Abstract
ASCT has been used in FL to achieve durable remissions either as consolidation therapy in first remission or as salvage therapy in the relapsed or refractory setting. The recent addition of R to chemotherapy regimens for FL has been shown to improve RFS and, in some studies, OS. The effectiveness of HDT and ASCT in pts with FL who have received prior therapy with R is unknown, although there is speculation that R may adversely affect outcome.
We retrospectively reviewed 128 consecutive patients with FL who underwent HDT and ASCT from 1/1994 through 12/2004. The analysis included 106 patients who were divided into No R and R groups. We excluded 19 patients who received R only as salvage therapy and three patients who received bone marrow rather than stem cells as their auto-graft. Patients were analyzed according to whether or not they had received an R-containing regimen prior to transplantation.
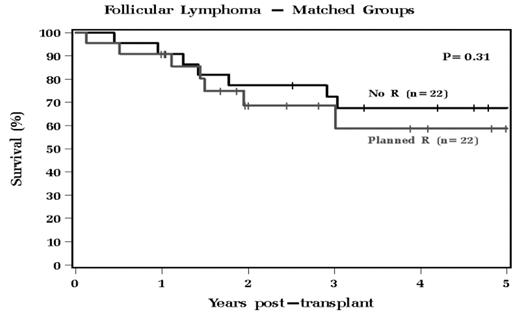
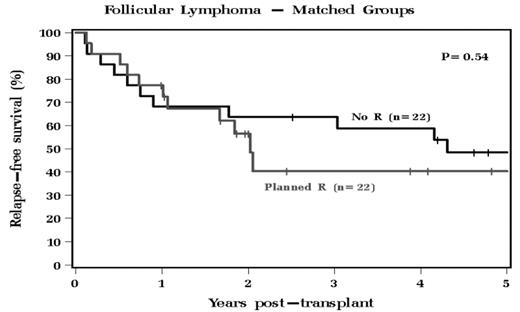
71 patients with No R therapy and 35 patients with R therapy were included. Characteristics between No R and R group are similar, with major difference being age, CD34+ dose, and length of follow-up. The No R group had a median OS that was not reached during follow up, with a median RFS of 49.9 months. The R group had a median OS of 63.2 months and a median RFS of 24.6 months. The OS and RFS were not significantly different between the groups. To control for potentially confounding factors between cohorts, we used propensity analysis to identify 22 patients among the R group who were well-matched to 22 patients in the No R group. Analysis was done for this well-matched cohort (Table 1), and OS and PFS were also found to have no significant difference (Figures 1 and 2).
In conclusion, we did not observe a difference in outcome in the effectiveness of HDT and ASCT between FL patients who received R therapy and those who did not. Prospective studies are required to confirm these results.
Matched Groups by Rituximab Use
| . | No Rituximab . | Rituximab . | ||
|---|---|---|---|---|
| . | N . | % . | N . | % . |
| Age (years) | ||||
| mean +/− SD | 49 +/− 8 | 59 +/− 7 | ||
| median (range) | 51 (28–69) | 59 (49–75) | ||
| Stage | ||||
| I/II | 4 | 8.2 | 3 | 23.1 |
| III | 8 | 16.3 | 2 | 15.4 |
| IV | 37 | 75.5 | 8 | 61.5 |
| # of Prior Chemotherap | ||||
| mean +/− SD | 2.4 +/− 1.0 | 3.1 +/− 1.7 | ||
| median (range) | 2 (1–5) | 2 (1–7) | ||
| IPI | ||||
| Low, Low-Int | 29 | 59.2 | 13 | 100 |
| High-Int, High | 20 | 40.8 | 0 | 0.0 |
| . | No Rituximab . | Rituximab . | ||
|---|---|---|---|---|
| . | N . | % . | N . | % . |
| Age (years) | ||||
| mean +/− SD | 49 +/− 8 | 59 +/− 7 | ||
| median (range) | 51 (28–69) | 59 (49–75) | ||
| Stage | ||||
| I/II | 4 | 8.2 | 3 | 23.1 |
| III | 8 | 16.3 | 2 | 15.4 |
| IV | 37 | 75.5 | 8 | 61.5 |
| # of Prior Chemotherap | ||||
| mean +/− SD | 2.4 +/− 1.0 | 3.1 +/− 1.7 | ||
| median (range) | 2 (1–5) | 2 (1–7) | ||
| IPI | ||||
| Low, Low-Int | 29 | 59.2 | 13 | 100 |
| High-Int, High | 20 | 40.8 | 0 | 0.0 |
Follicular Lymphoma - Matched Groups
Follicular Lymphoma - Matched Groups
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal