Abstract
Introduction: On August 2002 an international multicentric trial on Fludarabine monophosphate (FAMP) plus Cyclophsphamyde (Cy) among previously untreated B-cell CLL, was activated. Our aim is to evaluate efficacy and toxicity of FAMP plus Cy in previously untreated B-cell CLL patients (pts). This is the second interim analysis after a fourth-year period.
Material and Methods: Treatment consists in three consecutive days of oral FAMP 40 mg/m2 (n=84) or i.v. FAMP 25 mg/m2 (n=13) plus i.v. Cy 600 mg/m2 on day 1 or Cy 250 mg/m2 from day 1 to 3, every 28 days × 6 cycles. Responses were assessed according to the National Cancer Institute working group criteria after cycle 3 and again after cycle 6. Since August 2002 to March 2006, 109 CLL pts from Argentina (n=95), Perú (n=11) and Uruguay (n=3) were enrolled for this protocol; eighty-nine were evaluated for response and toxicity. Median age: 64 years old (range: 44–81); male = 47, female = 42; Binet staging: A=14, B=45, C=30; median beta-2 microglobuline = 4.00 mg/dL (range: 1.3–9.2); median LDH = 341 UI/L (range: 101–762); among patients with available data the CD 38 expression more than 10% was 38% (22 of 58 pts). Blood counts at inclusion: median values (range); Lymphocytes: 32 ×109/L (2,7–137), Hb: 120 g/L (50–164), platelets: 175×109/L (10–364). Renal and hepatic parameters within normal range limits. Cytogenetic by banding was available in 27 cases: no alterations (n=17), +12 (n=1), del (6), del (12) (n=1), lost Y (n=1).
Results: At the time of this second interim analysis (March 2006), 56.2% (50 pts) had completed 6 cycles and 97.8% (87 pts) had undergone at least 3 cycles.
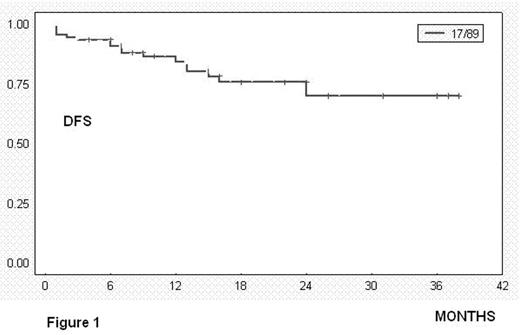
Overall responses: 92% = 81 pts (CR: 39% = 35 pts; PR: 52% = 46 pts); treatment failure: 9% = 7 pts. Evaluation for toxicity: 89 episodes of haematological toxicity and 7 episodes of infection grade 3–4 were reported after 436 cycles. Thirteen pts died: seven due infectious complications because of prolonged hematologic toxicity; one due to tumoral lysis syndrome, one due hemoptysis associated with lung cancer and the remaining four due disease progression. At 24 months, estimated DFS was 70% (figure 1, SE 7.6%) and estimated Overall Survival was 76% (figure 2, SE 7.4%). The median survival was not achieved in responders (PR and CR).
Conclusion: FAMP plus Cy combination as front-line treatment is effective in B-cell CLL. Haematologic toxicity is the most severe adverse events. The response rates to this therapy is quite similar to those reported for other multicentric trials and better than others GATLA protocols.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal