Abstract
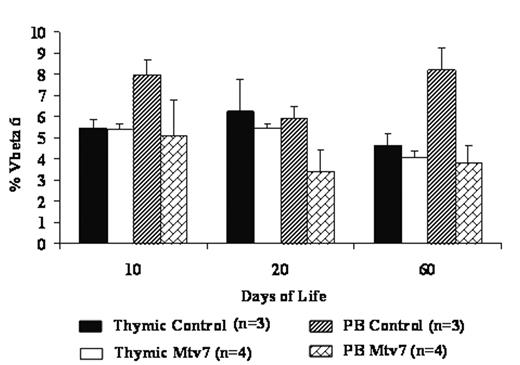
The developing fetal immune system provides a unique opportunity to manipulate normal immunologic development for therapeutic prenatal and anticipated postnatal interventions. In previous studies we have shown that allogeneic in utero hematopoietic cell transplantation (IUHCT) results in donor specific tolerance that can subsequently facilitate non-myeloablative postnatal cellular or organ transplants. It follows that in utero injection of transduced hematopoietic stem cells (HSC) could potentially induce tolerance to a transgene encoded protein. We hypothesized that expression of a transduced antigenic protein by HSC and their progeny would alter thymic T cell development resulting in deletion of antigen specific T-cells. To test this hypothesis, we used the mammary tumor virus (MTV) superantigen system to evaluate the effect of IUHCT of transduced HSC on T cell development. In this system, expression of different MTV oncogenes by different I-E+ strains of mice results in deletion of T cells expressing the relevant Vβ T cell receptor. Specifically, mice which are Mtv7+ delete T cells expressing the Vβ6 T-cell receptor. In this study, CD150+CD48− enriched Balb/c (I-E+ Mtv7−) HSC were transduced with an HIV-based lentivirus expressing MTV7 under an MND promoter. 1.5E+05 transduced cells were injected intravascularly via the vitelline vein into E14 Balb/c fetuses. Non-injected age matched naive Balb/c mice served as the control group. The peripheral blood (PB) and thymuses of injected fetuses and control mice were harvested at day of life (DOL) 10, 20 and 60 and analyzed by flow cytometry for T lymphocyte Vβ6 expression. Additionally, the T cell composition of the thymus was assessed at DOL10 for CD4 and CD8 single positive (SP) and CD4/CD8 double positive (DP) cells. Thymic flow cytometric analysis at DOL10 revealed that greater than 98% of the T cells were CD4CD8 DP, a stage that has not yet undergone negative selection. No significant difference was noted in the percentage of thymic Vβ6+ DP T-cells at this time point or at DOL20 and DOL60. In contrast, there was a significant decrease in the percentage of Vβ6+ peripheral blood SP cells in those mice injected with MTV7 transduced HSC relative to control mice at DOL10, DOL20 and DOL60 (p<0.05) (Fig 1). The current study supports the ability of enriched transduced HSC to induce deletion of transgene specific T cells after IUHCT. In the future, this strategy may be useful to promote tolerance for pre or postnatal cellular or gene therapy.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal