Abstract
Background. Low donor T-cell chimerism levels after allogeneic hematopoietic cell transplantation (HCT) with nonmyeloablative conditioning have been associated with high risks of both graft rejection and relapse/progression (
Methods. Conditioning regimen for HCT consisted of 2 Gy TBI with (n=15) or without (n=11) added fludarabine. 20 patients received PBSC from related, and 6 PBSC from HLA-matched unrelated donors. Peripheral blood stem cells (PBSC) were unmanipulated in 15 patients, and CD8-depleted in the 11 remaining patients. Indication for TBI/DLI included < 50% donor T-cell chimerism or decrement of donor T-cell chimerism by 20% (or 10% if below 60%). Depending of patient-donor relationship, DLIs were given at a dose of 1 to 10 × 107 T-cells/kg (median 2 × 107 T-cells/kg), 42 to 725 days (median 68.5 days) following HCT. Prophylactic postgrafting immunosuppression was pursued after DLI in all but one patients.
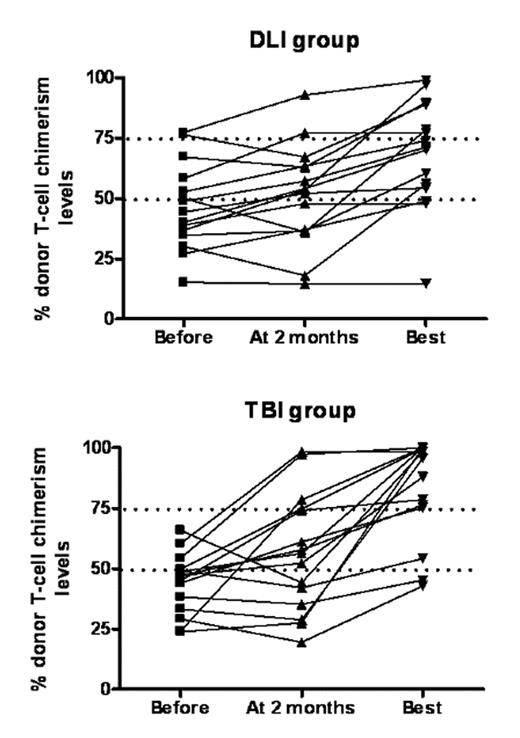
Results. Before DLI, donor T-cell chimerism levels ranged from 15 to 77% (median 44%) in the DLI group, versus from 24 to 66% (median 47%) in the TBI/DLI group (P=0.66). Two months after DLI, donor T-cell chimerism levels ranged from 15 to 93% (median 53%) in the DLI group (P=0.10 in comparison to before DLI), versus from 20 to 98% (median 56%) in the TBI/DLI group (P=0.04 in comparison to before DLI) (Figure 1). In addition, 0 of 15 patients in the DLI group versus 5 of 15 patients in the TBI/DLI group had > 25% increased T-cell chimerism levels 2 months after DLI (P=0.04). Highest T-cell chimerism levels after DLI ranged from 15 to 99% (median 71%) in the DLI group, versus from 43 to 100% (median 96%) in the TBI/DLI group (P=0.06). Further, 2 of 15 patients in the DLI group, versus 0 of 15 patients in the TBI/DLI group experienced graft rejection. In the DLI group, 3 patients developed grade II–IV acute GVHD (2 grade II and 1 grade IV), while 1 patient in the TBI/DLI group experienced grade II acute GVHD. TBI/DLI was followed by grade IV hematologic toxicities in most patients, but most of them remained treated in the outpatient clinic. One-year overall survival was 67% in the DLI group versus 73% in the TBI/DLI group (NS).
Conclusions. DLI increased donor T-cell chimerism levels in a number of patients when preceeded by low dose TBI. However, hematological toxicity of TBI/DLI was relatively high, with most patients requiring several RBC and Plt transfusions. Further studies are needed to assess its impact on HCT outcomes.
Disclosures: Off label use of fludarabine, cyclosporine, mycophenolate mofetil.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal