Abstract
Elevation of serum sialic acid and the ST6Gal-1 sialyltransferase is part of the hepatic system inflammatory response, but the contribution of ST6Gal-1 has remained unclear. Hepatic ST6Gal-1 elevation is mediated by P1, 1 of 6 promoters regulating the ST6Gal1 gene. We report that the P1-ablated mouse, Siat1ΔP1, and a globally ST6Gal-1–deficient mouse had significantly increased peritoneal leukocytosis after intraperitoneal challenge with thioglycollate. Exaggerated peritonitis was accompanied by only a modest increase in neutrophil viability, and transferred bone marrow–derived neutrophils from Siat1ΔP1 mice migrated to the peritonea of recipients with normal efficiency after thioglycollate challenge. Siat1ΔP1 mice exhibited 3-fold greater neutrophilia by thioglycollate, greater pools of epinephrine-releasable marginated neutrophils, greater sensitivity to G-CSF, elevated bone marrow CFU-G and proliferative-stage myeloid cells, and a more robust recovery from cyclophosphamide-induced myelosuppression. Bone marrow leukocytes from Siat1ΔP1 are indistinguishable from those of wild-type mice in α2,6-sialylation, as revealed by the Sambucus nigra lectin, and in the expression of total ST6Gal-1 mRNA. Together, our study demonstrated a role for ST6Gal-1, possibly from extramedullary sources (eg, produced in liver) in regulating inflammation, circulating neutrophil homeostasis, and replenishing granulocyte numbers.
Introduction
It has long been recognized that changes in carbohydrate structures, particularly in the sialylation of various plasma components, accompany severe inflammatory conditions1-3 and that cytokines regulate the glycosylation profiles of these plasma proteins.4-6 Changes in glycan structures of these glycoproteins, such as the α1-acid glycoprotein, have been shown to have predictive value in a number of diseases with an inflammatory component, such as rheumatoid arthritis,7-9 diabetes mellitus,10 and cancer.3,11,12 Upregulation of the sialyltransferase ST6Gal-1 and its secretion into the serum are also considered integral parts of the systemic inflammatory response.13,14 Despite many circumstantial inferences, whether ST6Gal-1 directly contributes to the inflammatory process has remained in contention.
ST6Gal-1 mediates the synthesis of the α2,6-sialyl linkage to generate the Siaα2,6Galβ1,4GlcNAc glycan structure that is widely distributed in most, if not all, mammalian tissues.15 Mice genetically engineered to be deficient in ST6Gal-1 are essentially unable to generate Siaα2,6Galβ1,4GlcNAc structures on glycoproteins, as demonstrated by the lack of binding to the lectin from Sambucus nigra.16 A single-copy gene, Siat1, encodes ST6Gal-1, which shares remarkable structural conservation between human, rat, and mouse homologues.17,18 Transcription initiation from a number of physically distinct promoter regions generates a family of mRNAs with identical coding domains that differ only in the 5′-untranslated regions.19-21 The level of ST6Gal-1 expression differs dramatically from tissue to tissue,22,23 and it is particularly high in liver,23,24 in B-lymphocytes,17,20 and in lactating mammary glands.21 A totally ST6Gal-1 deficient mouse (Siat1-null) exhibits a severely attenuated B-cell response, suggesting a requirement of this sialyltransferase and its cognate sialyl-glycan linkage in B-cell function.25
The P1 promoter mediates ST6Gal-1 expression in liver. ST6Gal-1 up-regulation during the systemic inflammatory response is mediated by the P1 promoter19,26 and is modulated by glucocorticoids and IL-6.5,27,28 P1 is also active in the intestinal epithelium of newborn animals,29 but its range of cellular and developmental specificity remains incompletely known. To address the function of ST6Gal-1 in inflammation, we have generated a mutant mouse, Siat1ΔP1, with specific ablation in the P1 region of Siat1.30 This mutation rendered the animal unable to up-regulate ST6Gal-1 expression during the systemic inflammatory response. Unlike the Siat1-null mouse, the Siat1ΔP1 mouse exhibits a normal B-cell response.30
Through the thioglycollate model of experimental peritonitis, we showed that Siat1-null and Siat1ΔP1 mice exhibit greater inflammatory cell migration into the peritoneum and that the more robust peritonitis was preceded by an exaggerated burst of granulocytes in the peripheral blood. Our results suggest a previously unrecognized role of ST6Gal-1 in regulating inflammation, circulating neutrophil homeostasis, and myeloid differentiation.
Materials and methods
Animals, thioglycollate model of induced peritonitis, epinephrine-induced demargination of neutrophils, G-CSF– and cyclophosphamide-induced neutrophilia
Generation of the Siat1ΔP1 mouse by gene-targeted deletion of the P1 promoter of the Siat1 gene was described previously.30 Siat1ΔP1 mice were backcrossed 11 successive generations into the C57BL/6 background. For all experiments reported here, age- and sex-matched (typically 55- to 70-day-old animals) C57BL/6 wild-type animals were used as controls. Brewer's yeast thioglycollate (Becton Dickinson Microbiology, Baltimore, MD) was prepared in a 4% wt/vol sterile solution. To elicit inflammatory response and leukocyte emigration, 1 mL thioglycollate solution was administered intraperitoneally into each recipient animal. Where specified, a sample of peripheral blood (typically approximately 50 μL) was obtained by retro-orbital venous plexus sampling in polypropylene tubes containing EDTA (ethylenediaminetetraacetic acid). At indicated time points after thioglycollate challenge, animals were killed by CO2 asphyxiation, and the blood sample was withdrawn by cardiac puncture. Peritoneal cells were recovered by peritoneal lavage with 6 mL ice-cold PBS. Typically, peritoneal lavage is free of red coloration, indicating the lack of red blood cell contamination. Red blood cells in peripheral blood were lysed in Tris-buffered ammonium chloride (pH 7.2) buffer, and total white blood cell counts were determined by flow cytometry. Bone marrow was harvested by flushing a single femoral bone with ice-cold PBS. Manual leukocyte differentials were performed in Wright-stained blood smears or cytospin preparations of red blood cell–lysed cell samples. Epinephrine-induced release of marginated neutrophils was performed according to Johnson et al31 except that epinephrine, 0.25 mg/kg body weight, was injected into wild-type and Siat1ΔP1 mice intravenously rather than intraperitoneally. Recombinant murine G-CSF (rmG-CSF; Chemicon International, Temecula, CA) diluted in sterile PBS was administered by tail vein injection at a dose of 20 μg/kg. Peripheral blood was obtained before injection, 30 minutes after injection, and 2 hours after injection. Cyclophosphamide, diluted in sterile PBS, was administered intraperitoneally (250 mg/kg). Peripheral blood was obtained before injection and 4, 5, 6, and 7 days after injection. All animal studies presented here have been approved by the Institute Animal Care and Use Committee of Roswell Park Cancer Institute. Unless otherwise stated, all data here are presented as the mean ± SD. Statistical significance was assessed by a 2-sided Student t test.
Flow cytometric profiling of inflammatory cells
Immunofluorescent staining and flow cytometric analysis of inflammatory cell subsets were performed as follows. Cells (0.5 × 106–2 × 106) were washed in PBS containing 0.5% BSA and 0.02% sodium azide (PAB). Fc receptor sites were blocked by incubation with goat serum (1:10; Gibco, Carlsbad, CA) and anti-CD16/32 (Fcγ III/II receptor) Fc block for 10 to 15 minutes. Samples were incubated with combinations of fluorescently labeled antibodies 1A8 (anti-Ly6G), 7/4 (anti–polymorphonuclear cell 40-kDa antigen; Serotec, Oxford, UK), M1/70 (anti–CD11b/Mac-1), Gr1 (anti-Ly6G, anti-Ly6C, clone RB6-8C5), and Sambucus nigra lectin (SNA; Vector Laboratories, Peterborough, UK), washed with PBS, and fixed in 1% formaldehyde. Biotin-conjugated reagents were visualized by incubation with streptavidin–Cychrome (BD PharMingen, San Diego, CA) for 30 minutes. Flow cytometric analysis was performed with a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, Franklin Lakes, NJ) and the WinList software package. For blood samples, heparin was included to prevent clotting, and red blood cells were lysed with red blood cell lysis buffer after the last incubation with antibody. Unless otherwise stated, all immunoreagents for flow cytometry were from BD PharMingen.
Ex vivo labeling of cells and reintroduction into recipients
Mice aged 10 to 11 weeks, either Siat1ΔP1 or C57BL/6 wild-type, were killed by CO2 asphyxiation, and bone marrow cells were recovered in cold sterile PBS. To apply labeling with PKH26-red (Sigma Chemical, St Louis, MO), cells were washed in RPMI medium (without serum), and 107 cells were resuspended in 1 mL Diluent C (Sigma) and rapidly added to 1 mL of 4 μM PKH26-red. The cells were incubated at 25°C for 5 minutes, terminated by the addition of 2.5% fetal calf serum. For carboxyfluorescein succinimidyl ester (CFSE) labeling, CFSE (3 μM) was added to 107 cells resuspended in 1 mL PBS. Cells were incubated at 37°C for 5 minutes, and labeling was stopped by the addition of fetal calf serum. After labeling, the cells were washed twice with cold PBS and counted by hemocytometer. Differentially labeled donor cells were recombined immediately before infusion into recipient animals. Recipient animals, either wild-type C57BL/6 or Siat1ΔP1, were anesthetized with tribromoethanol. Each recipient received pooled cells consisting of 107 cells intravenously from each labeled group. Concomitantly, 1 mL of 4% wt/vol thioglycollate was injected intraperitoneally to elicit peritonitis. After 6 hours, the recipient mice were killed by CO2 asphyxiation, and their peritoneal cells were recovered and assessed by flow cytometry.
Determination of cell viability
Peritoneal cells were washed, and their viability was determined by visual inspection for granulocytes with apoptotic nuclei after Wright-Giemsa staining. Flow analysis for FITC–annexin V and propidium iodide (BD PharMingen) was used as secondary confirmation for cell viability. Isolated cells were also maintained ex vivo in RPMI-1640 (with 10% FCS) at 37°C for 24 hours, and viability was redetermined to assess differences in spontaneous apoptosis.
Colony-forming cell assay
A total of 30 000 bone marrow nucleated cells in a volume of 0.1 mL were plated in 0.9 mL methylcellulose medium (MethoCult 3231; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 20 ng/mL rmG-CSF (Chemicon International) and 1 ng/mL rmIL-3 (Stem Cell Technologies) and were placed in a humidified incubator with 5% CO2 at 37°C. Colonies containing at least 50 cells were counted 4 days after incubation. The number of colonies per 1000 nucleated bone marrow cells was calculated.
RNA isolation and real time RT-PCR
Total RNA was isolated using TRIzol (Invitrogen), and cDNA was synthesized from 1 μg RNA using the iScript reverse transcriptase kit (Bio-Rad, Hercules, CA) according to manufacturer's instructions. Realtime PCR reactions, using iQ SYBR-green Supermix (Bio-Rad), were performed on the My iQ Single-Color Real-Time PCR Detection System (Bio-Rad). Primer pairs for each mRNA, available on request, were designed based on sequence information deposited in GenBank or have been previously published.20 Relative mRNA levels are derived from ΔCt, the difference of the threshold cycle value of the target mRNA and the Ct value for RPL32, a ribosomal protein mRNA used as a reference standard.
Results
More robust leukocyte emigration response to intraperitoneal thioglycollate challenge in ST6Gal-1–deficient mice
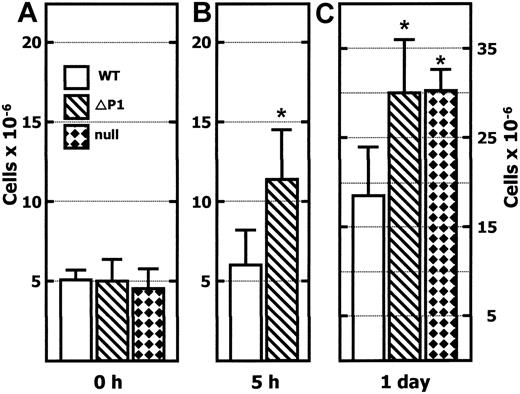
As shown in Figure 1A, resident cell numbers in the peritoneum are indistinguishable (approximately 5 × 106 cells/animal) between the C57BL/6 wild-type mouse and in the 2 independent ST6Gal-1–deficient mouse lines Siat1-null and Siat1ΔP1. We used the thioglycollate-induced model of peritonitis to demonstrate a significantly more robust acute inflammatory response in Siat1ΔP1 animals, such that within 5 hours the total peritoneal cell counts were elevated more than 2-fold in these mutant animals. Within the same time frame, peritoneal cell counts were only marginally elevated in the wild-type animals. Significant peritonitis was evident in animals of all genotypes by the next day, but the total peritoneal cell accumulation remained significantly and consistently greater in Siat1ΔP1 than in age- and sex-matched wild-type animals.
Greater leukocyte accumulation in the peritoneum ofSiat1ΔP1 andSiat1-null mice after intraperitoneal thioglycollate elicitation. Peritoneal lavage was recovered from wild-type C57BL/6 (WT), Siat1ΔP1 (ΔP1), and Siat1-null (null) animals in the absence of (0 hour; A), 5 hours after (B), and 1 day after (C) elicitation with 1 mL 4% wt/vol intraperitoneal thioglycollate. Viable cells were counted by hemocytometer after staining for trypan-blue exclusion. Age- and sex-matched mice were used. (A) n = 8WT, n = 8 ΔP1, and n = 4 null mice. (B) n = 8WT, n = 8 ΔP1 mice. (C) n = 8 WT, n = 8 ΔP1, and n = 4 null mice. *Mutant animal data points where statistically significant differences with corresponding WT have been reached. Statistical significance was noted as follows: P = .002, WT and ΔP1 (B); P = .002, WT and ΔP1 (C); and P = .001, WT and null (C). Error bars represent 1 standard deviation from the mean of each group.
Greater leukocyte accumulation in the peritoneum ofSiat1ΔP1 andSiat1-null mice after intraperitoneal thioglycollate elicitation. Peritoneal lavage was recovered from wild-type C57BL/6 (WT), Siat1ΔP1 (ΔP1), and Siat1-null (null) animals in the absence of (0 hour; A), 5 hours after (B), and 1 day after (C) elicitation with 1 mL 4% wt/vol intraperitoneal thioglycollate. Viable cells were counted by hemocytometer after staining for trypan-blue exclusion. Age- and sex-matched mice were used. (A) n = 8WT, n = 8 ΔP1, and n = 4 null mice. (B) n = 8WT, n = 8 ΔP1 mice. (C) n = 8 WT, n = 8 ΔP1, and n = 4 null mice. *Mutant animal data points where statistically significant differences with corresponding WT have been reached. Statistical significance was noted as follows: P = .002, WT and ΔP1 (B); P = .002, WT and ΔP1 (C); and P = .001, WT and null (C). Error bars represent 1 standard deviation from the mean of each group.
To confirm that the exaggerated peritonitis phenotype is the consequence of ST6Gal-1 deficiency, we also examined the Siat1-null mouse that was independently produced by Hennet et al.25 The Siat1-null mouse maintained resident peritoneal cell counts while at rest, similar to those observed in the wild-type and the Siat1ΔP1 mouse. However, 1 day after thioglycollate administration, the excessively robust peritonitis was reproduced in the Siat1-null mouse. Siat1-null and Siat1ΔP1 mice exhibited similar degrees of exaggerated peritonitis.
A more detailed time course of the peritonitis and the leukocyte differentials by Wright-Giemsa staining are summarized in Table 1. Differences in total peritoneal cell pools between Siat1ΔP1 and wild-type animals were most pronounced at the earlier stages of peritonitis (eg, 5 and 18 hours after thioglycollate elicitation). By 80 hours after elicitation, peritoneal cell counts in the mutant animals were only 20% greater than in their control counterparts. There was no significant difference in the composition of the inflammatory infiltrates (eg, granulocytes and monocyte-macrophage ratio) between the wild-type and mutant mice within the time course of this experiment. Granulocytes dominated the early stages and constituted up to 70% of the peritoneal cell population 5 hours after elicitation in wild-type and mutant mice. By 80 hours, less than 10% of the total population consisted of granulocytes.
Peritoneal leukocyte differentials in thioglycollate-challenged mice
Time after injection, genotype . | P . | Total leukocytes (n) . | Macrophages, % (n) . | Granulocytes, % (n) . | Lymphocytes, % (n) . |
|---|---|---|---|---|---|
| 0 h | — | ||||
| Wild type | 5.1 ± 0.6 (5) | 70.2 ± 1.1 (5) | 26.5 ± 3.6 (5) | 1.1 ± 0.8 (5) | |
| Siat1ΔP1 | 5.0 ± 1.4 (5) | 71.0 ± 2.4 (3) | 24.1 ± 2.8 (3) | 3.9 ± 1.3 (3) | |
| 5 h | .002 | ||||
| Wild type | 6.0 ± 2.2 (8) | 32.6 ± 5.0 (4) | 65.3 ± 5.4 (4) | 0.8 ± 0.4 (4) | |
| Siat1ΔP1 | 11.4 ± 3.1 (8) | 27.2 ± 5.0 (4) | 70.7 ± 5.5 (4) | 1.0 ± 1.1 (4) | |
| 18 h | .002 | ||||
| Wild type | 18.6 ± 5.4 (10) | 39.7 ± 3.3 (8) | 59.1 ± 3.6 (8) | 0.8 ± 0.2 (8) | |
| Siat1ΔP1 | 30.0 ± 6.0 (10) | 33.1 ± 3.2 (8) | 65.7 ± 3.5 (8) | 1.0 ± 0.5 (8) | |
| 80 h | .014 | ||||
| Wild type | 41.4 ± 7.8 (8) | 88.5 ± 3.7 (5) | 9.9 ± 3.7 (5) | 1.6 ± 0.4 (5) | |
| Siat1ΔP1 | 51.0 ± 6.0 (8) | 89.2 ± 1.7 (5) | 9.6 ± 1.5 (5) | 1.2 ± 0.4 (5) |
Time after injection, genotype . | P . | Total leukocytes (n) . | Macrophages, % (n) . | Granulocytes, % (n) . | Lymphocytes, % (n) . |
|---|---|---|---|---|---|
| 0 h | — | ||||
| Wild type | 5.1 ± 0.6 (5) | 70.2 ± 1.1 (5) | 26.5 ± 3.6 (5) | 1.1 ± 0.8 (5) | |
| Siat1ΔP1 | 5.0 ± 1.4 (5) | 71.0 ± 2.4 (3) | 24.1 ± 2.8 (3) | 3.9 ± 1.3 (3) | |
| 5 h | .002 | ||||
| Wild type | 6.0 ± 2.2 (8) | 32.6 ± 5.0 (4) | 65.3 ± 5.4 (4) | 0.8 ± 0.4 (4) | |
| Siat1ΔP1 | 11.4 ± 3.1 (8) | 27.2 ± 5.0 (4) | 70.7 ± 5.5 (4) | 1.0 ± 1.1 (4) | |
| 18 h | .002 | ||||
| Wild type | 18.6 ± 5.4 (10) | 39.7 ± 3.3 (8) | 59.1 ± 3.6 (8) | 0.8 ± 0.2 (8) | |
| Siat1ΔP1 | 30.0 ± 6.0 (10) | 33.1 ± 3.2 (8) | 65.7 ± 3.5 (8) | 1.0 ± 0.5 (8) | |
| 80 h | .014 | ||||
| Wild type | 41.4 ± 7.8 (8) | 88.5 ± 3.7 (5) | 9.9 ± 3.7 (5) | 1.6 ± 0.4 (5) | |
| Siat1ΔP1 | 51.0 ± 6.0 (8) | 89.2 ± 1.7 (5) | 9.6 ± 1.5 (5) | 1.2 ± 0.4 (5) |
Peritoneal leukocytes were recovered by peritoneal lavage without further treatment (0 h) and at 5, 18, and 80 hours after intraperitoneal thioglycollate injection. Differential counts (expressed as percentage of total population) of leukocyte subsets determined by staining of exudate smears. Percentage of granulocytes is the total of eosinophils and neutrophils. Total leukocyte counts are given in million per animal.
n indicates number of mice used for each determination; —, not applicable.
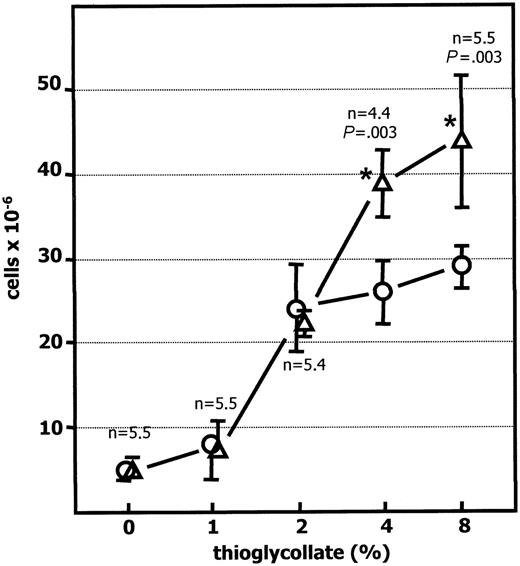
To assess whether the elevated peritonitis resulted from increased sensitivity to thioglycollate, Siat1ΔP1 and C57BL/6 wild-type cohorts were subjected to varying intraperitoneal dosages of thioglycollate, and the extent of peritonitis was evaluated 24 hours later. If the difference in peritoneal leukocytosis was principally related to heightened sensitivity to thioglycollate, then the difference in peritonitis should be most evident at lower concentrations of the stimulus. Figure 2 shows the absence of an exaggerated peritonitis phenotype at the lowest thioglycollate dosages (1% and 2%). An exaggerated peritonitis phenotype was only evident at the higher thioglycollate dosages (4% and 8%), which may suggest the presence of a greater pool of inflammatory cells in the Siat1ΔP1 mice available to emigrate under high-dose thioglycollate challenges.
Elicited Siat1ΔP1granulocytes have slightly enhanced viability
To examine whether delayed cell death in the peritoneal infiltrates contributed to the exaggerated peritonitis in ST6Gal-1 deficient animals, cytospin preparations from peritoneal cells of wild-type and Siat1ΔP1 animals were visually examined after Giemsa staining to score apoptotic neutrophils. As summarized in Table 2, the percentage of neutrophils with apoptotic nuclei was only slightly lower in Siat1ΔP1 (9.0% ± 2.7%) than in wild-type (13.3%± 3.0%) mice in cells recovered 6 hours after thioglycollate elicitation, but this difference was not statistically significant (P = .08). The difference in apoptotic index was more evident at 24 hours, when the percentage of apoptotic neutrophils was 30% in wild-type mice but only 18% in Siat1ΔP1 neutrophils (P = .013).
Decreased incidence of apoptosis in elicited PMNs from Siat1ΔP1 mice
Genotype . | 6-h elicited PMNs, % . | 24-h elicited PMNs, % . |
|---|---|---|
| Wild type | ||
| Mouse 1 | 9 | 25 |
| Mouse 2 | 14 | 31 |
| Mouse 3 | 14 | 34 |
| Mouse 4 | 16 | ND |
| Siat1ΔP1 | ||
| Mouse 1 | 7 | 19 |
| Mouse 2 | 13 | 16 |
| Mouse 3 | 8 | 19 |
| Mouse 4 | 8 | ND |
Genotype . | 6-h elicited PMNs, % . | 24-h elicited PMNs, % . |
|---|---|---|
| Wild type | ||
| Mouse 1 | 9 | 25 |
| Mouse 2 | 14 | 31 |
| Mouse 3 | 14 | 34 |
| Mouse 4 | 16 | ND |
| Siat1ΔP1 | ||
| Mouse 1 | 7 | 19 |
| Mouse 2 | 13 | 16 |
| Mouse 3 | 8 | 19 |
| Mouse 4 | 8 | ND |
Peritoneal cells were recovered from wild-type (n = 4) and Siat1ΔP1 mice (n = 4) elicited with 1 mL of 4% wt/vol thioglycollate for 6 or 24 hours. Cytospin preparations of cells were visually examined after Giemsa staining. Shown are the percentages of morphologically identified apoptotic neutrophils.
PMN indicates polymorphonuclear leukocyte; ND, not done.
The lower incidence of apoptotic granulocytes was confirmed by the use annexin V–FITC, which reveals the exposure of phosphatidylserine, an early event in cells undergoing apoptosis, and propidium iodide (PI) to measure permeability of the cell membrane. Using the criterion of viable cells being both annexin V negative and PI negative, the percentage of viable Siat1ΔP1 cells was slightly elevated compared with wild-type cells (81% and 75%, respectively) in cells recovered 24 hours after thioglycollate challenge. When these recovered cells were cultured ex vivo for an additional 24 hours, the difference in the percentage of viable cells persisted, with means of 67% and 54%, respectively, for Siat1ΔP1 and wild-type granulocytes (P = .019).
Exaggerated peritonitis insiat1ΔP1 mice is thioglycollate dosage dependent. Peritonitis was elicited in C57BL/6 wild-type (○) and Siat1ΔP1 (▵) mice with 1 mL of 0%, 1%, 2%, 4%, or 8% wt/vol intraperitoneal thioglycollate, as shown, and peritoneal cells were harvested 24 hours later and counted. N is the number of animals used in each determination. *Mutant animal data points at which statistically significant differences with corresponding WT have been reached. Statistical significance for the differences between wild-type and Siat1ΔP1 for 4% and 8% is as shown. Error bars indicate 1 standard deviation from the mean of each group.
Exaggerated peritonitis insiat1ΔP1 mice is thioglycollate dosage dependent. Peritonitis was elicited in C57BL/6 wild-type (○) and Siat1ΔP1 (▵) mice with 1 mL of 0%, 1%, 2%, 4%, or 8% wt/vol intraperitoneal thioglycollate, as shown, and peritoneal cells were harvested 24 hours later and counted. N is the number of animals used in each determination. *Mutant animal data points at which statistically significant differences with corresponding WT have been reached. Statistical significance for the differences between wild-type and Siat1ΔP1 for 4% and 8% is as shown. Error bars indicate 1 standard deviation from the mean of each group.
Transmigration efficiency is similar between Siat1ΔP1 and wild-type inflammatory cells
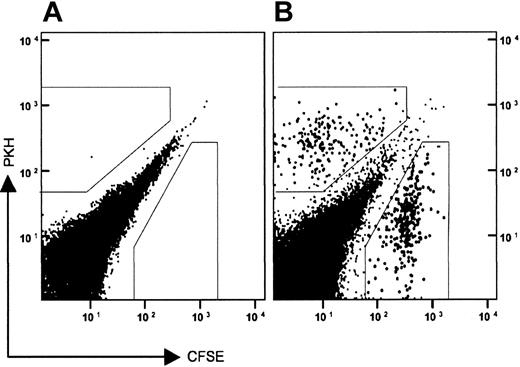
To test whether the exaggerated peritonitis in the Siat1ΔP1 mice resulted from more efficient inflammatory cell recruitment and transmigration into the peritoneum, the transmigration of ex vivo–labeled donor cells into the peritoneum of thioglycollate-challenged recipient animals was monitored. Bone marrow cells, which contain large numbers of functionally competent neutrophils,32 were recovered from donor animals and tagged ex vivo. Figure 3 shows a sample profile of the recovery of both CFSE- and PKH-labeled donor cells in the peritoneum after thioglycollate elicitation of the recipient. The recovered labeled cells displayed forward and side scatter characteristics consistent with granulocytes (data not shown). CFSE and PKH labeling also did not differentially affect transmigration efficiency. To demonstrate this, cells from a single donor animal were divided into 2 identical aliquots, separately labeled with either CFSE or PKH, and then recombined. The recombined pool, with a CFSE/PKH ratio of 1.3, was intravenously infused into recipient animals with the same genotype as the donor animal. After thioglycollate elicitation, CFSE- and PKH-labeled cells recovered in the peritoneum yielded a CFSE/PKH ratio of 1.3, which was identical to the ratio of the input donor cells (Table 3).
Recovery of CFSE- and PKH-labeled donor cells in peritoneum of thioglycollate-elicited recipients
. | Donor CFSE/PKH . | Recovered CFSE/PKH . |
|---|---|---|
| ΔP1-CFSE/ΔP1-PKH donor | ||
| ΔP1 recipient 1 | 1.30 | 1.3 |
| ΔP1 recipient 2 | 1.30 | 1.3 |
| ΔP1-CFSE/WT-PKH donor | ||
| WT recipient 1 | 1.24 | 1.3 |
| WT recipient 2 | 1.24 | 1.2 |
| WT recipient 3 | 1.24 | 1.2 |
| WT recipient 4 | 1.24 | 1.0 |
| ΔP1 recipient 1 | 1.24 | 0.8 |
| ΔP1 recipient 2 | 1.24 | 1.2 |
| ΔP1 recipient 3 | 1.24 | 1.2 |
| ΔP1 recipient 4 | 1.24 | 1.2 |
. | Donor CFSE/PKH . | Recovered CFSE/PKH . |
|---|---|---|
| ΔP1-CFSE/ΔP1-PKH donor | ||
| ΔP1 recipient 1 | 1.30 | 1.3 |
| ΔP1 recipient 2 | 1.30 | 1.3 |
| ΔP1-CFSE/WT-PKH donor | ||
| WT recipient 1 | 1.24 | 1.3 |
| WT recipient 2 | 1.24 | 1.2 |
| WT recipient 3 | 1.24 | 1.2 |
| WT recipient 4 | 1.24 | 1.0 |
| ΔP1 recipient 1 | 1.24 | 0.8 |
| ΔP1 recipient 2 | 1.24 | 1.2 |
| ΔP1 recipient 3 | 1.24 | 1.2 |
| ΔP1 recipient 4 | 1.24 | 1.2 |
Total bone marrow cells from donors labeled with CFSE of PKH-26 were pooled and injected into tail veins of recipient animals. At the same time, 1 mL of 4% wt/vol thioglycollate was introduced intraperitoneally into recipients. After 6 hours, peritoneal aspirate was recovered from recipients and examined by flow cytometry.
To determine whether Siat1ΔP1 cells differed from wild-type cells in transmigration efficiency, CFSE-tagged Siat1ΔP1 cells and PKH-tagged wild-type cells were combined to a ratio of 1.24, and the pooled cells were infused by tail vein injection into recipient animals. Peritoneal aspirates, recovered from the recipients 6 hours after thioglycollate elicitation, retained the input donor CFSE/PKH ratio regardless of recipient genotype (Table 3). These data demonstrated that inflammatory cells of both genotypes were recruited with the same efficiency into the peritoneum of either Siat1ΔP1 or C57BL/6 wild-type recipients.
Elevated neutrophilia in ST6Gal-1–deficient mice
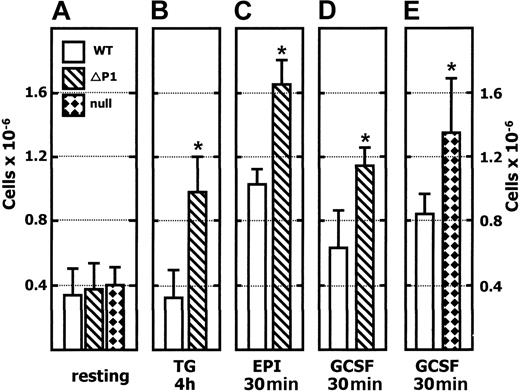
The thioglycollate dosage data shown in Figure 2 suggested an increased availability of inflammatory cells in the Siat1ΔP1 mouse. We sought confirmation of this notion by examining the peripheral blood compartment, which is the immediate source of white blood cells migrating to inflamed sites. As shown in Figure 4A, there was no evidence of an elevated pool of granulocytes in the peripheral blood of ST6Gal-1–deficient animals of either genotype (eg, Siat1ΔP1 and Siat1-null) in the unstimulated state. However, 4 hours after an intraperitoneal thioglycollate challenge, there was a 2.5-fold elevation of blood granulocyte counts in the Siat1ΔP1 that was absent in the C57BL/6 wild-type cohorts. The neutrophilia in Siat1ΔP1 mice was transient, and granulocyte counts returned to resting levels within 18 hours (data not shown).
PKH26- and CFSE-tagged donor cells migrate to the peritonea of thioglycollate-elicited recipients. Total bone marrow cells from a Siat1ΔP1 mouse were separately labeled with either PKH26-red or CFSE. The tagged donor cells were recombined, and pooled cells were injected by tail vein into a recipient mouse, also Siat1ΔP1. Concomitant with the transfer of the tagged donor cells, peritonitis was elicited by intraperitoneal injection of 1 mL of 4% (wt/vol) thioglycollate. Peritoneal cells were recovered 6 hours later, and the presence of tagged donor cells was profiled by flow cytometry. (A) Recipient animal that did not receive tagged donor cells. (B) Recipient animal receiving 107 pooled donor cells. Gates (boxed regions, B) were used to determine the PKH/CFSE ratios of tagged cells, as summarized in Table 2. Typically, up to 0.4% of the labeled donor cells are recovered in the peritonea of the thioglycollate-elicited recipients.
PKH26- and CFSE-tagged donor cells migrate to the peritonea of thioglycollate-elicited recipients. Total bone marrow cells from a Siat1ΔP1 mouse were separately labeled with either PKH26-red or CFSE. The tagged donor cells were recombined, and pooled cells were injected by tail vein into a recipient mouse, also Siat1ΔP1. Concomitant with the transfer of the tagged donor cells, peritonitis was elicited by intraperitoneal injection of 1 mL of 4% (wt/vol) thioglycollate. Peritoneal cells were recovered 6 hours later, and the presence of tagged donor cells was profiled by flow cytometry. (A) Recipient animal that did not receive tagged donor cells. (B) Recipient animal receiving 107 pooled donor cells. Gates (boxed regions, B) were used to determine the PKH/CFSE ratios of tagged cells, as summarized in Table 2. Typically, up to 0.4% of the labeled donor cells are recovered in the peritonea of the thioglycollate-elicited recipients.
To evaluate the idea that Siat1ΔP1 mice have an increased reservoir of inflammatory cells, we examined the total peripheral granulocyte pool that included not only those cells freely circulating in the blood but also those that adhered reversibly to the vascular endothelium. Epinephrine infusion can rapidly and specifically release cells from this marginated pool into the systemic circulation.33-35 The resultant epinephrine-induced neutrophilia was used to estimate the difference in overall pool size of peripheral granulocytes between wild-type and Siat1ΔP1 animals.31,36 As shown in Figure 4B, there was significantly greater neutrophilia in the blood of Siat1ΔP1 (1.6 × 106/mL) animals than in their wild-type cohorts (1 × 106/mL) 30 minutes after epinephrine infusion. Differences between Siat1ΔP1 and wild-type animals disappeared within 2 hours; however, the overall level of blood neutrophils, though diminished, remained above resting levels (data not shown). Doubling the epinephrine dosage, to 0.5 mg/kg body weight, did not result in greater neutrophilia from either Siat1ΔP1 or wild-type animals (data not shown), indicating an efficient maximal release of marginated neutrophils at 0.25 mg/kg epinephrine.
Another significant granulocyte reservoir exists in the bone marrow.32 However, there was no difference in overall bone marrow cellularity between wild-type and Siat1ΔP1 mice (31.7 ± 3.4 × 106 and 31.7 ± 4.7 × 106 nucleated cells/femur from 4 wild-type and 4 age-sex-matched Siat1ΔP1 mice; data not shown, with mature banded and segmented granulocytes constituting 43.7% and 42.6% of the population for each genotype, respectively; Table 4).
Bone marrow differentials between WT and Siat1Δ P1 mice
. | WT . | Siat1ΔP1 . | P* . |
|---|---|---|---|
| Total myeloid cells, % | |||
| Proliferative | 10.0 ± 1.8 | 13.8 ± 0.6 | < .005 |
| Myeloblasts | 0.9 | 1.4 | |
| Promyeloblasts | 3.0 | 4.8 | |
| Myelocytes | 6.2 | 7.5 | |
| Nonproliferative | 55.1 ± 5.4 | 55.5 ± 1.9 | .90 |
| Metamyelocytes | 11.5 | 12.9 | |
| Band neutrophils | 19.4 | 19.1 | |
| Segmented neutrophils | 24.3 | 23.5 | |
| Total monocytes, % | 0.86 ± 0.70 | 1.52 ± 0.61 | .21 |
| Total lymphocytes, % | 17.2 ± 5.8 | 14.2 ± 2.3 | .32 |
| Total erythroid cells, % | |||
| Proliferative | 6.2 ± 1.3 | 5.6 ± 0.8 | .43 |
| Rubriblasts | 0.8 | 1.2 | |
| Prorubricytes | 1.6 | 1.6 | |
| Rubricytes | 3.8 | 2.8 | |
| Nonproliferative | 10.6 ± 2.1 | 9.5 ± 4.5 | .47 |
| Metarubricytes | 10.6 | 9.5 | |
| Myeloid-erythroid ratio | 3.97 ± 0.72 | 4.66 ± 0.60 | .19 |
| Myeloid proliferative-nonproliferative ratio | 0.18 ± 0.04 | 0.25 ± 0.01 | < .01 |
| Erythroid proliferative-nonproliferative ratio | 0.59 ± 0.15 | 0.64 ± 0.18 | .73 |
. | WT . | Siat1ΔP1 . | P* . |
|---|---|---|---|
| Total myeloid cells, % | |||
| Proliferative | 10.0 ± 1.8 | 13.8 ± 0.6 | < .005 |
| Myeloblasts | 0.9 | 1.4 | |
| Promyeloblasts | 3.0 | 4.8 | |
| Myelocytes | 6.2 | 7.5 | |
| Nonproliferative | 55.1 ± 5.4 | 55.5 ± 1.9 | .90 |
| Metamyelocytes | 11.5 | 12.9 | |
| Band neutrophils | 19.4 | 19.1 | |
| Segmented neutrophils | 24.3 | 23.5 | |
| Total monocytes, % | 0.86 ± 0.70 | 1.52 ± 0.61 | .21 |
| Total lymphocytes, % | 17.2 ± 5.8 | 14.2 ± 2.3 | .32 |
| Total erythroid cells, % | |||
| Proliferative | 6.2 ± 1.3 | 5.6 ± 0.8 | .43 |
| Rubriblasts | 0.8 | 1.2 | |
| Prorubricytes | 1.6 | 1.6 | |
| Rubricytes | 3.8 | 2.8 | |
| Nonproliferative | 10.6 ± 2.1 | 9.5 ± 4.5 | .47 |
| Metarubricytes | 10.6 | 9.5 | |
| Myeloid-erythroid ratio | 3.97 ± 0.72 | 4.66 ± 0.60 | .19 |
| Myeloid proliferative-nonproliferative ratio | 0.18 ± 0.04 | 0.25 ± 0.01 | < .01 |
| Erythroid proliferative-nonproliferative ratio | 0.59 ± 0.15 | 0.64 ± 0.18 | .73 |
Female mice between 55 and 65 days of age were analyzed. Differentials were based on 500 cell counts. Total values are expressed as percentages of total population and, where shown, ± SD. Additional cells, including plasma cells and binucleated cells, consisted of less than 0.5%. Cells not included in the counts were the occasional macrophages, mitotic figures, and megakaryocytes.
Difference between WT and Siat1Δ P1.
G-CSF, in addition to stimulating proliferation and differentiation of granulocyte precursors, can stimulate the release of the bone marrow reservoir into peripheral circulation.37,38 Intravenous infusion of rmG-CSF (20 μg/kg body weight) elicited 1.5- to 2-fold neutrophilia within 30 minutes in C57BL/6 wild-type animals but significantly greater neutrophilia in Siat1ΔP1 (Figure 4D) and Siat1-null (Figure 4E) animals. G-CSF–elicited neutrophilia was transient, and the blood granulocyte pool returned to resting levels within 2 hours (data not shown).
Expanded granulopoietic capacity in ST6Gal-1 deficiency
Bone marrow differentials were performed to assess the granulopoietic status of resting C57BL/6 wild-type and Siat1ΔP1 mice. Siat1ΔP1 mice had a skewed myeloid compartment with a statistically significant increase in total percentage of proliferative level myeloid cells that resulted in an elevated proliferative-nonproliferative myeloid cell ratio, with no difference in the erythroid compartment (Table 4).
Colony-forming cell assays were performed on extracted bone marrow cells to estimate the relative number of granulocyte precursor level cells. Siat1ΔP1 marrow cells, when cultivated ex vivo in semisolid media in the presence of rmG-CSF and IL-3, yielded 2.82 ± 0.45 CFU/1000 input cells, which was a statistically significant difference (P < .003) from the 1.99 ± 0.13 CFU/1000 cells observed from bone marrow cells of matched wild-type animals (data not shown).
To test the concept of a more robust granulopoietic capacity in the Siat1ΔP1 mouse, we used cyclophosphamide to induce reversible myelosuppression with neutropenia with a nadir on the fourth day, and to examine the compensatory mechanism resulting in rebound neutrophilia.39,40 As summarized in Table 5, Siat1ΔP1 animals exhibited better compensatory recovery, as evidenced by 2-fold and 1.6-fold greater granulocyte counts in peripheral blood on days 5 and 6, respectively. These data, together with the comparative bone marrow differentials of resting animals and the ex vivo colony-forming cell assays, support the notion of an elevated granulopoietic capacity in the Siat1ΔP1 mouse that contributes to the enhanced neutrophilic response.
Siat1ΔP1 has improved recovery from cyclophosphamide-induced myelosuppression
Day . | WT, × 109/L . | Siat1ΔP1, ×109/L . | P . |
|---|---|---|---|
| 0 | 0.335 ± 0.150 | 0.370 ± 0.166 | .391 |
| 4 | 0.036 ± 0.007 | 0.055 ± 0.006 | .007 |
| 5 | 0.142 ± 0.078 | 0.287 ± 0.090 | .050 |
| 6 | 1.59 ± 0.42 | 2.55 ± 0.41 | .016 |
| 7 | 0.323 ± 0.245 | 0.241 ± 0.099 | .557 |
Day . | WT, × 109/L . | Siat1ΔP1, ×109/L . | P . |
|---|---|---|---|
| 0 | 0.335 ± 0.150 | 0.370 ± 0.166 | .391 |
| 4 | 0.036 ± 0.007 | 0.055 ± 0.006 | .007 |
| 5 | 0.142 ± 0.078 | 0.287 ± 0.090 | .050 |
| 6 | 1.59 ± 0.42 | 2.55 ± 0.41 | .016 |
| 7 | 0.323 ± 0.245 | 0.241 ± 0.099 | .557 |
Age- and sex-matched female Siat1ΔP1 (n = 29) and C57BL/6 (WT; n = 32) mice received 200 mg/kg cyclophosphamide on day 0. Peripheral blood was withdrawn and granulocyte counts were determined, as described under the first subheading within “Materials and methods.” Values are means ± SEM. For counts taken on days 4-7, n = 4 for each genotype. P values, calculated using the Student t test, compare WT and Siat1ΔP1 blood.
Siat1ΔP1 mice have no major alterations in α2,6-sialylation profile or in sialyltransferase expression in bone marrow cells
Flow cytometry using 1A8-FITC and 7/4-PE segregated marrow cells into 3 major subsets41,42 (Figure 5). The R1 subset consisted exclusively of mature segmented and band neutrophils, the R2 subset was a mixture of monocytes and myelocytes, and the R3 subset was a heterogeneous mixture of cell types including blasts, as revealed by Giemsa-stained cytospin preparations of flow-sorted subsets (data not shown). The degree of α2,6-sialylation of the total marrow population and the 3 subsets was assessed using the SNA lectin conjugated to biotin. There was no noticeable difference in SNA profiles among wild-type and Siat1ΔP1 cells in the unfractionated (total) cells or in any of the fractionated subsets including the mature granulocyte pool (R1).
Greater neutrophilia inSiat1ΔP1 andSiat1-null animals. Peripheral blood was collected from mice in the absence of treatment (resting, A) or after the following administrations: TG 4h, 4 hours after intraperitoneal 4% (wt/vol) thioglycollate (B); EPI 30m, 30 minutes after intravenous 0.25 mg/kg epinephrine (C); GCSF 30m, 30 minutes after intravenous G-CSF administration (D-E). Collected blood was analyzed by flow cytometry after the lysis of red blood cells, and the granulocyte population (7/4+/1A8+) was calculated by taking the percentage of the appropriate PE-7/4 and FITC-1A8 events against total events for each flow cytometric acquisition. *Mutant animal data points at which statistically significant differences with corresponding WT have been reached. (A) n = 21, WT; n = 21, Siat1ΔP1; n = 4, Siat1-null. (B) n = 4, WT; n = 4 Siat1ΔP1; P = .003. (C) n = 4, WT; n = 4, Siat1ΔP1; P < .001. (D) n = 8, WT; n = 6 Siat1ΔP1; P < .001. (E) n = 4, WT; n = 4 Siat1-null; P = .03. Error bars indicate 1 standard deviation from the mean of each group.
Greater neutrophilia inSiat1ΔP1 andSiat1-null animals. Peripheral blood was collected from mice in the absence of treatment (resting, A) or after the following administrations: TG 4h, 4 hours after intraperitoneal 4% (wt/vol) thioglycollate (B); EPI 30m, 30 minutes after intravenous 0.25 mg/kg epinephrine (C); GCSF 30m, 30 minutes after intravenous G-CSF administration (D-E). Collected blood was analyzed by flow cytometry after the lysis of red blood cells, and the granulocyte population (7/4+/1A8+) was calculated by taking the percentage of the appropriate PE-7/4 and FITC-1A8 events against total events for each flow cytometric acquisition. *Mutant animal data points at which statistically significant differences with corresponding WT have been reached. (A) n = 21, WT; n = 21, Siat1ΔP1; n = 4, Siat1-null. (B) n = 4, WT; n = 4 Siat1ΔP1; P = .003. (C) n = 4, WT; n = 4, Siat1ΔP1; P < .001. (D) n = 8, WT; n = 6 Siat1ΔP1; P < .001. (E) n = 4, WT; n = 4 Siat1-null; P = .03. Error bars indicate 1 standard deviation from the mean of each group.
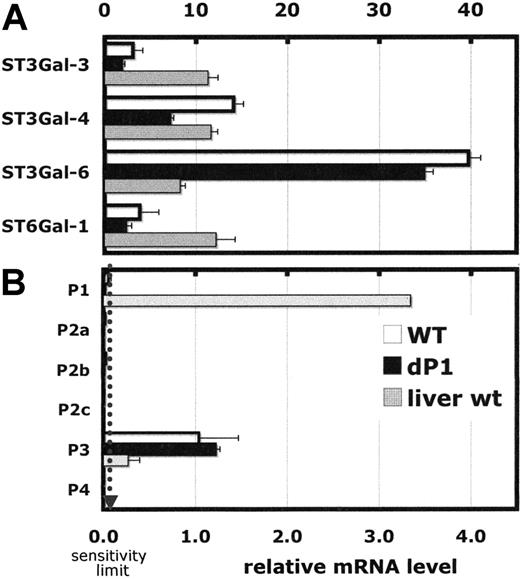
Real-time RT-PCR analysis was performed to assess the expression, on the mRNA level, of ST6Gal-1 and the α2,3-sialyltransferases that can potentially compete for precursor acceptor substrate structures, Galβ1,4GlcNAc. Among the sialyltransferases examined, ST3Gal-6 was highest and 4-fold greater than that in resting liver, shown as a reference (Figure 6A). ST3Gal-6 is believed to be a principal sialyl transferase participating in the synthesis of sialyl-Lewis structures and selectin ligand formation.43 In contrast, ST6Gal-1 mRNA was only one fourth that found in resting liver. Moreover, ST6Gal-1 mRNA was detected primarily in the R3 population, with only marginally detectable levels elsewhere, including the R1 fraction of mature band and segmented granulocytes (data not shown). ST6Gal-1 mRNA expression in marrow cells was derived predominantly from the P3 promoter (Figure 6B), visualized by the presence of Exon O sequence unique to the 5′-untranslated region of P3 transcripts.20 Use of the P1 promoter in wild-type marrow cells was below the sensitivity limit of the procedure. Overall, there was no significant difference, quantitatively or qualitatively, in ST6Gal-1 mRNA levels between Siat1ΔP1 and wild-type marrow–derived cells. To address the possibility that P1 may be active in precursor myeloid cells not abundant in the resting marrow, marrow from animals in rebound from cyclophosphamide-induced myelosuppression was examined. ST6Gal-1 mRNA in both wild-type and Siat1ΔP1 cells in animals 6 days after cyclophosphamide treatment was negligible, and use of P1 in wild-type cells was below detection limits (data not shown).
Flow cytometric analysis ofsiat1ΔP1 and WT bone marrow cells. Bone marrow cells were labeled with FITC-1A8 (anti-Ly6G), PE-7/4 (anti–polymorphonuclear cell, 40-kDa antigen), and biotinylated SNA, as described in “Flow cytometric profiling of inflammatory cells.” Unfractionated bone marrow cells (Total), and differentially flow-sorted populations R1, R2, and R3 were analyzed for SNA binding. SNA binding profiles of wild-type (dashed lines) and Siat1ΔP1 (solid lines) cells are compared.
Flow cytometric analysis ofsiat1ΔP1 and WT bone marrow cells. Bone marrow cells were labeled with FITC-1A8 (anti-Ly6G), PE-7/4 (anti–polymorphonuclear cell, 40-kDa antigen), and biotinylated SNA, as described in “Flow cytometric profiling of inflammatory cells.” Unfractionated bone marrow cells (Total), and differentially flow-sorted populations R1, R2, and R3 were analyzed for SNA binding. SNA binding profiles of wild-type (dashed lines) and Siat1ΔP1 (solid lines) cells are compared.
Real-time PCR analysis of selected genes in bone marrow of wild-type andSiat1ΔP1 mice. Expression of selected genes (A) and different mRNA forms of ST6Gal1 (B) were measured by real-time PCR relative to RPL32, a ribosomal protein mRNA, as reference standard. The procedure involved in analysis of 1 μg total RNA in duplicate. Sensitivity limit is denoted by the dotted line. Liver RNA is shown for comparison. Error bars represent 1 standard deviation from the mean of each group.
Real-time PCR analysis of selected genes in bone marrow of wild-type andSiat1ΔP1 mice. Expression of selected genes (A) and different mRNA forms of ST6Gal1 (B) were measured by real-time PCR relative to RPL32, a ribosomal protein mRNA, as reference standard. The procedure involved in analysis of 1 μg total RNA in duplicate. Sensitivity limit is denoted by the dotted line. Liver RNA is shown for comparison. Error bars represent 1 standard deviation from the mean of each group.
Discussion
Early studies have identified serum ST6Gal-1 as an acute-phase reactant and have demonstrated a correlation between elevated hepatic ST6Gal-1 expression, altered serum sialic acid levels, and inflammatory conditions. Nevertheless, the contribution of ST6Gal-1 to the development and management of inflammation has remained unproven. Our results point to a previously unrecognized role for ST6Gal-1 in attenuating acute neutrophilic inflammation and in regulating myelopoiesis.
Animals with ST6Gal-1 deficiency developed an exaggerated acute neutrophilic response. The data are compelling that this phenotype is a consequence of ST6Gal-1 deficiency and not a confounding secondary effect. First, the exaggerated peritonitis phenotype was observed in Siat1-null and in Siat1ΔP1 mice. These are independently generated mutant lines produced by 2 separate laboratories pursuing completely different strategies. The Siat1ΔP1 mutation is a promoter ablation, whereas the Siat1-null mutation was a disrupted coding region located more than 30 kb from the ablated region in Siat1ΔP1. Second, the Siat1ΔP1 mice used in these studies have been backcrossed to C57BL/6 for 11 generations to minimize genetic background effects.
Disruption of other glycosyltransferase genes, notably the α1,3/4-fucosyltransferases required for the elaboration of selectin-ligand glycan structures, has generated defects in the overall efficiency of leukocyte recruitment to sites of inflammation and to peripheral lymph nodes.44,45 We examined this mechanism in the Siat1ΔP1 mouse by monitoring the recovery of differentially PKH- or CFSE-labeled mutant and wild-type donors cells in the peritonea of recipient animals after thioglycollate challenge. Based on an invariant CFSE/PKH ratio between the donor pool and those cells recovered from the peritonea of recipients (Table 3), we concluded that altered efficiency of inflammatory cell recruitment from circulation is not a principal mechanism for the more robust peritonitis response exhibited by the Siat1ΔP1 mouse.
Neutrophils constitutively undergo apoptosis with a short half-life,46 and possible deviations to this program that can lead to altered severity of peritonitis. We observed that peritoneal neutrophils from Siat1ΔP1 mice exhibited a slight and consistent increase in cell viability. However, the rapidity of the onset of the exaggerated peritonitis phenotype (within 5 hours) makes the slightly increased cell viability an unlikely principal mechanism responsible for the exaggerated peritonitis.
Our data support a model wherein an increased reservoir of available inflammatory cells exists in animals with ST6Gal-1 deficiency. This elevated supply is sequestered because the baseline circulating granulocyte count was similar between Siat1ΔP1, Siat1-null, and C57BL/6 wild-type mice (Figure 4A). In the Siat1ΔP1 mouse, a portion of these excess cells putatively adhere reversibly to the vascular endothelium and can be reintroduced into circulation within 30 minutes of an epinephrine infusion (Figure 4C). Based on the relative differences in epinephrine-induced neutrophilia and assumption of 2.5 mL blood in each mouse, we estimate the reservoir of marginated granulocytes in Siat1ΔP1 is 2-fold larger than that in their wild-type cohorts, which extrapolates to an upper limit of 2.5 × 106 excess cells in the marginated pool. Our finding that the exaggerated peritonitis is only present at higher but not at the lower concentrations of thioglycollate (Figure 2) further supports the model of elevated supply of available inflammatory cells and not necessarily greater sensitivity to inflammatory stimulus.
In the thioglycollate model of peritonitis, a 3-fold greater transient elevation in inflammatory cell numbers in systemic circulation precedes peritoneal leukocytosis in the Siat1ΔP1 but not wild-type animals (Figure 4B). This thioglycollate-induced transient neutrophilia lends further support for the idea of an inflated supply of inflammatory cells in the Siat1ΔP1 mouse. However, there was an average excess of 15 × 106 cells in the peritoneum of each Siat1ΔP1 mice (Figure 2), and it is highly unlikely that the expanded marginated reservoir can account totally for this difference. A reservoir of mature granulocytes also exists in the bone marrow, but we have found no evidence that the marrow reservoir is altered in the ST6Gal-1–deficient mice. However, our observation also indicates that ST6Gal-1 deficiency results in a heightened response to G-CSF in mobilization of the marrow granulocyte reservoir (Figure 4D-E).
Our data also point to an increased granulopoietic capacity to replenish the pool of available inflammatory cells in Siat1ΔP1 mice, as evident from increased proliferative level myeloid cells in bone marrow differentials of the Siat1ΔP1 mouse, increased CFU-G when Siat1ΔP1 bone marrow cells were cultured ex vivo in the presence of G-CSF, and improved recovery from cyclophosphamide-induced myelosuppression.
The role of ST6Gal-1 and its cognate sialyl-glycan product in B-cell functionality25 is well established. It is, at least in part, attributable to the requirement for α2,6-sialic acid containing ligand for the B-cell receptor accessory molecule CD22.47 However the widespread tissue distribution of ST6Gal-1 and the existence of at least 6 spatially distinct Siat1 promoter regions have perpetuated ideas that ST6Gal-1 also has function outside the B-cell compartment. Our data demonstrated that ST6Gal-1 deficiency impacts leukocytic inflammatory response on multiple levels, including myeloid cell regeneration, resulting in greater granulopoietic capacity, greater sensitivity to G-CSF–induced mobilization of bone marrow granulocytes, and an expanded reservoir of inflammatory cells in the periphery. The kinetics of the peritonitis was consistent with the view that the initial hours of the exaggerated response are driven by more efficient mobilization of an expanded reservoir of granulocytes. Thereafter, the exaggerated response in ST6Gal-1–deficient animals was likely sustained by an increase granulopoietic capacity to replenish the granulocyte supply.
The detailed biochemical pathway through which insufficient ST6Gal-1 expression perturbs inflammatory cell homeostasis remains to be elucidated. One possibility is the potential for ST6Gal-1 to divert the precursor substrate, Galβ1,4GlcNNAc, away from the synthesis of ligands for selectin-mediated adhesion; it is well established that selectin/selectin ligand–mediated interactions play pivotal roles in the homing and adherence of leukocytes to high endothelial venules and to sites of inflammation.48-50 Another possibility is that ST6Gal-1 may mask relevant structures from recognition by receptors such as galectin-3. Galectin-3 has previously been implicated in the maintenance of granulocyte numbers during acute peritonitis,51 in providing protection from apoptosis,52 as a chemoattractant for monocytes,53 and in the inhibition of BM-CSF–driven bone marrow cell proliferation.54 Moreover, ST6Gal-1 may provide the ligands for Siglecs, a family of sialic acid–recognizing receptors, many of which are present in myeloid lineage cells.55-57 Consistent with our conclusion of elevated granulopoietic capacity in ST6Gal-1–deficient animals, a number of earlier reports associated ST6Gal-1 with the maintenance of hematologic homeostasis. Up-regulation of ST6Gal-1 accompanies myeloid maturation, prompting a suggestion for ST6Gal-1 in governing the release of myeloid cells from the bone marrow.58 Reports also indicate increased ST6Gal-1 expression in activated endothelial cells59 and in CD34+/– progenitor cells.60 Up-expression of α2,6-linked sialic acid and ST6Gal-1 in the endothelium has been shown to inhibit VCAM-1–dependent adhesion under flow conditions.61
Our findings point to a novel idea that extramedullary ST6Gal-1 directly or indirectly modulates myelopoiesis and neutrophil homeostasis. Presumably, the phenotypes of the Siat1ΔP1 mouse result from altered sialylation in specific cell types in which the P1 promoter is normally used. Earlier we identified P1 as the promoter mediating elevated hepatic ST6Gal-1 expression during the hepatic acute-phase response.19,27 Therefore, it is significant that P1-mediated ST6Gal-1 mRNA was not detected in wild-type bone marrow cells by RT-PCR, a highly sensitive method for quantifying gene expression. Moreover, SNA profiling revealed little difference in overall α2,6-sialylation patterns between Siat1ΔP1 and wild-type major marrow cell populations. The possibility remains that an extinguished P1 promoter alters sialylation in cell subsets (eg, granulocyte precursors) that do not make up a significant percentage of the overall marrow population or in the stromal cells that support and provide the microenvironment for granulopoiesis.
In this report, we have focused predominantly on Siat1ΔP1, a mouse with a significantly more restricted ST6Gal-1 deficiency than the Siat1-null animal and yet a similar extent of acute peritonitis on thioglycollate challenge. Given that the Siat1ΔP1 mutant retains the ability to express ST6Gal-1 from the remaining promoter regions, our data suggest a model wherein the requirement of ST6Gal-1 in inflammation is largely mediated by the P1 promoter.
We previously reported that the Siat1ΔP1 mouse responded to intraperitoneal inoculation of the pathogen Salmonella by an unusually robust infiltration of neutrophils into the peritoneal space,30 and in this report we have reproduced the exaggerated acute neutrophilic peritonitis phenotype using the sterile agent, thioglycollate. Equally important, we had shown earlier that the Siat1ΔP1 mouse does not share the humoral defect displayed by the Siat1-null mouse and therefore demonstrated that the requirement of ST6Gal-1 in the humoral response is met by Siat1 promoters other than P1.
Taken together, these studies indicate that ST6Gal-1, presumably through α2,6-sialyl modifications of Galβ1,4GlcNAc terminal structures, is important in regulating acute inflammation, especially in neutrophil hematologic homeostasis and in B-cell responses. Furthermore, these studies show that Siat1 promoters have distinct roles in regulating specific ST6Gal-1 functions. Further studies will use Siat1-null and Siat1ΔP1 mice to elucidate the mechanism by which the expression of ST6Gal-1 at different sites modulates key innate and antigen-specific responses.
Prepublished online as Blood First Edition Paper, July 18, 2006; DOI 10.1182/blood-2006-04-014779.
Supported by National Institutes of Health (NIH) grant AI056082 (J.T.L.), The Roswell Park Alliance Foundation (J.T.L., B.H.S.), and NIH grant CA098852 (J.R.O.). This research made use of core facilities supported in part by the Roswell Park Cancer Institute's National Cancer Institute–funded Cancer Center support grant CA16056.
The authors declare no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr V. E. Valli (Department of Pathobiology, University of Illinois at Urbana-Champaign) for invaluable advice and input. The Siat1-null mouse (ST6GalKO) was provided by the Consortium for Functional Glycomics.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal