Abstract
Background: Acute myeloid leukemia (AML) is the most common type of leukemia among adults in the U.S. However, data on the effects of treatment on survival for large numbers of AML patients in clinical practice are limited.
Methods: Using the linked SEER-Medicare database, we evaluated mortality for AML patients 65+ years of age diagnosed between 1999 and 2002 who received chemotherapy versus no chemotherapy. Patients were followed from diagnosis until death or the database cut-off (December 31, 2003). The presence of chemotherapy treatment was identified based on procedure codes and diagnosis-related groups. The two study cohorts were matched on age, sex, comorbidity (Deyo adaptation of the Charlson Comorbidity Index), geographical region, teaching hospital status, and myelodysplastic syndrome (MDS) in the prior 12 months. Mortality rates were estimated via the Kaplan-Meier technique. Subgroup analyses were performed by level of comorbidity, age group, and teaching hospital status.
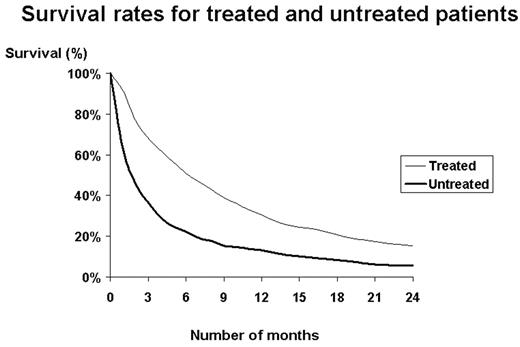
Results: A total of 3,317 elderly patients with AML who were eligible for fee-for-service Medicare coverage were identified. Of these, 1,193 (36%) received chemotherapy and 2,124 did not receive treatment. Treated patients were found to be younger and had fewer comorbidities compared to untreated patients. After matching, there were 888 patients in both cohorts. Mean age was 75 years and 59% were male. Fifty-three percent of patients received care at teaching hospitals. The median Charlson score was 1.0 and 24% were diagnosed with prior MDS. Median survival was 4.4 months longer for the treated cohort than for the untreated cohort (6.1 versus 1.7 months; P<0.0001) (see Figure).
Conclusions: This large observational study of elderly patients with AML illustrates that chemotherapy treatment is associated with improved survival. This survival gain is slightly less than that observed in other population-based AML studies that did not match on key prognostic factors, such as age and comorbidity levels.
Survival rates for treated and untreated patients
Disclosures: Dr. Karsten and Ms. Cahill are full-time employees of Vion Pharmaceuticals, Inc.; Dr. Menzin has received consulting fees from the study sponsor, Vion Pharmaceuticals, Inc.; Dr. Karsten and Ms. Cahill both hold stock in Vion Pharmaceuticals, Inc.; Funding for this study was provided by Vion Pharmaceuticals, Inc., New Haven, CT.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal