defective CD18 integrins have a disease known as leukocyte adhesion deficiency (LAD-I), which is associated with recurrent bacterial infections that the immune system fails to clear, as well as massive leukocytosis.3
The Mac-1 integrin (also called αMβ2 or CD11b/CD18) is a member of the CD18 integrin family. It is expressed mainly on cells of the myeloid lineage, such as monocytes and neutrophils, but is also found on T cells. This integrin is involved in phagocytosis, adhesion to, and migration through the endothelium as well as other functions, such as regulation of apoptosis and degranulation.1,2 In line with its many functions, Mac-1 is the most promiscuous integrin of the CD18 family. It binds to a wide range of ligands, including the blood coagulation protein fibrinogen,4 the adhesion ligands intercellular adhesion molecule-1 and -2 (ICAM-1 and -2)5,6 and the complement protein iC3b.7 More recently discovered partners for Mac-1 include, for example, the LDL-receptor–related protein8 and the matrix metalloproteinase MMP9.9
Lately, important development has been made in the understanding of the structure and function of the integrin receptors; however, their regulation is still incompletely understood. Leukocyte integrins on resting cells are not able to bind their ligands, but, when the cell has received an activating stimulus, for example, by chemokines displayed on or released from the activated endothelium, an intracellular signaling cascade is initiated which mediates a change in the integrin to make it adhesive. This process is called “inside-out” signaling or “activation,” and it affects both the structure of the extracellular domains to increase affinity for ligands, as well as integrin clustering in the cell membrane and altered cytoskeletal contacts.10-12
The ligands of Mac-1 bind to different sites in the integrin extracellular domains, the so-called inserted or I domain in CD11b being an important binding site for ligands.12 Also the CD18 I-like domain contains ligand-binding sites, and, additionally, it is involved in regulating I domain conformational changes.12 Unlike another CD18 family member, LFA-1 (CD11a/CD18), Mac-1 has not been much studied for inside-out signaling, and proximal events in Mac-1 integrin activation are poorly understood.
The inside-out activation of the LFA-1 integrin has been extensively studied, and several proximal elements for its activation have been identified.11,13,14 It has recently been shown that adhesion through LFA-1 in T cells is regulated by phosphorylation of both its intracellular domains.14 The CD11a phosphorylation site (Ser1140) is constitutively phosphorylated and is necessary for chemokine- and ligand-induced integrin activation, which involves changes in the conformation/affinity in individual integrin molecules.14 As CD11a, also CD11b has been previously shown to be constitutively phosphorylated on serine in resting leukocytes.15,16 Indeed, there is only one serine residue in the CD11b cytoplasmic domain (Ser1126). To investigate the possibility that the CD11b phosphorylation site is involved in adhesion regulation, we have mutated the CD11b phosphorylation site and investigated adhesion to diverse Mac-1 ligands after activation, as well as integrin-mediated functions in vivo.
Materials and methods
Reagents
Phorbol 12,13-dibutyrate (PDBu) and bovine serum albumin (BSA) were obtained from Sigma-Aldrich (Zwijndrecht, the Netherlands). Human serum albumin (HSA) was from the Central Laboratory, Blood Transfusion Service, Swiss Red Cross, Bern, Switzerland. Recombinant human ICAM-1, ICAM-2-Fc, and SDF-1α were purchased from R&D Systems (Minneapolis, MN), human complement iC3b from Calbiochem (La Jolla, CA). Optifect and OptiMEM I Reduced Serum Medium were obtained from Invitrogen (Carlsbad, CA). The CD11b cytoplasmic domain peptides (KLGFFKRQYKDMMSEGGPPGAEPQ [BP] and KLGFFKRQYKDMMpSEGGPPGAEPQ [PP], where pS marks phosphoserine) were synthesized by Fmoc chemistry and checked by mass spectrometry.17
Monoclonal antibodies
MEM170 (anti-CD11b) was a gift from Dr V. Horejsi (Institute of Molecular Genetics, Prague, Czech Republic). The human leukocyte integrin CD18 chain antibody R7E4 has been reported previously.18 IB4, a CD18 antibody that recognizes CD11/CD18 heterodimers, was a gift from M.A. Arnaout (Massachusetts General Hospital, Boston, MA). mAb24 (anti-CD18) was a gift from N. Hogg (Imperial Cancer Research Fund, London, United Kingdom). KIM127 (anti-CD18) was a kind gift from M. Robinson (Celltech, Slough, United Kingdom). CBRM1/5 was from Santa Cruz Biotechnology (Santa Cruz, CA). CD11b phosphospecific antibodies were rabbit polyclonal antibodies made as described.14 The CD11b cytoplasmic domain peptide KLGFFKRQYKDMMpSEGGPPGAEPQ was used as an antigen.
cDNA constructs
The cDNA for full-length CD11b was subcloned into pcDNA3. The S1126A mutation was made by site-directed mutagenesis.19 Both constructs were checked by sequencing.
Transfections and cell lines
The human T-cell lymphoma cell line clone J-β2.7, which lacks CD11 chains,20 was a gift from N. Hogg. Wt-CD11b and S1126A-CD11b pcDNA3 were transfected into the J-β2.7 cells using the Optifect transfection reagent according to the manufacturer's instructions. Transfected Jurkat cells were purified using magnetic cell sorting (MACS) columns (Miltenyi Biotech, Surrey, United Kingdom), using the antibodies MEM170 or IB4. The integrin expression and the condition of transfectants were analyzed by flow cytometry. Wt-CD11a-Jβ2.7 and S1140A-CD11a-Jβ2.7 cells have been described.14 The HMEC-1 endothelial cells were grown as described previously.9 Human neutrophils were isolated as previously described.9
Flow cytometry and adhesion assays
The flow cytometry assays to detect surface expressed integrins were done as described,14 using IB4 and MEM170 antibodies. Cell adhesion assays were performed as reported,14 except for modifications as follows. For the adhesion assay with denatured BSA, the protein was denatured for 5 minutes in 95°C and was coated at 10 μg/mL or 1 mg/mL. The adhesion medium was RPMI1640, 40 mM HEPES, 0.1% BSA, 2 mM CaCl2. Three percent HSA was used as a blocking agent for at least 2 hours. Cells were stimulated with either 100 nM PDBu or 5 mM MgCl2/1 mM EGTA, and the R7E4 inhibition of adhesion was made at 10 μg/mL mAb concentration for 30 minutes at 37°C. For mAb24, KIM127, and CBRM1/5 reporter antibody assays, transfected J-β2.7 cells were reacted with mAb24 or KIM127 (5 μg/mL) in the presence of activators for 20 minutes at 37°C. Cells were instantly stained with Alexa-fluor–conjugated anti–mouse IgG Abs on ice for 20 minutes and analyzed by flow cytometry. mAb24, KIM127, and CBRM1/5 expression was reported as mean fluorescence intensity.
Soluble ICAM-2Fc and iC3b binding assays
J-β2.7 transfectants were incubated in 25 μL RPMI 1640, 40 mM HEPES, 1 mM MgCl2 in the presence of 200 μg/mL ICAM-2Fc or iC3b at 37°C for 10 minutes. After removal of the unbound ligand by washing with PBS, the cells were incubated with either FITC-conjugated anti–human IgG-Fc–specific antiserum (for ICAM-2-Fc) (Jackson Immunoresearch Laboratories, West Grove, PA) or FITC-conjugated C3c antibody, which recognizes iC3b (Dako, Glostrup, Denmark) on ice for 20 minutes. Cells were analyzed by flow cytometry. The fluorescence was compared with integrin expression levels and reported as the percentage of MEM170 staining.
Transendothelial migration assay
HMEC-1 cells were cultured for 5 days (until confluent) on gelatin (0.5% in PBS) coated Transwell membranes (8-μm pore size; Costar, Bucks, United Kingdom) in RPMI 1640, 10% FCS, 1% penicillin/streptomycin/L-glutamine. The endothelium was activated with SDF-1α at a concentration of 100 ng/mL for 4 hours. Jβ2.7 tranfectants (1 × 106cells/well) were pipetted onto the endothelial cell layer in 100 μL volume and left to migrate for 4 hours. Migrated cells were collected from the wells and counted.
Cell dissemination in vivo
Animal experiments were approved by the ethics committee of the University of Helsinki. Jβ2.7 cells were labeled with 125I by the lactoperoxidase method21 and intravenously injected into Balb/c mice (n = 3 per group). Mice were killed 1 hour after inoculation, and organs were harvested, weighed, and the radioactivities were determined using a Wallac-LKB γ-counter.
Results
Ser1126 phosphorylation of CD11b in human neutrophils
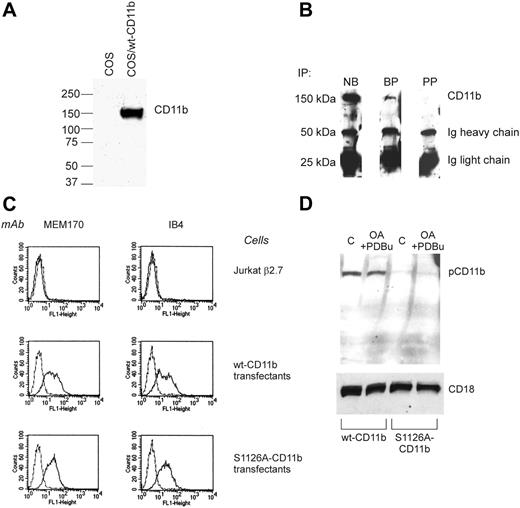
We have recently reported that the phosphorylation of CD11a on Ser1140 in the cytoplasmic domain mediates integrin activation by affinity regulation.14 The CD11b-chain of the integrin has previously been shown to be phosphorylated on serine in resting cells.15 To study phosphorylation of Ser1126 in cells, we made a polyclonal phosphospecific antibody to pSer1126-CD11b. The antibody was purified using the phosphopeptide antigen and tested on lysates from nontransfected COS cells and cells transfected with wild-type CD11b (Figure 1A). The antibody recognized CD11b specifically and did not recognize any proteins from nontransfected cells. In addition, in a dot blot, it recognized the phosphopeptide much better than the unphosphopeptide (not shown). To investigate phosphorylation of CD11b in human leukocytes, the CD11b polypeptide was immunoprecipitated from neutrophil lysates. Used as such, the antibody recognized CD11b in Western blots (Figure 1B). To block any antibody that recognized the unphosphorylated form of the integrin, the antibody was treated with the unphosphopeptide. This led to a significant decrease in binding to CD11b; however, the antibody still recognized the CD11b chain, indicating that it is phosphospecific, and that CD11b is indeed phosphorylated on Ser1126 in neutrophils (Figure 1B). Treatment of the phosphospecific antibody with the phosphopeptide antigen before Western blotting completely abolished recognition of CD11b.
CD11b is phosphorylated on Ser1126 in neutrophils. (A) COS cells were transfected with wt Mac-1 or left untransfected. Cell lysates were analyzed by Western blotting with the pCD11b antibody. (B) Western blot of CD11b/CD18 integrin immunoprecipitated from lysed peripheral blood neutrophils detected with pAb P-CD11b. NB indicates no blocking peptide present; BP, blocking peptide present, PP, CD11b phosphopeptide present. (C) Fluorescence-activated cell sorting (FACS) analysis of transfectants shows that Mac-1 heterodimers are not expressed on untransfected Jβ2.7 cells (2 top panels). The expression level of wt Mac-1 is comparable to that of the S1126A–Mac-1 transfectants (2 bottom panels). MEM170, CD11b-antibody; IB4, CD18-antibody. (D) Wt and S1126A-CD11b–transfected Jβ2.7 cells were pretreated with 1.5 μM okadaic acid (OA) and activated with 200 nM PDBu for 30 minutes or left untreated (C). Lysates from cells were analyzed by Western blotting with the pCD11b antibody (top). CD18 is shown as a loading control (bottom). Dotted lines represent the background fluorescence.
CD11b is phosphorylated on Ser1126 in neutrophils. (A) COS cells were transfected with wt Mac-1 or left untransfected. Cell lysates were analyzed by Western blotting with the pCD11b antibody. (B) Western blot of CD11b/CD18 integrin immunoprecipitated from lysed peripheral blood neutrophils detected with pAb P-CD11b. NB indicates no blocking peptide present; BP, blocking peptide present, PP, CD11b phosphopeptide present. (C) Fluorescence-activated cell sorting (FACS) analysis of transfectants shows that Mac-1 heterodimers are not expressed on untransfected Jβ2.7 cells (2 top panels). The expression level of wt Mac-1 is comparable to that of the S1126A–Mac-1 transfectants (2 bottom panels). MEM170, CD11b-antibody; IB4, CD18-antibody. (D) Wt and S1126A-CD11b–transfected Jβ2.7 cells were pretreated with 1.5 μM okadaic acid (OA) and activated with 200 nM PDBu for 30 minutes or left untreated (C). Lysates from cells were analyzed by Western blotting with the pCD11b antibody (top). CD18 is shown as a loading control (bottom). Dotted lines represent the background fluorescence.
Mutation of the Ser1126 phosphorylation site does not influence cell-surface expression of integrin heterodimers
To examine the effect of phosphorylation of Ser1126 on integrin activation, we mutated the serine to alanine and made stable transfectants of Jβ2.7 cells. The transfectants were examined for surface expression of integrin heterodimers (Figure 1C). Mutation of the serine in the CD11b cytoplasmic domain did not influence surface expression of the integrin heterodimers, as examined by flow cytometry with a CD11b and a CD18 antibody (Figure 1C). In addition, the heterodimers formed normally, because they could be immunoprecipitated with the IB4 antibody that only recognizes heterodimeric integrins (not shown). Thus, both wild-type and S1126A-mutated CD11b integrins were normally expressed on the surface of the leukocytes.
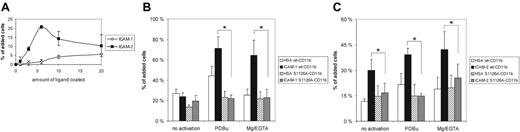
Mac-1 adhesion to ICAM-1 and ICAM-2 is attenuated when serine 1126 is mutated to alanine. (A) Jβ2.7 transfectants expressing wt CD11b were allowed to bind to wells coated with ICAM-1 or ICAM-2 at different ligand concentrations (microgram per milliliter of coated ligand) for 30 minutes. (B) Jβ2.7 transfectants expressing wt CD11b or S1126A-CD11b were allowed to bind to ICAM-1–coated wells (6 μg/mL coated ligand) without stimulation (control) or after stimulation with 100 nM PDBu or 5 mM MgCl2/1 mM EGTA for 30 minutes. Significant differences (P ≤ .02 for Mg/EGTA samples and P ≤ .002 for PDBu samples) in bracketed comparisons are indicated by a single asterisk. (C) As in panel B, except that ICAM-2 was used as the coated ligand. Significant differences (P ≤ .05 for unstimulated and Mg/EGTA-stimulated samples, P < .001 for PDBu-stimulated samples) in bracketed comparisons are indicated by a single asterisk. Error bars indicate SD.
Mac-1 adhesion to ICAM-1 and ICAM-2 is attenuated when serine 1126 is mutated to alanine. (A) Jβ2.7 transfectants expressing wt CD11b were allowed to bind to wells coated with ICAM-1 or ICAM-2 at different ligand concentrations (microgram per milliliter of coated ligand) for 30 minutes. (B) Jβ2.7 transfectants expressing wt CD11b or S1126A-CD11b were allowed to bind to ICAM-1–coated wells (6 μg/mL coated ligand) without stimulation (control) or after stimulation with 100 nM PDBu or 5 mM MgCl2/1 mM EGTA for 30 minutes. Significant differences (P ≤ .02 for Mg/EGTA samples and P ≤ .002 for PDBu samples) in bracketed comparisons are indicated by a single asterisk. (C) As in panel B, except that ICAM-2 was used as the coated ligand. Significant differences (P ≤ .05 for unstimulated and Mg/EGTA-stimulated samples, P < .001 for PDBu-stimulated samples) in bracketed comparisons are indicated by a single asterisk. Error bars indicate SD.
The Ser1126-phosphospecific antibody was then used to characterize CD11b from wt CD11b and S1126A-CD11b–stable transfectants (Figure 1D). The antibody recognized wt CD11b but not the S1126A-CD11b protein, confirming that CD11b was phosphorylated on Ser1126 in wt but not in mutant cells. Treatment of cells with a combination of okadaic acid and phorbol ester did not increase Ser1126 phosphorylation in cells (Figure 1D).
Adhesion to ICAM-1 and ICAM-2 is abolished for S1126A-CD11b
Using the wild-type and phosphorylation-site mutant cells, we first examined the Mac-1 integrin–mediated adhesion to the ligands ICAM-1 and ICAM-2. Using different amounts of coated ICAM-1, we determined a concentration that was in the steep portion of the dose-dependence curve (6 μg/mL) (Figure 2A), which was then used under different stimulatory conditions. Wt CD11b-transfected cells bound to coated ICAM-1 when cells were treated with phorbol ester (Figure 2B), an activating inside-out stimulus for integrins. In addition, adhesion was activated with Mg/EGTA, which bypasses intracellular signaling events and directly influences the extracellular domains of the integrin.12 The specificity of adhesion was shown by using nontransfected Jβ2.7 cells, which did not adhere to coated ICAM-1, and by blocking adhesion with the CD18 antibody R7E4 (not shown). When adhesion by S1126A-CD11b–transfected cells was examined, it was revealed that mutation of the CD11b phosphorylation site completely abolished activation of Mac-1 in response to phorbol ester and Mg/EGTA stimuli (Figure 2B).
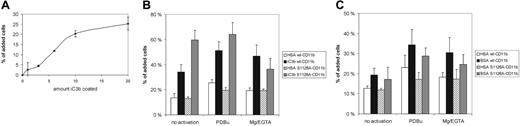
Mac-1 binding to iC3b and denatured BSA is not affected by the S1126A mutation. (A) Jβ2.7 transfectants expressing wt CD11b were allowed to bind to wells coated with iC3b at different ligand concentrations (microgram per milliliter of ligand used for coating) for 30 minutes. (B) Adhesion of transfected cells to coated iC3b (6 μg/mL) with or without activation was performed as described in “Materials and methods.” (C) As in panel B, except that denatured BSA (1 mg/mL) was used as a ligand. Error bars represent SD.
Mac-1 binding to iC3b and denatured BSA is not affected by the S1126A mutation. (A) Jβ2.7 transfectants expressing wt CD11b were allowed to bind to wells coated with iC3b at different ligand concentrations (microgram per milliliter of ligand used for coating) for 30 minutes. (B) Adhesion of transfected cells to coated iC3b (6 μg/mL) with or without activation was performed as described in “Materials and methods.” (C) As in panel B, except that denatured BSA (1 mg/mL) was used as a ligand. Error bars represent SD.
ICAM-2 is another ICAM-family member that binds to Mac-1.6 Thus, we measured Mac-1 integrin adhesion to coated ICAM-2 and noted a similar phenotype for activation as for ICAM-1 binding; that is, the phosphorylation site mutant could not bind after cell activation with phorbol ester (Figure 2C), even at a concentration which was optimal for wt CD11b-transfected cell binding (6 μg/mL) (Figure 2A). Very weak adhesion of S1126A mutant cells was observed to ICAM-2 after activation of cells with Mg2+/EGTA.
Adhesion to iC3b is normal for S1126A-CD11b
Mac-1 can bind to several other ligands than ICAM-1 and ICAM-2. One of the most important CD11b ligands is iC3b.7 Therefore, we measured adhesion of wt- and S1126A-CD11b–transfected cells to coated iC3b, at a ligand concentration which was in the steep part of the dose-dependence curve (Figure 3A), and therefore not saturated. Interestingly, we found that the wt CD11b and S1126A-CD11b cells bound at least equally well to iC3b (Figure 3B).
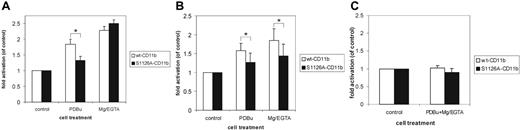
The S1126A mutation gives rise to modifications in activation epitopes. (A) The effects of the S1126A-CD11b mutation on expression of the CD18 activation reporter mAb24 were examined. Jβ2.7 cells were incubated with mAb24 (5 μg/mL) in the presence of phorbol ester (100 nM) or 5 mM Mg2+/1 mM EGTA, and mAb24 expression was analyzed by flow cytometry. Significant differences (P ≤ .01) in bracketed comparisons are indicated by a single asterisk. (B) As in panel A, except that KIM127 (5 μg/mL) was used instead of mAb24. Significant differences (P ≤ .05) in bracketed comparisons are indicated by a single asterisk. (C) As in panels A and B, except that CBRM1/5 was used, and both PDBu and Mg/EGTA were used during the activation. Error bars represent SD.
The S1126A mutation gives rise to modifications in activation epitopes. (A) The effects of the S1126A-CD11b mutation on expression of the CD18 activation reporter mAb24 were examined. Jβ2.7 cells were incubated with mAb24 (5 μg/mL) in the presence of phorbol ester (100 nM) or 5 mM Mg2+/1 mM EGTA, and mAb24 expression was analyzed by flow cytometry. Significant differences (P ≤ .01) in bracketed comparisons are indicated by a single asterisk. (B) As in panel A, except that KIM127 (5 μg/mL) was used instead of mAb24. Significant differences (P ≤ .05) in bracketed comparisons are indicated by a single asterisk. (C) As in panels A and B, except that CBRM1/5 was used, and both PDBu and Mg/EGTA were used during the activation. Error bars represent SD.
Denatured BSA has been previously shown to bind mainly to the Mac-1 α-I domain.22 To obtain information about the potential extracellular structures that are regulated by the S1126A-mutated CD11b, we used denatured BSA as a ligand. Binding of cells to coated, denatured BSA does not follow a normal dose-dependence curve, as has been reported previously23 (results not shown). We used 2 different concentrations of denatured BSA in our assays, 1 mg/mL, which has been previously used to show the complete dependence of the I domain for Mac-1 adhesion,22 and 10 μg/mL, a much lower concentration, in the same range as the other ligands used. Importantly, phorbol esters and Mg/EGTA treatment could activate S1126A-CD11b–transfected cell binding to denatured BSA, coated at either 1 mg/mL (Figure 3C), or 10 μg/mL (not shown). These results indicate that the Ser1126 mutation may not affect the conformation of the α-I domain of CD11b, at least not in a way that influences binding to denatured BSA.
Activation epitopes in the extracellular domains are influenced by the S1126A-CD11b mutation
We next examined whether the adhesion-deficient phenotype of the Mac-1 phosphorylation site mutant was due to an inability to undergo conformational changes in response to inside-out stimulation. Indeed, using mAb24, an antibody against an activation epitope in the β-I domain,24 we saw a reduced amount of mAb24 binding to the integrin extracellular domain in PDBu-treated cells but not to cells treated with Mg/EGTA, as compared with the wild-type CD11b-transfected cells (Figure 4A).
KIM127 is a reporter antibody for the active form of the integrin, which binds to the cysteine-rich repeat 2 in the extracellular region of the CD18 chain, and the binding correlates with the extension of the integrin extracellular domain.25,26 In contrast to mAb24, the KIM127 epitope showed decreased expression both in Mg/EGTA- and PDBu-treated cells for the phosphorylation site mutant as compared with wt CD11b cells. This result further indicates that the CD18 chain extracellular domain adopts a different conformation for the phosphorylation site mutant (Figure 4B).
S1126A-mutated integrins have lower affinity for a soluble ICAM ligand, and migration through SDF-1–activated endothelium is attenuated. (A) The effect of the S1126A mutation on soluble ICAM-2 binding of Mac-1 was determined. Jβ2.7 transfectants were incubated with 200 μg/mL ICAM-2Fc, and binding was determined by flow cytometry. (B) Jβ2.7 transfectants were incubated with 200 μg/mL iC3b, and binding was determined by flow cytometry. (C) Transendothelial migration through nonstimulated or SDF-1α–activated endothelium was determined as described in “Materials and methods.” Significant differences (P ≤ .02) in bracketed comparisons are indicated by a single asterisk. Error bars represent SD.
S1126A-mutated integrins have lower affinity for a soluble ICAM ligand, and migration through SDF-1–activated endothelium is attenuated. (A) The effect of the S1126A mutation on soluble ICAM-2 binding of Mac-1 was determined. Jβ2.7 transfectants were incubated with 200 μg/mL ICAM-2Fc, and binding was determined by flow cytometry. (B) Jβ2.7 transfectants were incubated with 200 μg/mL iC3b, and binding was determined by flow cytometry. (C) Transendothelial migration through nonstimulated or SDF-1α–activated endothelium was determined as described in “Materials and methods.” Significant differences (P ≤ .02) in bracketed comparisons are indicated by a single asterisk. Error bars represent SD.
The CBRM1/5 antibody has been reported to detect the “open” form of the I domain in CD11b. However, we could not detect any binding of CBRM1/5 to either wt or S1126A mutant cells either on unstimulated or phorbol ester–stimulated cells in the presence of magnesium (Figure 4C), indicating that in Jβ2.7 cells, the I domain of CD11b is in the “closed” conformation.
To compare the LFA-1 mutant with the Mac-1 mutant, we performed the same experiment for wild-type and S1140A–LFA-1 cells. KIM127 expression in these cells was normal in response to PDBu and Mg stimulation (not shown), showing that the extracellular domains of these 2 integrins of the same family are regulated differently.
Integrin affinity for ICAM ligands is reduced, and SDF-1α cannot activate migration through endothelium for the phosphorylation site mutant
On the basis of the activation epitope studies, we concluded that the mutant integrin could not undergo normal conformational changes in response to activation. Conformational changes of integrin extracellular domains have clearly been linked to affinity regulation of individual integrin molecules. To further study the mechanism of the decreased adhesion of mutant Mac-1 integrins to ICAMs, we investigated the affinity of Mac-1 for its ICAM ligands. For this, we used a soluble ligand binding assay.14 The binding of sICAM-1 to the cells is of low affinity and was below the detection limit in our assay. However, sICAM-2 bound well to the transfected cell and was thus used in our studies. Indeed, sICAM-2Fc bound well to wt CD11b-transfected Jβ2.7 cells (Figure 5A). Much weaker binding could be detected for S1126A-CD11b–transfected Jβ2.7 cells (Figure 5A). These results show that the affinity (or possibly, partially avidity) of the mutant Mac-1 integrin for ICAM ligands is lower than for the wild-type integrin. In contrast, the high affinity (binding) for iC3b was preserved for the S1126A-CD11b cells, as compared with wt CD11b-transfected cells, in good agreement with the adhesion results (Figure 5B).
One of the best characterized examples of integrin affinity modulation in a physiologic setting is the activation of integrin-dependent leukocyte arrest and migration through G protein–coupled receptors for chemokines.27 Therefore, we investigated the migration of wild-type and mutant CD11b-transfected cells through an SDF1α-stimulated endothelial cell layer in vitro. Wild-type cells were stimulated to migrate through endothelium activated with SDF-1α (Figure 5C). In contrast, no effect on migration could be seen in the presence of SDF-1α for mutant CD11b-transfected cells, but the basal migration was higher (Figure 5C).
Phosphorylation site mutation inhibits extravasation in vivo
To study the effect of the phosphorylation site mutant in a more physiologic setting, we examined cell extravasation in an in vivo model. 125I surface-labeled wt CD11b-Jβ2.7 and S1126A-CD11b-Jβ2.7 cells were injected into mice. The results showed that the phosphorylation site mutation of CD11b resulted in an increased amount of cells remaining in the peripheral circulation (Table 1). In addition, the infiltration to the lungs and spleen was dramatically reduced for the phosphorylation site mutant, whereas infiltration to other studied organs was not significantly affected. Thus, we conclude that the phosphorylation site mutation affects in vivo migration of leukocytes to target organs, presumably by interfering with Mac-1 integrin interactions with ICAM ligands on endothelium.
Effect of the CD11b phosphorylation site mutant on leukocyte extravasation in vivo
. | Percentage of injected dose/g tissue . | . | |
|---|---|---|---|
| Organ . | Wt CD11b/Jβ2.7 . | S1126A-CD11b/Jβ2.7 . | |
| Blood | 30 ± 4.7 | 42.2 ± 2.46 | |
| Heart | 17.1 ± 5.5 | 18.38 ± 4.98 | |
| Liver | 12.23 ± 2.16 | 15.35 ± 8.93 | |
| Kidney | 26.46 ± 2.32 | 24.14 ± 10.13 | |
| Lungs | 100.24 ± 37.75 | 6.02 ± 1.93 | |
| Muscle | 4.88 ± 2.98 | 2.04 ± 0.62 | |
| Femur | 10.96 ± 1.62 | 12.59 ± 3.18 | |
| Brain | 0.89 ± 0.37 | 0.099 ± 0.003 | |
| Spleen | 20.9 ± 4.4 | 2.07 ± 0.16 | |
. | Percentage of injected dose/g tissue . | . | |
|---|---|---|---|
| Organ . | Wt CD11b/Jβ2.7 . | S1126A-CD11b/Jβ2.7 . | |
| Blood | 30 ± 4.7 | 42.2 ± 2.46 | |
| Heart | 17.1 ± 5.5 | 18.38 ± 4.98 | |
| Liver | 12.23 ± 2.16 | 15.35 ± 8.93 | |
| Kidney | 26.46 ± 2.32 | 24.14 ± 10.13 | |
| Lungs | 100.24 ± 37.75 | 6.02 ± 1.93 | |
| Muscle | 4.88 ± 2.98 | 2.04 ± 0.62 | |
| Femur | 10.96 ± 1.62 | 12.59 ± 3.18 | |
| Brain | 0.89 ± 0.37 | 0.099 ± 0.003 | |
| Spleen | 20.9 ± 4.4 | 2.07 ± 0.16 | |
125I-surface-labeled wt and S1126A mutant cells were intravenously administered into Balb/c mice. At 1 hour after inoculation, mice were killed, and organs were harvested, weighed, and γ-counted. The result is given as mean ± SD (n = 3).
Discussion
In the present study, we demonstrate that phosphorylation of CD11b on the cytoplasmic Ser1126 is a major factor in the regulation of ICAM ligand binding and cellular extravasation. The major findings are as follows: (1) CD11b is phosphorylated on Ser1126 in resting human neutrophils, (2) mutation of the phosphorylation site leads to an inability of the integrin to become activated to bind ICAM-ligands, (3) the affinity for ICAMs of the mutated integrin is lower than that of the wild-type integrin, and (4) the phosphorylation site regulates chemokine-induced activation of migration through endothelium and severely diminishes in vivo dissemination of human leukocytes to lungs and spleen in a mouse model.
The Mac-1 integrin is involved in many different immunologic adhesion events to diverse ligands. It is now clear that phosphorylation of the α-chain plays a major role in the molecular mechanisms involved in certain selective activation events. The integrin CD11b chain is constitutively phosphorylated on Ser1126, and this phosphorylation site is required for adhesion events to ICAMs, as measured by a solid-phase adhesion assay. One major mechanism of regulating adhesion is through changes in the conformation of the extracellular domains of the integrin, thereby influencing its affinity for ligands. Several models for global conformational changes flowing through the integrin have been postulated, including the “switchblade”28 and “deadbolt” models.29 We have now shown that the CD11b cytoplasmic domain is indeed involved in controlling the global conformation of the integrin extracellular domain. Phorbol esters have been previously reported to change the conformation of the Mac-1 extracellular domain into the active conformation (with the open I domain, detected with the CBRM1/5 antibody).30
However, phorbol esters could not activate the phosphorylation site mutant, as measured by a solid-phase adhesion assay to ICAM-1 and ICAM-2 and by measuring the activation epitopes mAb24 and KIM127 in the extracellular domain of the β-chain. In the Jβ2.7-transfected cells, we could not detect any binding of the CBRM1/5 antibody during any stimulatory conditions for the wt or mutant cells. Phorbol esters have been reported to affect valency regulation of integrins, and it is possible that this clustering is involved in the regulation of Mac-1 by the Ser1126 phosphorylation site.
Mg2+ is thought to influence affinity of integrins by binding to the β-I–like domain MIDAS and ADMIDAS sites and thus changes the conformation of the β-I–like domain directly.12 We could see an increase in binding of mAb24 to the mutant integrin after Mg2+ stimulation. However, this change in conformation is apparently not enough to induce adhesion to coated ICAM-1, probably because the conformational changes cannot be further transmitted through the integrin. In addition, with another reporter antibody, KIM127, which primarily reports “extension” of the bent β2 integrins,25,26 we could see a decrease in activation after Mg2+ stimulation for the phosphorylation site mutant. These findings implicate that the mutant integrin extracellular domain is incapable of undergoing correct global conformational changes in response to phorbol ester or Mg2+ stimulation. It seems, however, that the mutation affects the flow of conformational changes in the integrin extracellular domains in such a way that binding only to certain ligands are affected. These results indicate that the mutant integrin adopts a conformation nonfavorable for ICAM-1 binding. This is further supported by its decreased binding of soluble ICAM-2, although the soluble ligand binding assay both for ICAM-2 and iC3b may partially measure also avidity of the interaction. Previously published results have shown that only certain antibodies, but not others, are capable of immunoprecipitating the phosphorylated form of CD11b,31 indicating that the phosphorylated species has a different conformation than the nonphosphorylated molecule, and that different pools of Mac-1 are capable of becoming activated to bind different ligands. Thus, constitutive phosphorylation of CD11b may profoundly regulate the function of this molecule, maybe by regulating the interaction with some integrin-activating factor in cells. One possible scenario could also be that cell activation results in modification of this factor and thereafter allows its interaction only with the phosphorylated integrin. Alternatively, the phosphorylation of CD11b is required for Mac-1 activation, but the activation may require binding of factors also to the β-chain, which occurs only after cell stimulation. When these 2 requirements are met (CD11b is phosphorylated and the β-chain has bound the activating factor), conformational changes are transmitted to the integrin extracellular domain, resulting in ICAM binding. In analogy, the α4-integrin is also constitutively phosphorylated. In its phosphorylated form it cannot bind paxillin, and this process excludes paxillin from the leading edge of the cell and regulates cell migration.32
Unfortunately, the phosphospecific antibody used in this study did not immunoprecipitate CD11b and, thus, could not be used to determine the stoichiometry of phosphorylation of CD11b on Ser1126; however, the stoichiometry of phosphorylation of the related integrin αL-chain has been reported to be rather high, in the range of 40%.14 In addition, using 2-dimensional gel electrophoresis, it was reported that alkaline phosphatase treatment shifted the single, broad band of CD11b to a more basic form, indicating that also CD11b is phosphorylated at a high stoichiometry.15
The binding of Mac-1 to the ligand iC3b was not negatively affected by the phosphorylation site mutation; actually, there was more constitutive binding to iC3b for the S1126A-CD11b–transfected cells. Indeed, iC3b binding sites are present in different parts of Mac-1, that is, the α-I domain,33,34 the β-propeller in CD11b,22 and the β-I–like domain in CD18.35 A combination of all these sites may give rise to the strong binding between Mac-1 and iC3b and probably explains why the binding to iC3b is not affected by CD11b phosphorylation. Probably the I domain is not the most essential iC3b binding site in the Jβ2.7 cell system, because it seems to be in the closed conformation, both in the wild-type and mutant integrin, also during stimulatory conditions. Instead, the β-I–like domain may be important for iC3b binding, because this domain has binding sites for both ICAM and iC3b; however, the amino acid residues responsible for binding are different for the 2 ligands, and it is possible that the phosphorylation site mutant exposes only the iC3b binding sites. We also investigated the binding of the transfected cells to other ligands with broad recognition footprints, that is, fibrinogen and Factor X22,36 ; unfortunately, these bound to untransfected Jβ2.7 cells as well as to CD11b-transfected cells and could therefore not be used to investigate the binding of Mac-1 and the phosphorylation site mutant.
These results show that we have identified a major proximal regulatory event for Mac-1–mediated adhesion. It is clear that the activation of Mac-1 can be regulated in different ways, resulting in specific ligand binding. In addition, the CD11b phosphorylation site is unique for this integrin, showing that regulation of integrin activation is a complex process, evidently not regulated in the same way for all integrins. However, together with the recently reported LFA-1 integrin regulation by phosphorylation on Ser1140 in the CD11a chain,14 these experiments strengthen the notion that integrin α-chain phosphorylation could be a more general mechanism of regulating integrin affinity. CD11a phosphorylation on Ser1140 regulates adhesion to ICAM-1 only during certain stimulatory conditions (by chemokines, ligands, or activating antibodies) but not others (phorbol ester and T-cell receptor stimulation).14 However, CD11b phosphorylation on Ser1126 regulates all tested ways of activation of adhesion to ICAM-1. This implicates that affinity regulation is the major regulatory mechanism for Mac-1–mediated adhesion, whereas LFA-1 uses either affinity or valency regulation, depending on the mode of activation.
Extravasation of leukocytes into the tissues is an important step in the defense against infections. However, this process may also cause serious tissue damage, for example, in autoimmune diseases, allograft rejection, and postischemic tissue destruction after myocardial infarction. CD11/CD18 integrin–ICAM interactions are necessary and sufficient for leukocyte arrest and transmigration through endothelium.1,2 Recently, it has been shown that dynamic shifts in β2-integrin affinity for ligands is a main mechanism involved in the regulation of leukocyte rolling, arrest, and transendothelial migration.37-39 One of the changes that happens in the transition from nonadherent, free leukocytes to rolling and firmly adherent leukocytes after chemokine stimulation is the expression of the KIM127 activation epitope.40,41 Both integrin affinity for ICAMs and the KIM127 epitope were down-regulated for the phosphorylation site mutant, and cells expressing the CD11b phosphorylation site mutant were deficient in chemokine-stimulated migration across an endothelial cell layer in vitro. Basal migration, however, was even higher than for the wild-type integrin-expressing cells. This static migration assay that is conducted without flow, evidently allows the cells to attach and detach more easily during the cell migration process, because of the lower affinity of the integrin for its ICAM ligands.
To study migration in a more physiologic setting, in which the blood flow will influence migration of cells through endothelium, the wild-type and mutant integrin-transfected Jβ2.7 cells were intravenously administered to Balb/c mice. Here, we took advantage of the fact that human β2-integrins have been shown to bind to murine ICAM-1 and ICAM-2.42,43 Indeed, the S1126 mutation dramatically decreased the extravasation of the cells to the spleen and lungs. These results show that the CD11b phosphorylation site is important in the regulation of in vivo migration of leukocytes through endothelium, through regulation of Mac-1 integrin affinity for ICAM-ligands. This is potentially useful in the therapeutic targeting of specific Mac-1 integrin functions, that is, adhesion to endothelial cells via ICAM-1 and ICAM-2, while leaving other functions intact, such as adhesion to foreign organisms coated with iC3b.
Prepublished online as Blood First Edition Paper, July 20, 2006; DOI 10.1182/blood-2006-03-013557.
Supported by the Academy of Finland, The Sigrid Jusélius Foundation, the Finnish Cancer Society, and the Magnus Ehrnrooth Foundation.
The authors declare no competing financial interests.
S.C.F. designed and performed the research and wrote the paper; M.V. performed the research and wrote the paper; M.S. and T.J.H. designed and performed part of the research; and C.G.G. obtained most of the research funding and participated in planning the research and writing the paper.
S.C.F. and M.V. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank M. Robinson, N. Hogg, V. Horejsi, and M.A. Arnaout for antibodies and cells and M. Aatonen and S. Kaukinen for expert technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal