Abstract
We previously reported that concurrent cyclosporine and plasma exchange(CSA/PE) is superior to corticosteroids and PE for acute TTP in terms of a decreased exacerbation rate (0% v. 47%) after stopping PE.(ASH 2005,Abstract# 1235) We now present similar data and serial analysis of ADAMTS13 activity and inhibitor concentration from two patient cohorts treated with CSA/PE.
Patients: Ten patients failing initial therapy with corticosteroids and PE (exacerbations or refractory) were treated with CSA/PE. This study led to a second cohort study of upfront therapy with CSA/PE in 11 patients with acute TTP.
Methods: CSA (2–3 mg/kg) orally was administered concurrent with daily PE. After remission (normal platelet count and LDH), 2 additional exchanges were performed on an every other day schedule. CSA was administered for 6 months and discontinued.
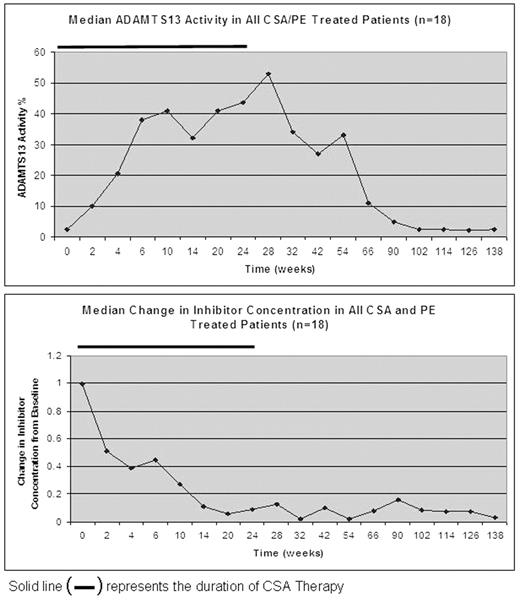
Results: In the exacerbation/refractory patients, 9/10 achieved remission after a median of 11 exchanges. One patient suffered an exacerbation and required one additional exchange to achieve a sustained (>30 d.) remission. Four patients relapsed 2,3,7, and 10 months after completing 6 months of CSA. In the CSA/PE upfront group, 10/11 achieved remission. One patient died of infectious and disease-related complications. Remission occurred after a median of 5 exchanges. No patients(0/10) suffered an exacerbation. Three patients recurred, 2 while taking CSA and one 2 weeks after stopping CSA. Median ADAMTS13 activity and the change in inhibitor concentration over time for all CSA/PE patients is shown in Figure 1.
Conclusions: CSA/PE improves clinical outcome and prevents exacerbations after tapering PE. CSA/PE also increased in vitro ADAMTS13 activity and suppressed the inhibitor, with both occurring well after the last PE. These findings coupled with the decline in median ADAMT13 activity after discontinuing CSA(Fig. 2) suggest the improvement in ADAMTS13 activity was a result of CSA therapy.
Presenting ADAMTS13 and Inhibitor Data
| . | N . | Med. ADAMTS13% Prior to Initial Therapy . | Med. ADAMTS13% Prior to CSA and PE . | Med. Inhibitor Conc. Prior to Initial Therapy(ug/mL) . | Med. Inhibitor Conc. Prior to CSA and PE(ug/mL) . | Remission Rate . | Exacerbation (30 d. Recurrence) Rate . |
|---|---|---|---|---|---|---|---|
| CSA and PE Exacerbation and Refractory | 10 | 2 (0–8) | 4 (0–20) | 587 (275–1,111) | 3,173 (372–9,845) | 9/10 | 1/10 |
| CSA and PE Upfront Therapy | 11 | N/A | 2 (0–60) | N/A | 571 (147–4,988) | 10/11 | 0/10 |
| . | N . | Med. ADAMTS13% Prior to Initial Therapy . | Med. ADAMTS13% Prior to CSA and PE . | Med. Inhibitor Conc. Prior to Initial Therapy(ug/mL) . | Med. Inhibitor Conc. Prior to CSA and PE(ug/mL) . | Remission Rate . | Exacerbation (30 d. Recurrence) Rate . |
|---|---|---|---|---|---|---|---|
| CSA and PE Exacerbation and Refractory | 10 | 2 (0–8) | 4 (0–20) | 587 (275–1,111) | 3,173 (372–9,845) | 9/10 | 1/10 |
| CSA and PE Upfront Therapy | 11 | N/A | 2 (0–60) | N/A | 571 (147–4,988) | 10/11 | 0/10 |
Effect CSA and PE on ADAMTS13 Activity and Inhibitor Concentration.
| . | . | Time (weeks) . | |||
|---|---|---|---|---|---|
| . | . | 0 . | 4 . | 10 . | 24 . |
| CSA and PE Exac. and Refractory | |||||
| N | 9 | 9 | 7 | 8 | |
| Median ADAMTS13 % | 4 (0–20) | 24 (4–39) | 42 (5–72) | 49 (7–116) | |
| Median Inhibitor Conc. (ug/mL) | 3,173 (372–9,845) | 294 (165–6,548) | 191 (41–4,228) | 204 (55–756) | |
| CSA and PE Upfront Therapy | |||||
| N | 10 | 8 | 5 | 4 | |
| Median ADAMTS13% | 2 (0–60) | 20 (5–104) | 32 (4–37) | 49 (7–116) | |
| Median Inhibitor Conc. (ug/mL) | 571 (147–4,988) | 270 (82–2,637) | 302 (110–378) | 204 (55–756) | |
| . | . | Time (weeks) . | |||
|---|---|---|---|---|---|
| . | . | 0 . | 4 . | 10 . | 24 . |
| CSA and PE Exac. and Refractory | |||||
| N | 9 | 9 | 7 | 8 | |
| Median ADAMTS13 % | 4 (0–20) | 24 (4–39) | 42 (5–72) | 49 (7–116) | |
| Median Inhibitor Conc. (ug/mL) | 3,173 (372–9,845) | 294 (165–6,548) | 191 (41–4,228) | 204 (55–756) | |
| CSA and PE Upfront Therapy | |||||
| N | 10 | 8 | 5 | 4 | |
| Median ADAMTS13% | 2 (0–60) | 20 (5–104) | 32 (4–37) | 49 (7–116) | |
| Median Inhibitor Conc. (ug/mL) | 571 (147–4,988) | 270 (82–2,637) | 302 (110–378) | 204 (55–756) | |
Figure
Disclosures: Off label use of cyclosporine in TTP is discussed.; Paid to review 2 medical malpractice cases involving TTP for two defendents in the action.; Speaker bureau for Glaxo-SmithKline and Novartis.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal