Abstract
In ARL, the addition of Rituximab (R) to CHOP chemotherapy may improve tumor response but the benefit may be offset by increased infectious deaths in patients with low CD4 cell counts (
Kaplan et al. Blood2005;106:1538
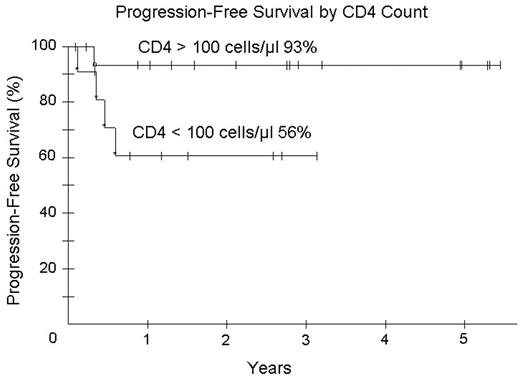
). We hypothesized that R with EPOCH will improve tumor kill, allow fewer chemotherapy cycles and reduce toxicity. Patients received EPOCH-R (in mg/m2/d etoposide 50, vincristine 0.4 and doxorubicin 10 all CIV d 1–4; and in mg/m2 cyclophosphamide 750 IV day 5, prednisone 60 po days 1–4 and rituximab 375 IV d 1, 5; and G-CSF sc d 6–15). Prophylactic IT MTX x 6 was administered and HAART was discontinued on all cycles. Cyclophosphamide was adjusted based on absolute neutrophil count (ANC) nadir. Response was assessed by CT and PET and patients received 1 cycle beyond CR for a minimum of 3 cycles. Characteristics of 30 enrolled patients: median (range) age 41 (9–61) years; IPI 3 (0–4); ECOG PS 1 (1–4), CD4 count 213 (0–674) cells/mm3; HIV viral load 55,900 (0–6,080,000) RNA copies/mL; male sex 25 (83%); LDH > N 22 (73%); stage IV 20 (67%) and histology DLBCL 26 (87%) and Burkitt lymphoma 4 (13%). Of 28 evaluable patients (2NE), median (range) cycles given is 3 (3–5) with CR/CRu in 25 (89%) and PR in 1 (3%) patients. All 4 patients with Burkitt lymphoma are in continuous remission. At 35 months median potential follow-up, PFS and OS are 80% and 65%, respectively. For patients with CD4 > and < 100 cells/mm3 OS is 93% and 28%, respectively. IPI did not impact OS and PFS. We assessed the predictive value of early (after cycle 2 or 3) PET scanning on subsequent relapse. This was evaluated in 23 patients in follow-up. 0 of 13 patients with a negative PET study progressed (100% negative predictive value) whereas 2 of 10 patients with a positive PET progressed (20% positive predictive value). One death from MAI occurred on treatment and toxicity over 91 cycles of therapy included fever/neutropenia on 27 (30%), ANC < 500/mm3 on 36 (40%), and platelets < 50,000 on 21 (23%) cycles. EPOCH-R was associated with less CD4 loss - median 128 cells/mm3 (range +154 to −639) compared to EPOCH alone (median 189 cells/mm3 (range +19 to −973)(Little et al. Blood2003;101:4653
). Abbreviated EPOCH-R is highly effective with acceptable tolerability in ARL and enables the administration of fewer treatment cycles (median 3 versus 6). Patients with CD4 < 100/mm3 have good tumor control with EPOCH-R with a PFS of 61% at 35 months, but survival continues to be limited by HIV-associated complications. In contrast, patients with high CD4 counts > 100/mm3 have an extremely favorable outcome with EPOCH-R. Albeit limited data, EPOCH-R was effective in all 4 patients with Burkitt lymphoma. PET scanning has a high negative but low positive predictive value for subsequent relapse. Accrual continues.Figure 1
Figure 2
Disclosures: EPOCH-R agents are ot all approved for HIV associated lymphoma.
Author notes
*
Corresponding author
2006, The American Society of Hematology
2006


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal