Abstract
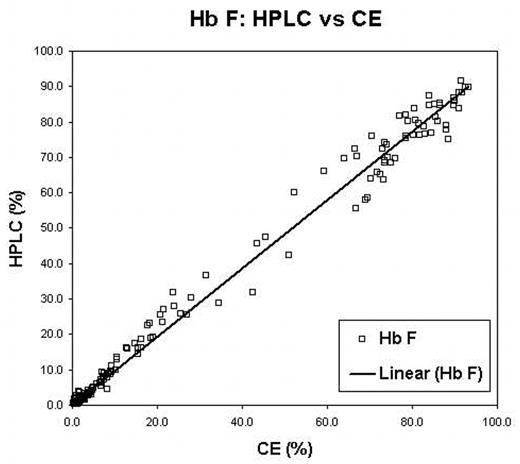
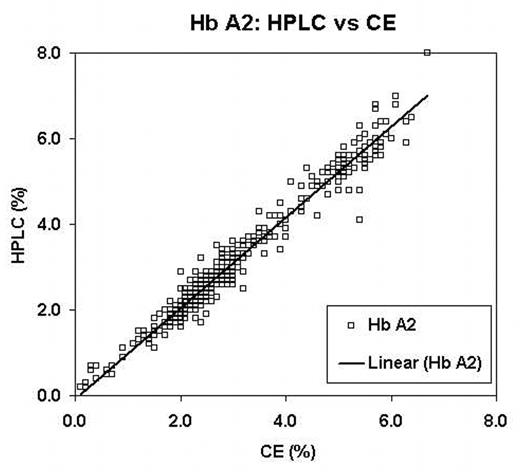
Traditional diagnosis of hemoglobinopathies rely on separation and accurate quantification of hemoglobin (Hb) fractions using alkaline and acid electrophoresis, isoelectric focusing (IEF) and/or High Performance Liquid Chromatography (HPLC). Recent reports have suggested capillary electrophoresis (CE) as an alternate method for screening. We evaluated a new CE system the Sebia CapillaryS 2 (Evry Cedex, France) and compared it to IEF and HPLC. For this evaluation samples referred to our laboratory for routine hemoglobinopathy screening or as cord bloods sent for confirmation of a variant detected as a result of newborn screening were analyzed using all three techniques. IEF was performed with the Resolve IEF kit (Perkin Elmer,Wallac Oy, Finland). HPLC was done on the BioRad Variant II (Munich, Germany) using the Hb A2/Hb A1c Dual program. The CE was performed using the “Capillarys Hemoglobin(E) kit”. Suspected rare variants were further investigated with DNA investigations, including PCR and direct nucleotide sequencing. Variant hemoglobins were detected in 156 of 764 samples (Table 1). The correlation between IEF, HPLC and CE was excellent. One variant (Hb Toulon) was not detected by the CE but ran with Hb F. The CE system did not report the Hb F value on samples with very low (< 1.0%) Hb F by HPLC. Two cases of Hb Constant Spring were identified by CE but not by either IEF or HPLC. The correlation of both Hb F and Hb A2 reported between the CE and HPLC systems were excellent (R > 0.99 and 0.98, Figure 1 and Figure 2 respectively). The positive bias of Hb A2 seen in HPLC when Hb S is present was lower in the CE. However, the CE system demonstrated a negative bias for Hb A2 if Hb C was present. CE was the only system capable of separating Hb A2 from Hb E. We conclude that CE is comparable to both IEF and HPLC for separation and quantification of hemoglobin fractions; this system has the added advantage of reporting Hb A2 in the presence of Hb E and consistently identifies Hb Constant Spring, which is not easily detected on IEF or HPLC. Further this CE system is user friendly and holds promise as a tool for newborn screening and routine laboratory hemoglobinopathy investigations.
Hemoglobinopathy Detected
| Hemoglobinopathy . | Number . |
|---|---|
| β Thalassemia | 99 |
| Hb S trait | 55 |
| Cord with Hb Barts | 28 |
| Hb C trait | 13 |
| Hb E trait | 12 |
| Hb D trait | 8 |
| α variant (not yet identified) | 5 |
| Hb Barts + Hb S trait | 4 |
| Hb Barts + Hb D trait | 3 |
| Hb S disease | 3 |
| Hb Barts + Hb E trait | 2 |
| Hb E +β Thalassemia | 2 |
| Hb H disease | 2 |
| Hb Constant Spring | 2 |
| Hb J Baltimore | 2 |
| Hb Toulon | 2 |
| δ variant (not identified) | 2 |
| Hb S + Hb C | 1 |
| Hb Barts + Hb C trait | 1 |
| Hb Q Thailand + --SEA/αα | 1 |
| Homozygous Hb E + Hb Constant Spring | 1 |
| Hb S + Hb Kenya | 1 |
| Hb J Roviga | 1 |
| Hb Beograd | 1 |
| Hb G Coushatta | 1 |
| Hb Sirian | 1 |
| Hb Titusville | 1 |
| B variant (not identified yet) | 1 |
| Total Abnormal | 255 |
| No hemoglobinopathy detected | 509 |
| Total | 764 |
| Hemoglobinopathy . | Number . |
|---|---|
| β Thalassemia | 99 |
| Hb S trait | 55 |
| Cord with Hb Barts | 28 |
| Hb C trait | 13 |
| Hb E trait | 12 |
| Hb D trait | 8 |
| α variant (not yet identified) | 5 |
| Hb Barts + Hb S trait | 4 |
| Hb Barts + Hb D trait | 3 |
| Hb S disease | 3 |
| Hb Barts + Hb E trait | 2 |
| Hb E +β Thalassemia | 2 |
| Hb H disease | 2 |
| Hb Constant Spring | 2 |
| Hb J Baltimore | 2 |
| Hb Toulon | 2 |
| δ variant (not identified) | 2 |
| Hb S + Hb C | 1 |
| Hb Barts + Hb C trait | 1 |
| Hb Q Thailand + --SEA/αα | 1 |
| Homozygous Hb E + Hb Constant Spring | 1 |
| Hb S + Hb Kenya | 1 |
| Hb J Roviga | 1 |
| Hb Beograd | 1 |
| Hb G Coushatta | 1 |
| Hb Sirian | 1 |
| Hb Titusville | 1 |
| B variant (not identified yet) | 1 |
| Total Abnormal | 255 |
| No hemoglobinopathy detected | 509 |
| Total | 764 |
Hb F: HPLC vs CE
Hb A2: HPLC vs CE
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal