Abstract
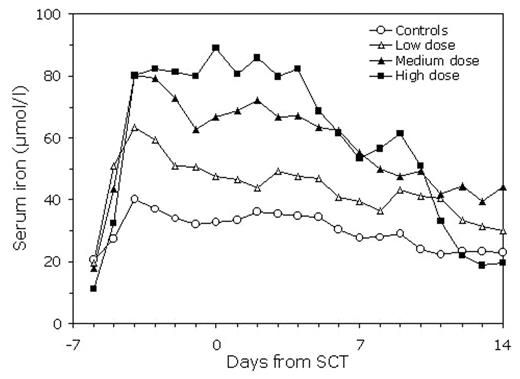
The myeloablative preconditioning for hematopoietic stem cell transplantation (SCT) disrupts the main route of body iron turnover by temporarily halting erythropoiesis. This is followed by rapid increase in transferrin saturation and appearance of non-transferrin-bound iron (NTBI) in plasma (Sahlstedt et al, Br J Haematol 2001). We have previously shown that considerable amounts of iron can in this situation be bound by a single intravenous dose of human plasma-derived ironless transferrin, apotransferrin (Sahlstedt al, Br J Haematol 2002). NTBI is cleared from circulation by the liver, to which iron-catalyzed generation of reactive oxygen species are considered to be potentially toxic as also possibly contributing to mucositis and idiopathic pneumonia syndrome. We studied the ability of three repeated apotransferrin dosing regimens to maintain the accumulating iron in a transferrin-bound form in 20 patients receiving cyclophosphamide 120mg/kg and total body irradiation 12 Gy before SCT. The low-dose group received a total dose (including loading and maintenance doses) of 340 mg/kg, the medium-dose group 610 mg/kg and the high-dose group 1040 mg/kg starting on day −6 before SCT. Serum transferrin, total iron, NTBI and transferrin saturation were analyzed from daily samples until day +14. A significantly higher increase in serum transferrin and in serum total iron was detected in the high-dose group compared with the other dose groups and controls. On the second day after start of preconditioning, the mean increase in serum iron was 48 μmol. At the same time, the 24-hour increment in serum transferrin after the apotransferrin doses decreased by 45% pointing to uptake of transferrin-bound iron by tissues. Serum iron levels remained significantly more elevated in the high-dose group compared with controls for about 10 days, after which their decrease coincided with appearance of reticulocytes in peripheral blood. In 5/8 patients in the high-dose group, no detectable NTBI was found during the study period, whereas in the controls 75% of the samples were NBTI positive. In conclusion, even with repeated administration of relatively large doses of apotransferrin, full transferrin saturation and appearance of NTBI can be prevented in only part of the patients. Achieving complete iron binding with apotransferrin in all SCT patients would apparently require very high apotransferrin doses. The remarkable rise in transferrin saturation indicates that about 180 μmol or iron per day is initially released into circulation after start of preconditioning therapy.
Disclosures: Freja Ebeling, Jaakko Parkkinen, Leni von Bonsdorff and Hanna Salo have been employed or are still employed by the Finnish Red Cross Blood Transfusion Service, which produced the study drug.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal